Submitted:
04 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Studied groups
2.2. Assessment of psoriasis severity
2.3. miRNA isolation
2.4. Quantitative RT-PCR (RT-qPCR)
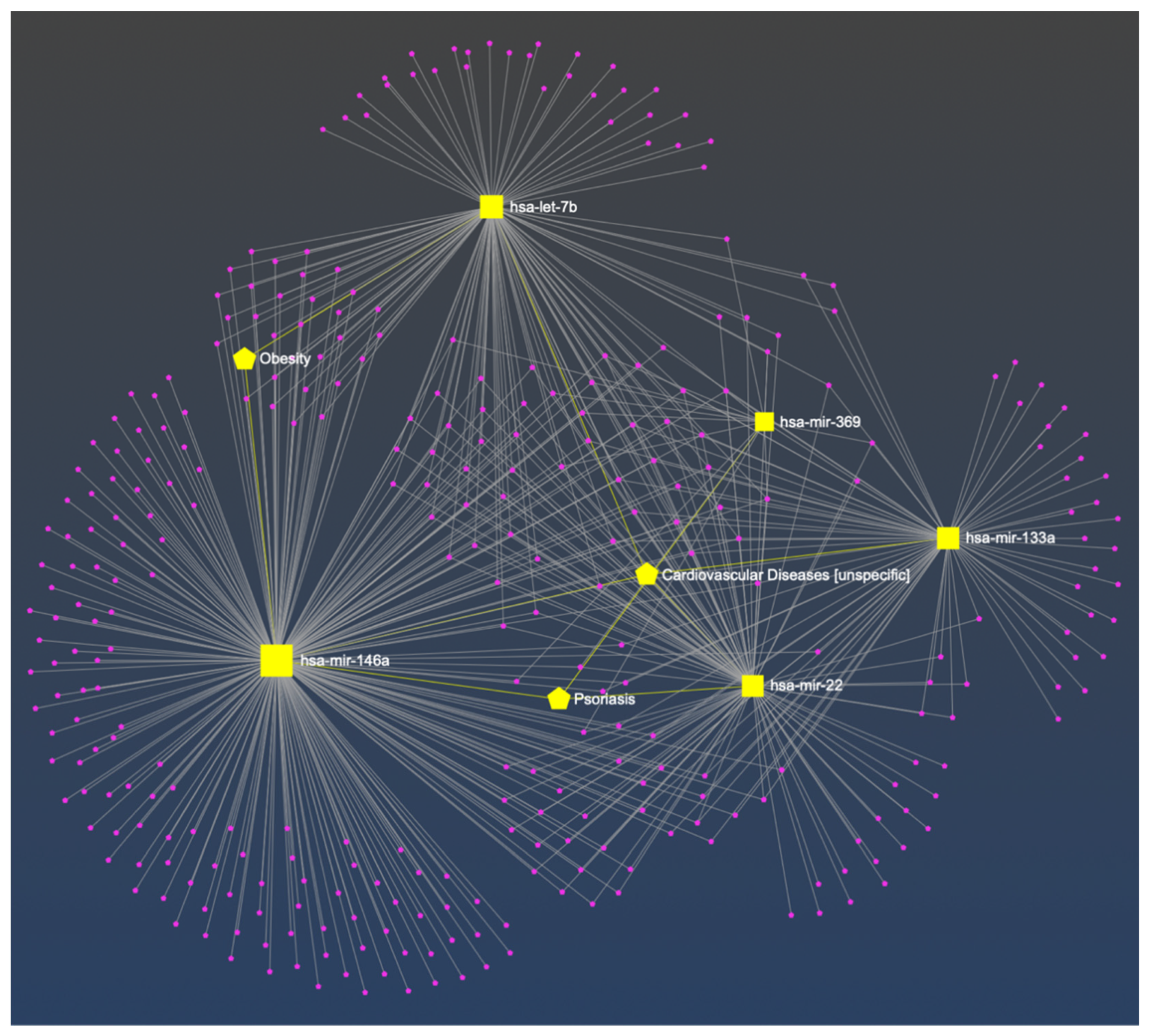
2.5. The constructed miRNA-disease network.
2.6. Data analysis
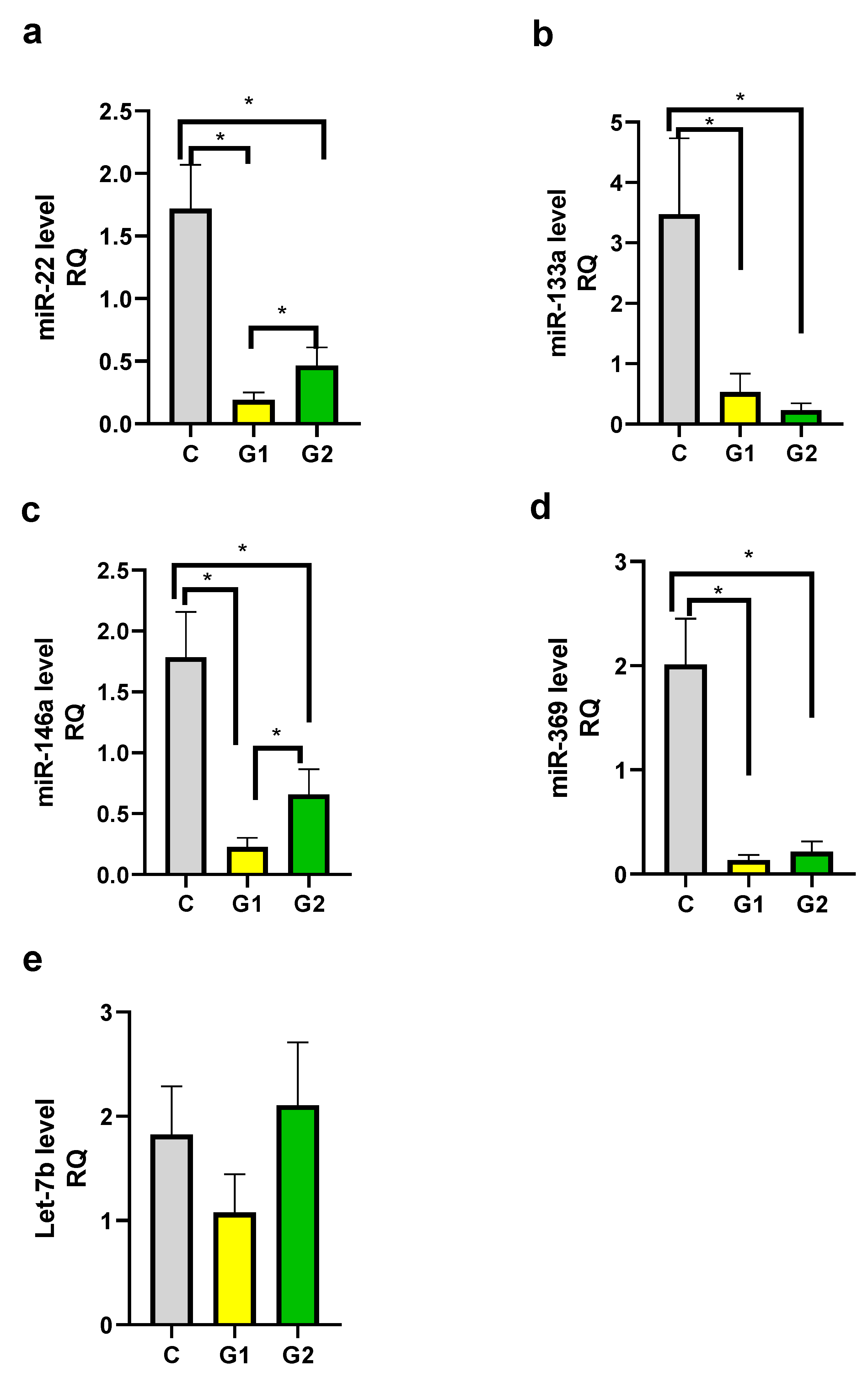
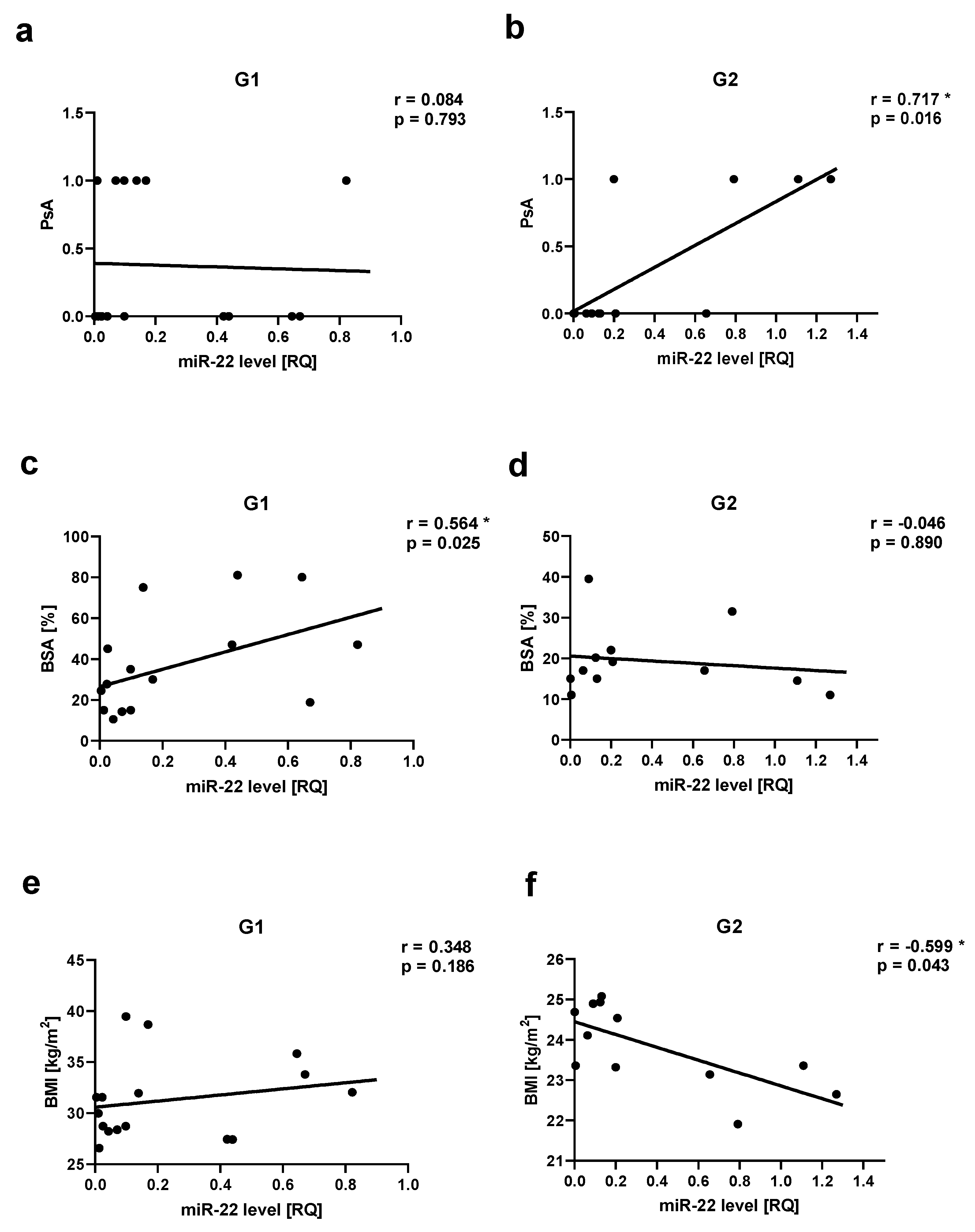
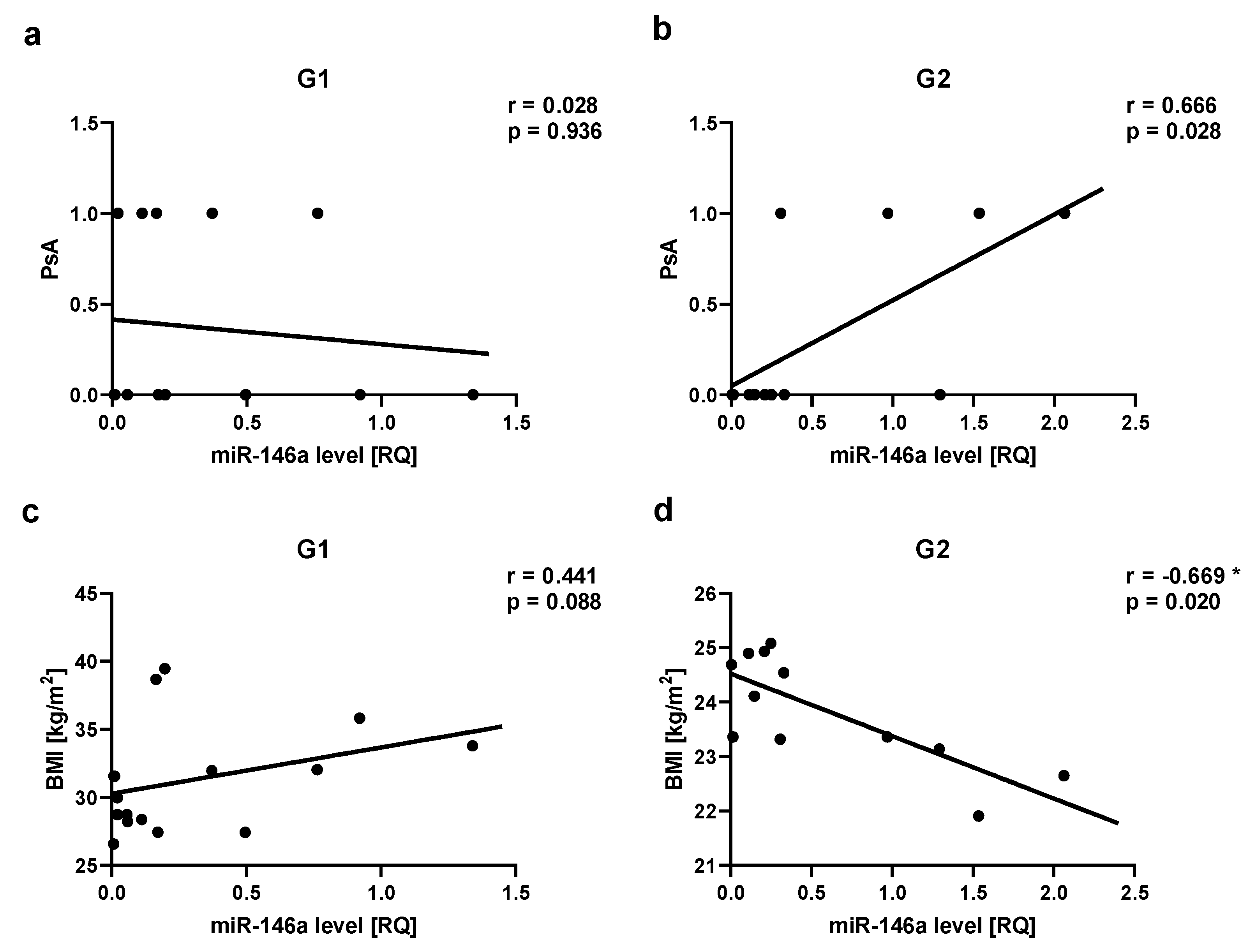
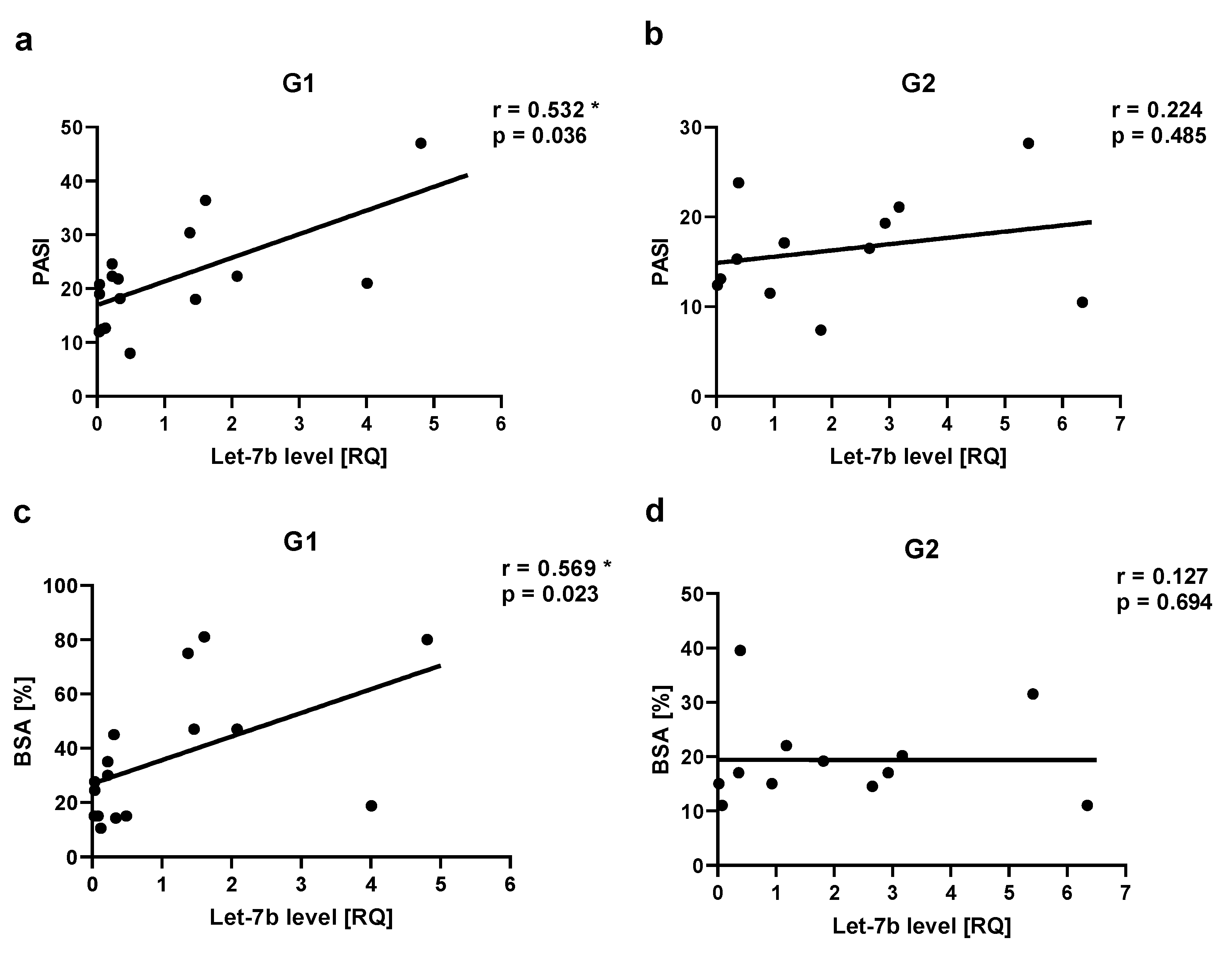
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dopytalska, K.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. The Role of Epigenetic Factors in Psoriasis. Int. J. Mol. Sci. 2021, 22, 9294. [CrossRef]
- Srivastava, A.K.; Chand Yadav, T.; Khera, H.K.; Mishra, P.; Raghuwanshi, N.; Pruthi, V.; Prasad, R. Insights into interplay of immunopathophysiological events and molecular mechanistic cascades in psoriasis and its associated comorbidities. J Autoimmun. 2021, 118, 102614. [CrossRef]
- Di Meglio, P.; Nestle, F.O. The role of IL-23 in the immunopathogenesis of psoriasis. F1000 Biol Rep. 2010, 2, 40. [CrossRef]
- Ogawa, K.; Okada, Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [CrossRef]
- Nedoszytko, B.; Szczerkowska-Dobosz, A.; Stawczyk-Macieja, M.; Owczarczyk-Saczonek, A.; Reich, A.; Bartosińska, J.; Batycka- Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; et al. Pathogenesis of psoriasis in the “omic” era. Part II. Genetic, genomic and epigenetic changes in psoriasis. Postep. Dermatol. Alergol. 2020, 37, 283–298. [CrossRef]
- Kisiel, B.; Kisiel, K.; Szymański, K.; Mackiewicz, W.; Biało-Wójcicka, E.; Uczniak, S.; Fogtman, A.; Iwanicka-Nowicka, R.; Koblowska, M.; Kossowska, H.; et al. The association between 38 previously reported polymorphisms and psoriasis in a Polish population: High predicative accuracy of a genetic risk score combining 16 loci. PLoS ONE 2017, 12, e0179348. [CrossRef]
