Antimicrobial resistance (AMR) alone is expected to kill more people than cancer and car accidents combined with a death rate of approximately 700000 people per annum and a projected 10 million deaths by 2050 at global level.
Antibiotics are a subset of antimicrobial medications employed in countering pathogenic activity. AMR, arising due to loss of effectiveness of antibiotics, indicates to increasing difficulty in treating common infections and rising post-procedural infections/mortality, forcing physicians to resort to higher-grade alternatives. The primary consequences of this phenomenon are its contribution to creation of multi-drug resistant variants which ultimately results in higher mortality (e.g., multi drug resistant streptococcus aureus MRSA) alongside increased disease burden and mortality (rates) especially in regions where with limited medical access in low- and middle-income countries (1). This is especially true in India, one of the nations with the highest disease burden in the world(GHS Index Score: 60.2) alongside a multivariate (mixed?) and poorly regulated healthcare system which leads to high susceptibility for AMR development. Recent AMR studies demonstrate incidence of resistant infections in not only a hospital/care setting but also in the wider community due to increased availability of over-the-counter medications and failure of users to complete the dosage requirement(will need a reference). The increased ineffectiveness of treatment caused by AMR adds to sociocultural biases against modern medicine.
In the following sections, this review aims to explore the factors contributing to , mechanisms of development of, and overview of the Indian policy-based approach to combating AMR.
Given the significance of geographical variability within the environmental aspect of AMR development, single-model approaches will not be effective. Moreover, this does not pose as much a risk of being a leading cause of AMR in developed countries as much as it does in developing countries such as those in SE Asia, serving as a driver of environmental AMR in India (2, 3).
Between 2000 and 2010, consumption in pharmaceuticals increased by nearly 35% across countries with 76% of the contribution to this increase arising from India, Brazil, Russia, and China among others (4). AMR incidence in India contributed to by its growing role in the global pharmaceutical market with a CAGR of around 17% between 2008-09 and 2013-14 alongside developing into a key supplier of Active Pharmaceutical Ingredients (APIs) for drug manufacturing processes internationally (5). The lack of understanding of medication combinations even among medical practitioners, along with the broad availability of illegal antimicrobials, exacerbated by insufficient government control, acts as a breeding ground for AM abuse (6, 7).
Various forms of contaminated water are observed at different levels across the nation, with three principal repositories. Firstly, it has been estimated pharmaceutical industry wastewater contains several kilograms of antibiotics discarded into water every day (8). Secondly, of the 30-90% of all antimicrobials excreted as such via human excreta, only 20-30% of this waste output undergoes treatment as municipal wastewater with the rest discarded into nearby water bodies. Finally, hospital wastewater, bearing the highest level of antimicrobial consumption is discharged without treatment into surface water. The water arising from such places can be said to be the richest sources of AMR bacteria and has been implicated in driving genotoxic alterations and modifying bacterial strains into more resistant manifestations (9)
Alternative sources of contamination include livestock discard wherein direct or indirect environmental contamination due to animal excreta. This problem, while being well-documented in the Netherlands, has not been extensively studied in an Indian context (10) However, it can be assumed to be worsened in the latter context due to overcrowded shelters, widespread inappropriate disposal of animal excrement and corpses in livestock farms (11). Agricultural manure and sludge also contain components replete with bacteria and medicines derived from biological waste water treatment. The antibacterial composition of both varies depending on the source; for example, manure has an abundance of medications such as oxytetracycline and doxycycline, whereas sludge contains pharmaceuticals that are generally less water-soluble such as Ciprofloxacin and norfloxacin (12). The gene responsible for conferring resistance (resistosome) has been found to persist long after the manure or sludge has been decomposed (13, 14). Heavy metal exposure to antimicrobials demonstrates responses of resistance development i.e., via target alterations, decreased membrane permeability with metal-resistant bacteria more likely to develop drug resistance as well (15, 16).
The following major pathways of drug resistance development have been identified: drug inactivation/alteration, drug binding site modification, porin loss, and the formation of efflux pumps for efficient antibiotic clearance from the intracellular compartment (17-20).