- Kisielnicka, A.; Sobalska-Kwapis, M.; Purzycka-Bohdan, D.; Nedoszytko, B.; Zabłotna, M.; Seweryn, M.; Strapagiel, D.; Nowicki, R.J.; Reich, A.; Samotij, D.; Szczęch, J.; Krasowska, D.; Bartosińska, J.; Narbutt, J.; Lesiak, A.; Barasińska, P.; Owczarczyk-Saczonek, A.; Czerwińska, J.; Szepietowski, J.C.; Batycka-Baran, A.; Czajkowski, R.; Górecka-Sokołowska, M.; Rudnicka, L.; Czuwara, J.; Szczerkowska-Dobosz, A. The Analysis of a Genome-Wide Association Study (GWAS) of Overweight and Obesity in Psoriasis. Int. J. Mol. Sci. 2022, 23, 7396. [CrossRef]
- Owczarczyk-Saczonek, A.; Purzycka-Bohdan, D.; Nedoszytko, B.; Reich, A.; Szczerkowska-Dobosz, A.; Bartosińska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; et al. Pathogenesis of psoriasis in the “omic” era. Part III. Metabolic disorders, metabolomics, nutrigenomics in psoriasis. Postep. Dermatol. Alergol. 2020, 37, 452–467. [CrossRef]
- Purzycka-Bohdan, D.; Kisielnicka, A.; Bohdan, M.; Szczerkowska-Dobosz, A.; Sobalska-Kwapis, M.; Nedoszytko, B.; Nowicki, R.J. Analysis of the Potential Genetic Links between Psoriasis and Cardiovascular Risk Factors. Int J Mol Sci. 2021, 22, 9063. [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [CrossRef]
- Furrow, R.E.; Christiansen, F.B.; Feldman, M.W. Environment-Sensitive Epigenetics and the Heritability of Complex Diseases. Genet. 2011, 189, 1377–1387. [CrossRef]
- Fogel, O.; Richard-Miceli, C.; Tost, J. Epigenetic Changes in Chronic Inflammatory Diseases. Adv. Protein Chem. Struct. Biol. 2017, 106, 139–189. [CrossRef]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 44, 863–872. [CrossRef]
- Zeng, C.; Tsoi, L.C.; Gudjonsson, J.E. Dysregulated epigenetic modifications in psoriasis. Exp Dermatol. 2021, 30, 1156-1166. [CrossRef]
- Sonkoly, E.; Wei, T.; Janson, P.C.; Sääf, A.; Lundeberg, L.; Tengvall- Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; Ståhle, M.; Pivarcsi, A. MicroRNAs: Novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007, 2, e610. [CrossRef]
- Hawkes, J.E.; Nguyen, G.H.; Fujita, M.; Florell, S.R.; Callis Duffin, K.; Krueger, G.G.; O’Connell, R.M. microRNAs in Psoriasis. J Invest Dermatol. 2016, 136, 365-371. [CrossRef]
- Liu, Y.; Liu, Q. MicroRNAs as regulatory elements in psoriasis. Open Med, 2016, 11, 336–40. [CrossRef]
- Liu, Q.; Wu, D.H.; Han, L.; Deng, J.W.; Zhou, L.; He, R.; Lu, C.J.; Mi, Q.S. Roles of microRNAs in psoriasis: Immunological functions and potential biomarkers. Exp Dermatol. 2017, 26, 359-367. [CrossRef]