In addition to the above factors affecting and overview of AMR genesis, bacteria also differ in the mechanisms of AMR development. The numerous examples of this are observed in AmpC (type of b-lactamase-mediated) drug resistance in Enterobacteriaceae, metallo-beta-lactamase resistance in Gram-negative bacteria, vancomycin-resistant enterococci (VRE), and the XDR mycobacterium tuberculosis species which is resistant to fluoroquinolones and second-line injectable drugs such as amikacin, capreomycin, and kanamycin (21). Additionally, resistance against key last-resort drugs (carbapenems and colistin) have been identified among Gram-negative organisms (22).
Multidrug resistant strains, also referred to as the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) have also been on the rise (23).
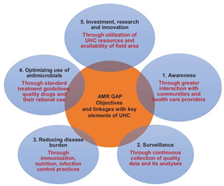
The implementation of AMR policies is inhibited by its sheer complexity alongside the presence of various competing national priorities equally demanding a strategic, efficient solution. This is especially true in developing countries like India already combating multiple issues for creation of a stand-alone national program due to technical and financial difficulties. As a result, it is necessary to identify a point of entry for AMR implementation in India. The consensus lies in the fact that the WHOGAP cannot work without Universal Health Coverage (UHC). This is because UHC is more than health financing involving all components of the health system as well, it impacts all key aspects of the WHO GAP (24):
The WHO developed a Global Action Plan global in response to the AMR problem, the nature of which demands the establishment of epidemiological surveillance systems (26).
The WHO Global action plan on AMR encompasses 5 objectives covered at 3 levels – the member state, the secretariat and national, international partners’ action(s):
An effective strategy for addressing AMR demands access to effective therapy for common infections in combination of reduction of resistance emergence risk. The AMR goals have been proven to be aligned with UHC.
Withal, the implementation of UHC is in accordance with UN Sustainable Development Goal 3 by creating opportunities for increased and equalized healthcare access alongside reducing infectious disease burden. Moreover, the WHOGAP ‘ensuring, for as long as possible, continuity of successful treatment and prevention of infectious diseases with effective and safe medicines that are quality assured, used in a responsible way and accessible to all who need them’ (25).
Nonetheless, one of the most ideal approaches to combating AMR resistance on a policy level is the One Health (OH) approach, which posits that disease emergence can be attributed to various complex interacting factors.
The OH approach elucidated above is aligned with global efforts to address the problem of AMR, the key strategies of which include launching a global awareness campaign to inform the public about the damage that improper and excessive usage of antimicrobials may cause while simultaneously enhance and reinforce hygiene practices to stop the spread of diseases (26, 27). Furthermore, the OH method intends to reduce antimicrobial resistance and their irrational use in agriculture, improve global drug resistance monitoring, and find and comprehend novel processes of resistance acquisition and possible hazards (28). Moreover, this method encourages quick and innovative clinical diagnosis creation and concurrent application of vaccinations and other alternatives. Improvement of incentives offered to encourage investment in developing new medications and enhancing existing ones adds to ease of diagnosis (29).
Increased awareness of and employment of infectious disease specialists makes the establishment of a worldwide innovation fund for early-stage investigation of novel therapies ultimately paving the way for a worldwide alliance to fight AMR effectively via international cooperation.
The benefits of the OH approach include its transdisciplinary and multisectoral approach which facilitates the consideration of non-healthcare associated factors such as financial burden (30).
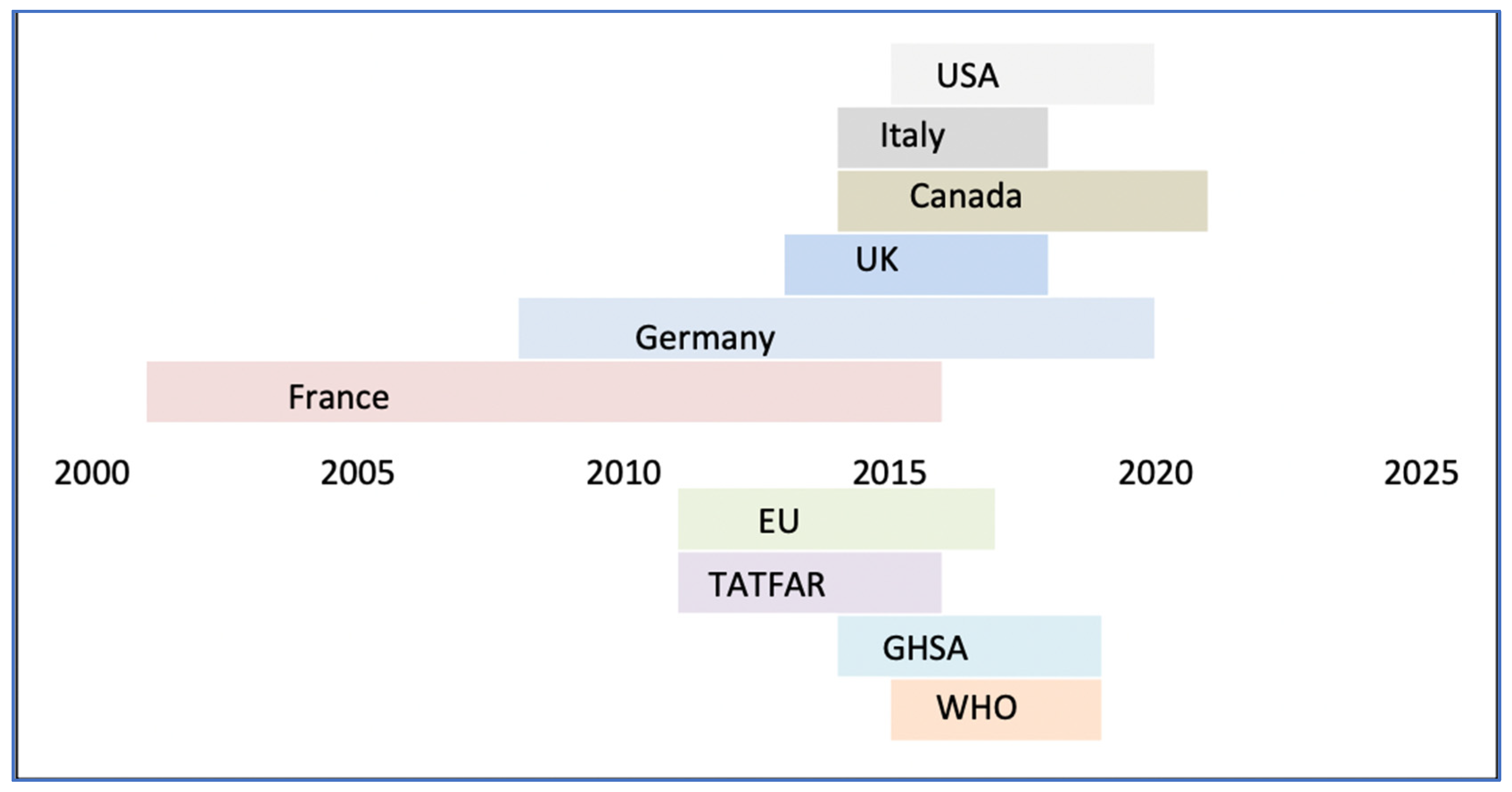
Despite the efficacy of the OH approach, a policy-based approach to AMR has been notably lacking outside the G7 countries. In addition to plans established by intergovernmental organizations, there are several existing G7-led collaborative ventures on AMR issues, due for revision every 5 years.
The G7 countries’ policy enforcement differs, with the US seeking to expedite regulatory processes around bacterial testing for AMR and France planning to limit antibiotic prescriptions in both the human and veterinary sectors. In contrast, Canada is exploring using the orphan drug framework to help incentivize the submission of new medicinal methods. Overall, country action plans emphasize the importance of interdisciplinary efforts for AMR implementation and regulation.