- Xiao, S.; Liu, X.; Wang, X.; Lv, H.; Zhao, J.; Guo, X.; Xian, F.; Ji, Y.; Zhang, G. Plasma MicroRNA Expression Profiles in Psoriasis. J Immunol Res. 2020, 16, 1561278. [CrossRef]
- Alatas, E.T.; Kara, M.; Dogan, G.; Akn Belli, A. Blood microRNA expressions in patients with mild to moderate psoriasis and the relationship between microRNAs and psoriasis activity. An Bras Dermatol, 2020, 95, 702e7. [CrossRef]
- Domingo, S.; Solé, C.; Moliné, T.; Ferrer, B.; Cortés-Hernández, J. MicroRNAs in Several Cutaneous Autoimmune Diseases: Psoriasis, Cutaneous Lupus Erythematosus and Atopic Dermatitis. Cells. 2020, 9, 2656. [CrossRef]
- Leal, B.; Carvalho, C.; Ferreira, A.M.; Nogueira, M.; Brás, S.; Silva, B.M.; Selores, M.; Costa, P.P.; Torres, T. Serum Levels of miR-146a in Patients with Psoriasis. Mol Diagn Ther. 2021, 25, 475-485. [CrossRef]
- Kumar, S.; Han, J.; Li, T.; Qureshi, A.A. Obesity, waist circumference, weight change and the risk of psoriasis in US women: Risk of psoriasis in US women. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1293–1298. [CrossRef]
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2016, 232, 633-639. [CrossRef]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [CrossRef]
- Owczarczyk-Saczonek, A.; Placek W. Compounds of psoriasis with obesity and overweight. Postepy Hig Med Dosw. 2017, 71, 761-772. [CrossRef]
- Ko, S.-H.; Chi, C.-C.; Yeh, M.-L.; Wang, S.-H.; Tsai, Y.-S.; Hsu, M.-Y. Lifestyle changes for treating psoriasis. Cochrane Database Syst. Rev. 2019, 7, 1–68. [CrossRef]
- Hsu, S.; Green, L.J.; Lebwohl, M.G.; Wu, J.J.; Blauvelt, A.; Jacobson, A.A. Comparable efficacy and safety of brodalumab in obese and nonobese patients with psoriasis: Analysis of two randomized controlled trials. Br. J. Derm. 2020, 182, 880–888. [CrossRef]
- Norden, A.; Rekhtman, S.; Strunk, A.; Garg, A. Risk of psoriasis according to body mass index: A retrospective cohort analysis. J. Am. Acad. Dermatol. 2021, 86, 1020–1026. [CrossRef]