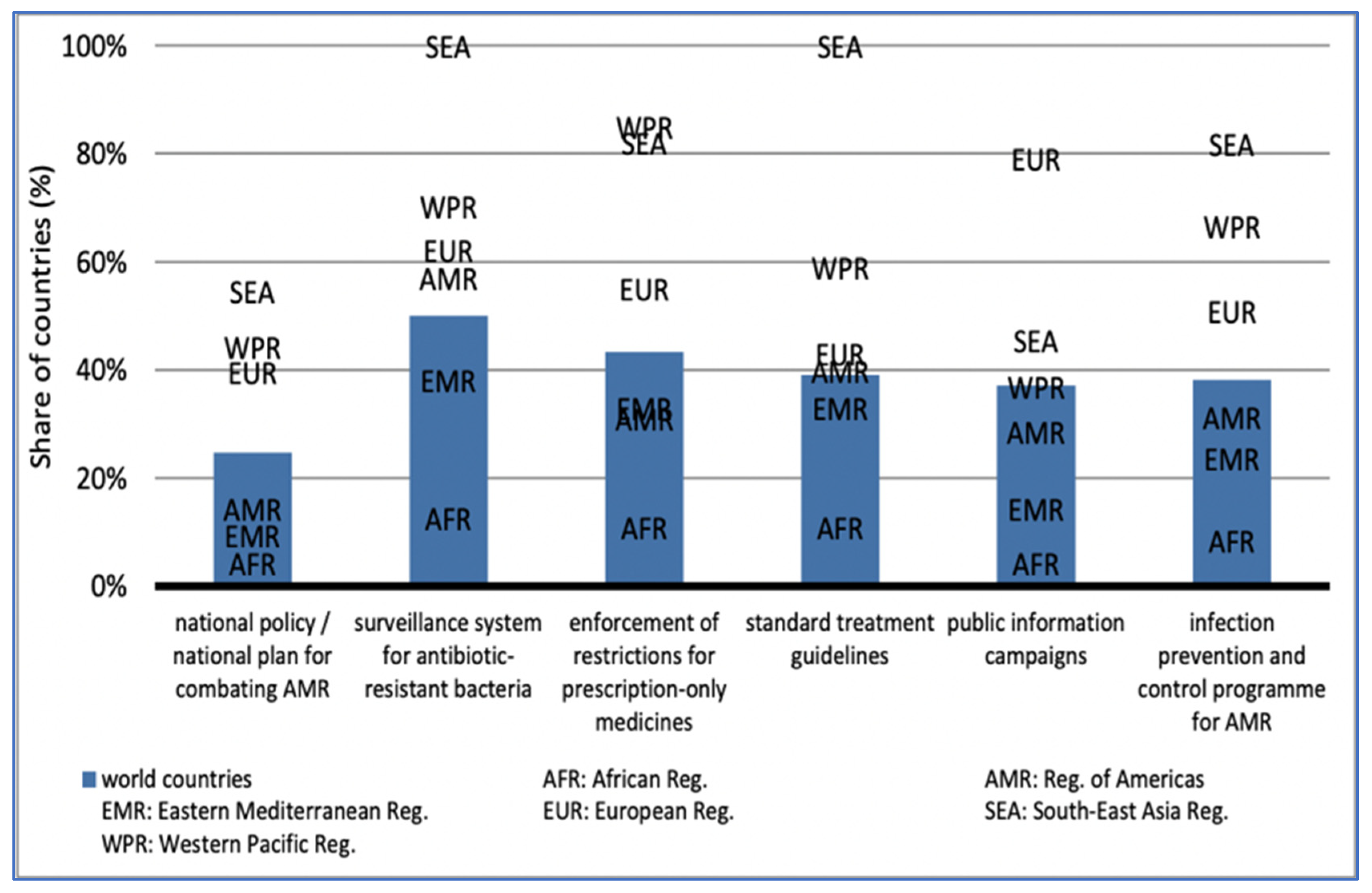
Given the large prevalence of AMR outside of the G7 countries, south-east Asia (55%) and the western Pacific (44%) are among the most prominent in national AMR programs. The Americas (14%), the Eastern Mediterranean (10%), and Africa (4%) have the least prominence:
Evaluation of Viable Policies
Some of the most optimal policies aforementioned have tried to prevent the formation of novel AMR strains and limit their spread, hence playing a critical role in lowering the health and economic burden imposed by them. While the financial burden on available healthcare amenities due to AMR is a reality, the best target to curb inappropriate drug use has yet to be ascertained.
The two-pronged approach followed to combat AMR resistance followed comprises of emergence (EA) and transmission avoidance (TA).