- Barros, G.; Duran, P.; Vera, I.; Bermúdez, V. Exploring the Links between Obesity and Psoriasis: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 7499. [CrossRef]
- Budu-Aggrey, A.; Brumpton, B.; Tyrrell, J.; Watkins, S.; Modalsli, E.H.; Celis-Morales, C.; Ferguson, L.D.; Vie G.Å.; Palmer, T.; Fritsche, L.G.; et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. PLoS Med. 2019, 16, e1002739. [CrossRef]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb., 2010, 17; 332-341. [CrossRef]
- Carrascosa, J.M.; Rocamora, V.; Fernandez-Torres, R.M.; Jimenez- Puya, R.; Moreno, J.C.; Coll-Puigserver, N.; Fonseca, E. Obesity and psoriasis: Inflammatory nature of obesity, relationship between psoriasis and obesity, and therapeutic implications. Actas Dermosifiliogr., 2014, 105. [CrossRef]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 bias. Eur. J. Immunol., 2009, 39, 2629-2635. [CrossRef]
- Ahmed, M.; Gaffen, S.L. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev., 2010; 21: 449-453. [CrossRef]
- Price, N.L.; Fernández-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta. 2016, 1861, 2104–2110. [CrossRef]
- Landrier, J.F.; Derghal, A.; Mounien. L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells. 2019; 8, 859. [CrossRef]
- Gharanei, S.; Shabir, K.; Brown, J.E.; Weickert, M.O.; Barber, T.M.; Kyrou, I.; Randeva, H.S. Regulatory microRNAs in Brown, Brite and White Adipose Tissue. Cells. 2020, 9, 2489. [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [CrossRef]
- Boehncke, W.H.; Boehncke, S.; Tobin, A.M.; Kirby, B. The “psoriatic march”: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303-307. [CrossRef]
- Hajer, G.R., Van Haeften, T.W., Visseren, F.L.J. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Hear. J. 2008, 29, 2959–2971. [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum 2006, 54, 2665–2673. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [CrossRef]
- miRNet - a miRNA-centric network visual analytics platform. Available online: https://www.mirnet.ca/miRNet/home.xhtml (accessed on 14 May 2023).
- 45. World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation, 894. World Health Organ Tech Rep Ser; 2000, 1–253.
- Pischon, T. Use of obesity biomarkers in cardiovascular epidemiology. Dis Markers 2009, 26, 247–263. [CrossRef]
- Nimptsch, K.; Konigorski, S.; Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019, 92, 61-70. [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr. 2010, 64, 2-5. [CrossRef]
- Michalak-Stoma, A.; Bartosińska, J.; Kowal, M.; Raczkiewicz, D.; Krasowska, D.; Chodorowska, G. IL-17A in the Psoriatic Patients’ Serum and Plaque Scales as Potential Marker of the Diseases Severity and Obesity. Mediators Inflamm. 2020 5, 7420823. [CrossRef]
- Sileno, S.; Beji, S.; D’Agostino, M.; Carassiti, A.; Melillo, G.; Magenta, A. microRNAs involved in psoriasis and cardiovascular diseases. Vasc Biol. 2021 Jun 3;3(1):R49-R68. [CrossRef]
- Kadye, R.; Stoffels, M.; Fanucci, S.; Mbanxa, S.; Prinsloo, E. A STAT3 of Addiction: Adipose Tissue, Adipocytokine Signalling and STAT3 as Mediators of Metabolic Remodelling in the Tumour Microenvironment. Cells, 2020, 9, 1043. [CrossRef]
- van Boven, N.; Akkerhuis, K.M.; Anroedh, S.S.; Rizopoulos, D.; Pinto, Y.; Battes, L.C.; Hillege, H.L.; Caliskan KC, Germans T, Manintveld OC; et al. Serially measured circulating miR-22-3p is a biomarker for adverse clinical outcome in patients with chronic heart failure: The Bio-SHiFT study. Int J Cardiol. 2017, 235, 124-132. [CrossRef]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res. 2013,112, 1234-1243. [CrossRef]
- Figueiredo, R.; Adão, R.; Leite-Moreira, A.F.; Mâncio, J.; Brás-Silva, C. Candidate microRNAs as prognostic biomarkers in heart failure: A systematic review. Rev Port Cardiol. 2022, 41, 865-885. [CrossRef]
- Yang, F.; Chen, Q.; He, S.; Yang, M.; Maguire, E.M.; An, W.; Afzal, T.A.; Luong, L.A.; Zhang, L.; Xiao, Q. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 2018, 137, 1824–1841. [CrossRef]
- Ghorbani, S.; Sezavar, S.H.; Bokharaei-Salim, F.; Ataei-Pirkooh, A.; Tavakoli, A.; Javanmard, D.; Sadri-Nahand, J.; Kiani, S.J.; Ghaffari, H.; Beikzadeh, L.; et al. Expression levels of miR-22, miR-30c, miR-145, and miR-519d and their possible associations with inflammatory markers among patients with coronary artery disease. ARYA Atheroscler. 2022, 18, 1-10. [CrossRef]
- Wang, Y.; Chang, W.; Zhang, Y.; Zhang L.; Ding, H.; Qi, H.; Xue, S.; Yu, H.; Hu, L.; Liu, D.; et al. Circulating miR-22-5p and miR-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. J Cell Physiol. 2019, 234, 4778–4786. [CrossRef]
- Tu, Y.; Wan, L.; Zhao, D.; Bu, L.; Dong, D.; Yin, Z.; Cheng, Z.; Shen, B. In vitro and in vivo direct monitoring of miRNA-22 expression in isoproterenol-induced cardiac hypertrophy by bioluminescence imaging. Eur J Nucl Med Mol Imaging. 2014, 41, 972–984. [CrossRef]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circulation Research 2013, 112, 1234–1243. [CrossRef]
- Gurha, P.; Wang, T.; Larimore, A.H.; Sassi, Y.; Abreu-Goodger, C.; Ramirez, M.O.; Reddy, A.K.; Engelhardt, S.; Taffet G.E.; Wehrens X.H.; et al. microRNA-22 promotes heart failure through coordinate suppression of PPAR/ERR-nuclear hormone receptor transcription. PLoS ONE 2013, 8, e75882. [CrossRef]
- Gupta, S.K.; Foinquinos, A.; Thum, S.; Remke, J.; Zimmer, K.; Bauters, C.; de Groote, P.; Boon, R.A.; de Windt, L.J.; Preissl, S.; et al. Preclinical Development of a MicroRNA-Based Therapy for Elderly Patients With Myocardial Infarction. J Am Coll Cardiol. 2016, 68, 1557-1571. [CrossRef]
- Xia, P.; Fang, X.; Zhang, Z.H.; Huang, Q.; Yan, K.X.; Kang, K.F.; Han, L.; Zheng, Z.Z. Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol Lett. 2012, 148, 151-162. [CrossRef]
- García-Rodríguez, S.; Arias-Santiago, S.; Blasco-Morente, G.; Orgaz-Molina, J.; Rosal-Vela, A.; Navarro, P.; Magro-Checa, C.; Martínez-López, A.; Ruiz, J.C.; Raya, E.; Naranjo-Sintes, R.; Sancho, J.; Zubiaur, M. Increased expression of microRNA-155 in peripheral blood mononuclear cells from psoriasis patients is related to disease activity. J Eur Acad Dermatol Venereol. 2017, 31, 312-322. [CrossRef]
- Hermann, H.; Runnel, T.; Aab, A.; Baurecht, H.; Rodriguez, E.; Magilnick, N.; Urgard, E.; Šahmatova, L.; Prans, E.; Maslovskaja, J.; et al. miR-146b Probably Assists miRNA-146a in the Suppression of Keratinocyte Proliferation and Inflammatory Responses in Psoriasis. J. Investig. Dermatol. 2017, 137, 1945–1954. [CrossRef]
- Løvendorf, M.B.; Mitsui, H.; Zibert, J.R.; Røpke, M.A.; Hafner, M.; Dyring- Andersen, B.; Bonefeld, C.M.; Krueger, J.G.; Skov, L. Laser capture microdissection followed by next-generation sequencing identifies disease-related microRNAs in psoriatic skin that reflect systemic microRNA changes in psoriasis. Exp Dermatol. 2015, 24, 187–193. [CrossRef]
- Shen, H.; Wang, D.; Zhan, M.; Ding, H.; Zhao, H. MicroRNA-146a and microRNA-146b deficiency correlates with exacerbated disease activity, and their longitude increment relates to etanercept response in psoriasis patients. J Clin Lab Anal. 2022, 36, e24198. [CrossRef]
- Koga, Y.; Jinnin, M.; Ichihara, A.; Fujisawa, A.; Moriya, C.; Sakai, K.; Fukushima, S.; Inoue, Y.; Ihn, H. Analysis of expression pattern of serum microRNA levels in patients with psoriasis. J Dermatol Sci. 2014, 74, 170-171. [CrossRef]
- Srivastava, A.; Nikamo, P.; Lohcharoenkal, W.; Li, D.; Meisgen, F.; Xu Landén, N.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol. 2017, 139, 550-561. [CrossRef]
- Xiuli, Y.; Honglin, W. miRNAs Flowing Up and Down: The Concerto of Psoriasis. Front Med (Lausanne). 2021, 26, 646796. [CrossRef]
- Meisgen, F.; Xu Landén, N.; Wang, A.; Réthi, B.; Bouez, C.; Zuccolo, M.; Gueniche, A.; Ståhle, M.; Sonkoly, E.; Breton, L.; Pivarcsi, A. MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J Investig Dermatol. 2014, 134,1931– 1940. [CrossRef]
- Shams, K.; Kurowska-Stolarska, M.; Schütte, F.; Burden, A.D.; McKimmie, C.S.; Graham, G.J. MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2. J Biol Chem. 2018, 293, 3003–3012. [CrossRef]
- Lu, L.F.; Boldin, M.P.; Chaudhry, A.; Lin, L.L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of miR-146a in controlling treg cell-mediated regulation of Th1 responses. Cell. 2010, 142, 914–929. [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M. R.; Sabbatinelli, J.; Bonafè, M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res Rev. 2021, 70, 101374. [CrossRef]
- Mortazavi-Jahromi, S.S.; Aslani, M.; Mirshafiey, A. A comprehensive review on miR-146a molecular mechanisms in a wide spectrum of immune and non-immune inflammatory diseases. Immunol Lett. 2020, 227, 8-27. [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; Laaksonen, R.; Lehtimäki, T. miR-21.; miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011, 219, 211-217. [CrossRef]
- Cheng, H.S.; Besla, R.; Li, A.; Chen, Z.; Shikatani, E.A.; Nazari-Jahantigh, M.; Hammoutène, A.; Nguyen, M.A.; Geoffrion, M.; Cai, L.; Khyzha, N.; Li, T.; MacParland, S.A.; Husain, M.; Cybulsky, M.I.; Boulanger, C.M.; Temel, R.E.; Schober, A.; Rayner, K.J.; Robbins, C.S.; Fish, J.E. Paradoxical Suppression of Atherosclerosis in the Absence of microRNA-146a. Circ Res. 2017, 121, 354-367. [CrossRef]
- Cao, J.; Zhang, K.; Zheng, J.; Dong, R. MicroRNA-146a and -21 cooperate to regulate vascular smooth muscle cell proliferation via modulation of the Notch signaling pathway. Mol Med Rep. 2015, 11, 2889-2895. [CrossRef]
- Chen, T.; Li, Z.; Jing, T.; Zhu, W.; Ge, J.; Zheng, X.; Pan, X.; Yan, H.; Zhu, J. MicroRNA-146a regulates the maturation process and pro-inflammatory cytokine secretion by targeting CD40L in oxLDL-stimulated dendritic cells. FEBS Lett. 2011, 585, 567-573. [CrossRef]
- Wang, H.J.; Huang, Y.L.; Shih, Y.Y.; Wu, H.Y.; Peng, C.T.; Lo, W.Y. MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflamm. 2014, 2014, 379537. [CrossRef]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854-860. [CrossRef]
- Oh, J.G.; Watanabe, S.; Lee, A.; Gorski, P.A.; Lee, P.; Jeong, D.; Liang, L.; Liang, Y.; Baccarini, A.; Sahoo, S.; et al. miR-146a suppresses SUMO1 expression and induces cardiac dysfunction in maladaptive hypertrophy. Circ Res. 2018, 123, 673–685. [CrossRef]
- Barraclough, J.Y.; Joglekar, M.V.; Januszewski, A.S.; Martínez, G.; Celermajer, D.S.; Keech, A.C.; Hardikar, A.A.; Patel, S. A MicroRNA Signature in Acute Coronary Syndrome Patients and Modulation by Colchicine. J Cardiovasc Pharmacol Ther. 2020, 25, 444-455. [CrossRef]
- Xue, S.; Zhu, W.; Liu, D.; Su, Z.; Zhang, L.; Chang, Q.; Li, P. Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol Med. 2019, 25, 18. [CrossRef]
- Guo, M.; Mao, X.; Ji, Q.; Lang, M.; Li, S.; Peng, Y.; Zhou, W.; Xiong, B.; Zeng, Q. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol. 2010, 88, 555-564. [CrossRef]
- Hijmans, J.G.; Diehl, K.J.; Bammert, T.D.; Kavlich, P.J.; Lincenberg, G.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of Overweight and Obesity on Circulating Inflammation-Related microRNA. Microrna. 2018, 7, 148-154. [CrossRef]
- Al-Rawaf, H.A. Circulating microRNAs and adipokines as markers of metabolic syndrome in adolescents with obesity. Clin Nutr. 2019, 38, 2231-2238. [CrossRef]
- Benbaibeche, H.; Hichami, A.; Oudjit, B.; Haffaf, E.M.; Kacimi, G.; Koceïr, E.A.; Khan, N.A. Circulating mir-21 and mir-146a are associated with increased cytokines and CD36 in Algerian obese male participants. Arch Physiol Biochem. 2022, 128, 1461-1466. [CrossRef]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.X.; Liu, N.; Rudnicki, M.A.; Kuang, S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013, 9, e1003626. [CrossRef]
- Yin, H.; Pasut, A.; Soleimani, V.D.; Bentzinger, C.F.; Antoun, G.; Thorn, S.; Seale, P.; Fernando, P.; Van Ijcken, W.; Grosveld, F.; et al. MicroRNA-133 Controls Brown Adipose Determination in Skeletal Muscle Satellite Cells by Targeting Prdm16. Cell Metab. 2013, 17, 210–224. [CrossRef]
- Trajkovski, M.; Ahmed, K.; Esau, C.C.; Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012, 14, 1330–1335. [CrossRef]
- Valente, M.H.; Gomes, F.M.D.S.; Benseñor, I.J.M.; Brentani, A.; Escobar, A.M.D.U.; Grisi, S. Relation between Birth Weight, Growth, and Subclinical Atherosclerosis in Adulthood. BioMed. Res. Int. 2015, 2015, 926912. [CrossRef]
- Wu, L.; Van Kaer, L. Contribution of lipid-reactive natural killer T cells to obesity-associated inflammation and insulin resistance. Adipocyte 2013, 2, 12–16. [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [CrossRef]
- Vijgen, G.H.E.J.; Bouvy, N.D.; Teule, G.J.J.; Brans, B.; Schrauwen, P.; Lichtenbelt, W.D.V.M. Brown Adipose Tissue in Morbidly Obese Subjects. PLoS ONE 2011, 6, e17247. [CrossRef]
- Wang, Y.; Cai, L.; Wu, Y.; Wilson, R.F.; Weston, C.; Fawole, O.; Bleich, S.N.; Cheskin, L.J.; Showell, N.N.; Lau, B.D.; et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 547–565. [CrossRef]
- Bostjancic, E.; Brandner, T.; Zidar, N.; Glavac, D.; Stajer, D. Down- regulation of miR-133a/b in patients with myocardial infarction correlates with the presence of ventricular fibrillation. Biomed Pharmacother. 2018, 99, 65–71. [CrossRef]
- Bostjancic E.; Zidar N.; Stajer D.; Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 2010, 115, 163–169. [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nature Medicine 2007, 13, 613–618. [CrossRef]
- Shi, L.; Yu, C.; Tian, X.; Ma, C.; Wang, L.; Xia, D.; Cui, C.; Chen, X.; Jiang, T.; Gu, Y.; et al. Effect of microRNA-133a-3p/matrix metalloproteinase-9 axis on the growth of atherosclerotic vascular smooth muscle cells. Exp Ther Med. 2019,18 4356–4362. [CrossRef]
- Liao, X.B.; Zhang, Z.Y.; Yuan, K.; Liu, Y.; Feng, X.; Cui, R.R.; Hu, Y.R.; Yuan, Z.S.; Gu, L.; Li, S.J.; et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 2013, 154, 3344–3352. [CrossRef]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011, 4, 446–454. [CrossRef]
- Guo, S.; Zhang, W.; Wei, C.; Wang, L.; Zhu, G.; Shi, Q.; Li, S.; Ge, R.; Li, K.; Gao, L.; Gao, T.; Wang, G.; Li, C. Serum and skin levels of miR-369-3p in patients with psoriasis and their correlation with disease severity. Eur J Dermatol. 2013, 23, 608-613. [CrossRef]
- Scalavino, V.; Liso, M.; Cavalcanti, E.; Gigante, I.; Lippolis, A.; Mastronardi, M.; Chieppa, M,; Serino, G. miR-369-3p modulates inducible nitric oxide synthase and is involved in regulation of chronic inflammatory response. Scientific Reports 2020, 10, 15942. [CrossRef]
- Wang, J.; Chen, X.; Huang, W. MicroRNA-369 attenuates hypoxia-induced cardiomyocyte apoptosis and inflammation via targeting TRPV3. Braz J Med Biol Res. 2021, 54, e10550. [CrossRef]
- Liang, J.; Bai, S.; Su, L.; Li ,C.; Wu, J.; Xia, Z .; Xu, D. A subset of circulating microRNAs is expressed differently in patients with myocardial infarction. Mol Med Rep. 2015, 12, 243–247. [CrossRef]
- Wu, Y.; Liu, L.; Bian, C.; Diao, Q.; Nisar, M.F.; Jiang, X.; Bartsch, J.W.; Zhong, M.; Hu, X.; Zhong, J.L. MicroRNA let-7b inhibits keratinocyte differentiation by targeting IL-6 mediated ERK signaling in psoriasis. Cell Commun Signal. 2018, 16, 58. [CrossRef]
- Mirzaei, R.; Zamani, F.; Hajibaba, M.; Rasouli-Saravani, A.; Noroozbeygi, M.; Gorgani, M.; Hosseini-Fard, S.R.; Jalalifar, S.; Ajdarkosh, H.; Abedi, S.H.; Keyvani, H.; Karampoor, S. The pathogenic.; therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J Neuroimmunol. 2021, 358, 577640. [CrossRef]
- Pasquali, L.; Svedbom, A.; Srivastava, A.; Rosén, E.; Lindqvist, U.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. Circulating microRNAs in extracellular vesicles as potential biomarkers for psoriatic arthritis in patients with psoriasis. J Eur Acad Dermatol Venereol. 2020, 34, 1248-1256. [CrossRef]
- Li, Y.; He, X.N.; Li, C.; Gong, L.; Liu, M. Identification of Candidate Genes and MicroRNAs for Acute Myocardial Infarction by Weighted Gene Coexpression Network Analysis. Biomed Res Int. 2019, 2019, 5742608. [CrossRef]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective effect of let-7 mirna family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes 2017, 66, 2266–2277. [CrossRef]
- Perez-Sanchez, C.; Font-Ugalde, P.; Ruiz-Limon, P.; Lopez-Pedrera, C.; Castro-Villegas, MC.; Abalos-Aguilera, M.C.; Barbarroja, N.; Arias-de la Rosa, I.; Lopez-Montilla, M.D.; Escudero-Contreras A.; et al. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Human Molecular Genetics 2018, 27, 875–890. [CrossRef]
- Wade, S.M.; McGarry, T.; Wade, S.C.; Fearon, U.; Veale, D.J. Serum MicroRNA Signature as a Diagnostic and Therapeutic Marker in Patients with Psoriatic Arthritis. J Rheumatol. 2020, 47, 1760-1767. [CrossRef]
- Mantravadi, S.; Ogdie, A.; Kraft, W.K. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev Clin Pharmacol 2017, 10, 899– 910. [CrossRef]
- Costa, L.; Ramonda, R.; Ortolan, A.; Favero, M.; Foti, R.; Visalli, E.; Rossato, M.; Cacciapaglia, F.; Lapadula, G.; Scarpa, R. Psoriatic arthritis and obesity: The role of anti-IL-12/IL-23 treatment. Clin Rheumatol. 2019, 38, 2355-2362. [CrossRef]
- Costa, L.; Caso, F.; Atteno, M.; Del Puente, A.; Darda, M.A.; Caso, P.; Ortolan, A.; Fiocco, U.; Ramonda, R.; Punzi, L.; Scarpa R. Impact of 24-month treatment with etanercept, adalimumab, or methotrexate on metabolic syndrome components in a cohort of 210 psoriatic arthritis patients. Clin Rheumatol. 2014, 33, 833-839. [CrossRef]
- Shan, J.; Zhang, J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: A systematic review and meta-analysis. Joint Bone Spine 2018, 86, 173–183. [CrossRef]
- Singh, S.; Facciorusso, A.; Singh, A.G.; Casteele, N.V.; Zarrinpar, A.; Prokop, L.J.; Grunvald, E.L.; Curtis, J.R.; Sandborn, W.J. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS ONE, 2018, 13, e0195123. [CrossRef]
- Chatzikyriakidou, A.; Voulgari, P.V.; Georgiou, I.; Droso,s A.A. The role of microRNA-146a (miR-146a) and its target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scand J Immunol. 2010, 71, 382-385. [CrossRef]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal models of psoriasis-highlights and drawbacks. J Allergy Clin Immunol. 2021, 147, 439-455. [CrossRef]
- Mabuchi, T.; Singh, T.P.; Takekoshi, T.; Jia, G.F.; Wu, X.; Kao, M.C.; Weiss, I.; Farber, J.M.; Hwang, S.T. CCR6 is required for epidermal trafficking of γδ-T cells in an IL-23-induced model of psoriasiform dermatitis. J Invest Dermatol. 2013, 133, 164-171. [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; Lubberts, E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009, 182, 5836-5845. [CrossRef]
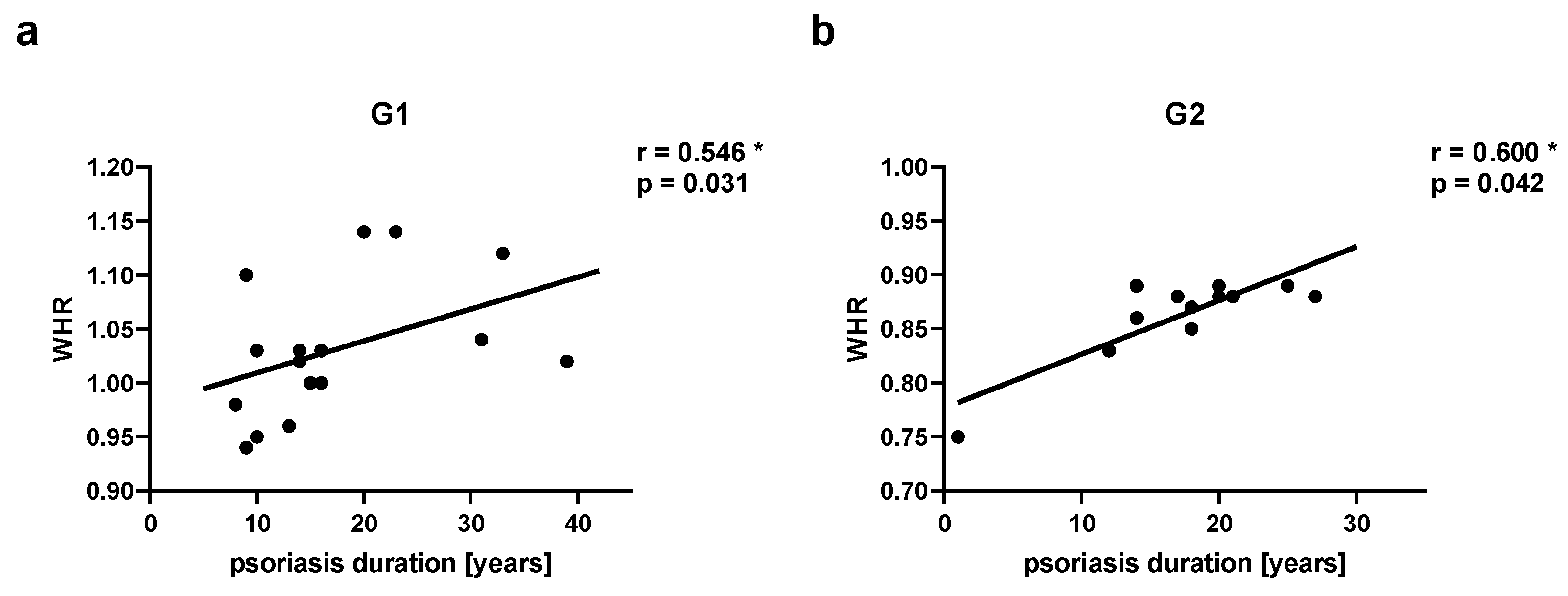
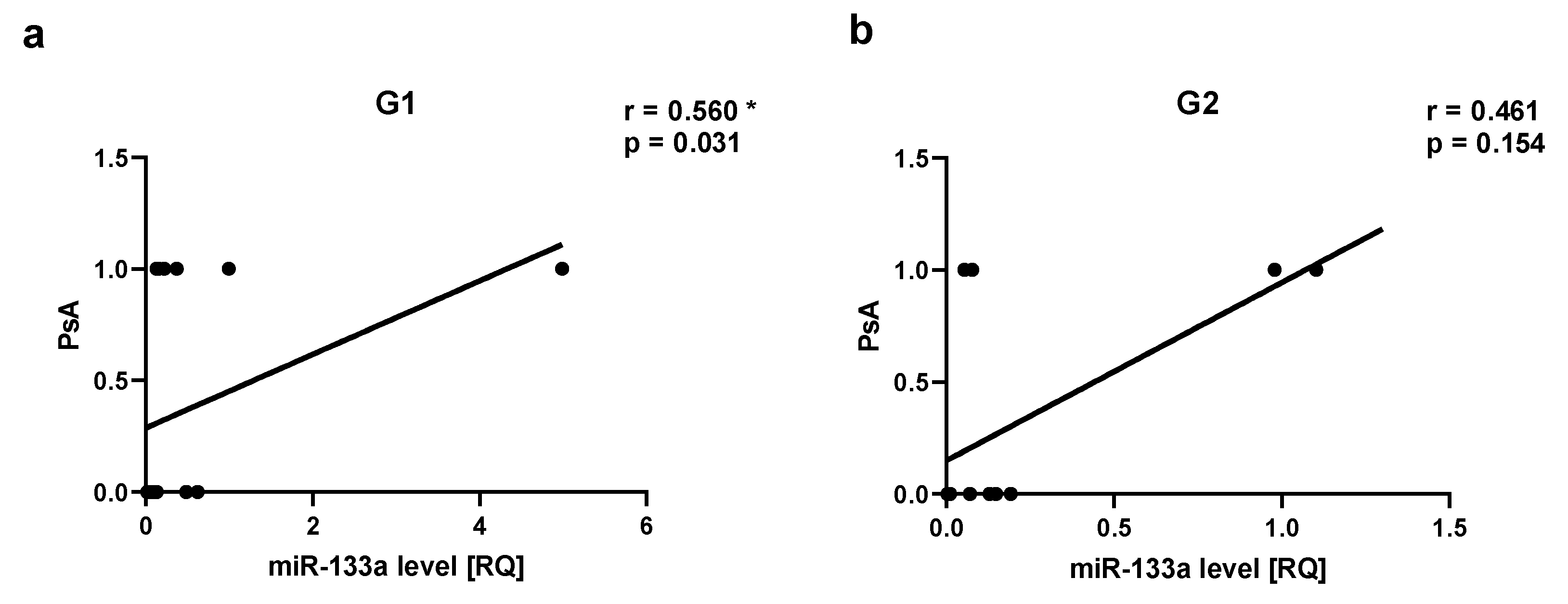
| CHARACTERISTIC | PSORIATIC PATIENTS | CONTROL GROUP (N=16) Mean (SEM) |
|
|---|---|---|---|
| GROUP 1 (N=16) Mean (SEM) |
GROUP 2 (N=12) Mean (SEM) |
||
| Age (years) | 45.9 (13.5) | 40 (12.3) | 38.4 (8.3) |
| Duration of psoriasis (years) | 17.5 (9.2) | 17.25 (6.7) | - |
| Psoriatic arthritis (PsA)* | 6 (37.5%) | 4 (33.3%) | - |
| PASI | 21.7 (9.7) | 16.35 (5.98) | - |
| BSA | 36.3 (24.2) | 19.4 (8.4) | - |
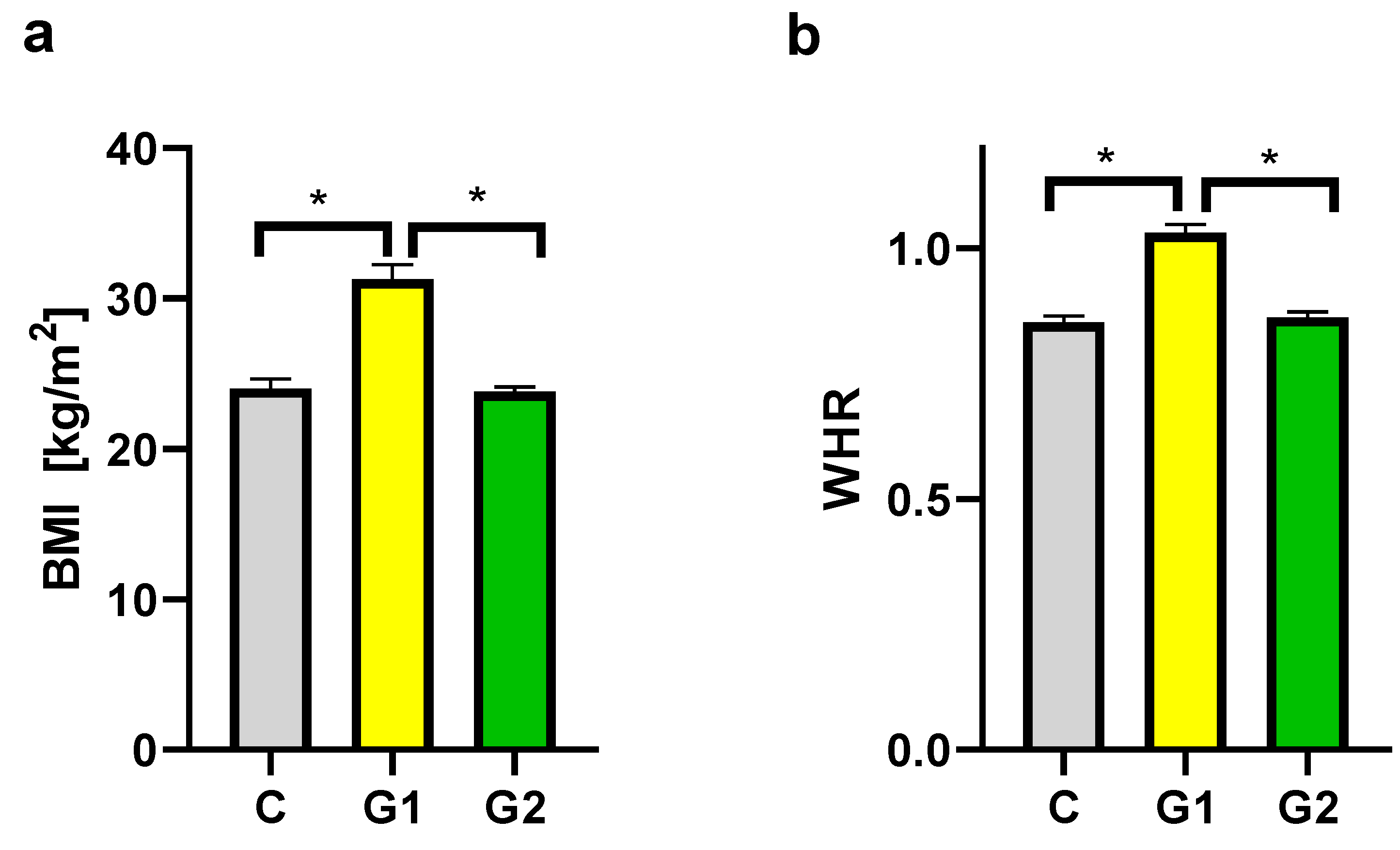
| BMI | 31.3 (3.9) | 23.8 (1.0) | 24.0 (2.4) |
| BMI in patients with PsA | 31.6 (3.8) | 22.8 (0.7) | 24.0 (2.4) |
| WHR | 1.03 (0.1) | 0.86 (0.04) | 0.85 (0.05) |
| WHR in patients with PsA | 1.08 (0.06) | 0.88 (0.01) | 0.85 (0.05) |
| Lipid disturbances* | 10 (62.5%) | 6 (50%) | 3 (18.75%) |
| Hypertension* | 9 (56.25%) | 6 (50%) | 2 (12.5%) |
| Hyperglycemia* | 3 (18.75%) | 1 (8.3%) | 1 (6.25%) |
| Smoking addiction* | 6 (37.5%) | 8 (66.7%) | 5 (31.25%) |
| Alcohol abuse* | 2 (12.5%) | 4 (33.3%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