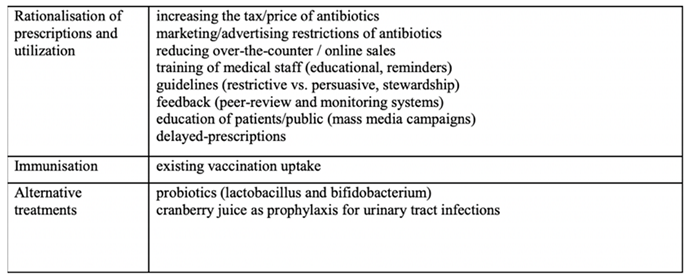
EA consists of six components: rationalization of prescription and utilization, increased immunity, consideration of alternative treatments, establishment of antimicrobial stewardship programs, enhanced immunization, and enforcement of price policies to discourage consumption of certain goods in order to restrict public access to a specific antibiotic or redirect to a change in the antibiotic mix. The following table address the components addressed (30):
Table 1.
Addressing the components and functionality of emergence avoidance in counter-AMR measures (30).
Table 1.
Addressing the components and functionality of emergence avoidance in counter-AMR measures (30).
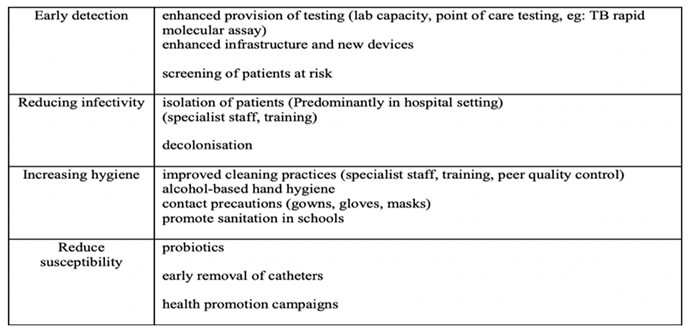
TA entails early detection, infectivity and susceptibility reduction, hygiene increments, encapsulated by the following table:
Table 2.
Addressing the components and functionality of transmission avoidance in counter-AMR measures (30).
Table 2.
Addressing the components and functionality of transmission avoidance in counter-AMR measures (30).
Given that hospital settings are often considered hotspots for the clinical manifestation of AMR-related problems, most interventions focus on this setting. The rationale behind considering thus is also justified on the basis of the maximum costs and consequences incurred from these settings. However, it is necessary to consider the community setting in tackling AMR to determine the efficacy of policies.
The AMR problem is nowhere epitomized as much as it is in India by not only being the largest consumer of antimicrobials globally compounded with widespread misuse but also having a rapidly expanding pharmaceutical industry. These factors both facilitate the growth of antimicrobials and of considerable discharge of antibiotic-laden waste into the environment (31).
- (c)
Artificial Intelligence (AI) in AMR
AI has demonstrated significant performance in AMR control: sequencing-based AI applications have been used to study AMR to collect clinical data for involvement in clinical decision support systems, thereby assisting physicians in not only monitoring AMR trends to foster rational antibiotic use, but also in designing new antibiotics and in synergistic drug combination studies (32).
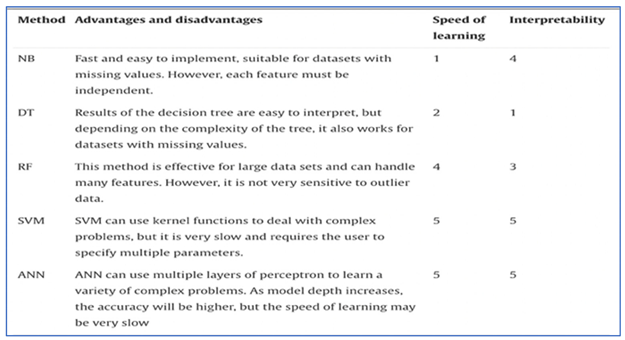
The following are some of the most often utilized AI algorithms for AMR: NB (Naive Bayes), DT (Decision Tree), RF (Random Forest), SVM (Support Vector Machine), ANN (Artificial Neural Network).
Nave Bayes (NB) is a classification approach with separate assumptions for each feature that calculates the input/output joint probability distribution for a given training dataset (33). It is utilised to identify the primary resistance component, extract resistant patterns, and predict the likelihood of unsuccessful therapy due to AMR (34, 35).
Classification with decision trees (DT) involves three steps: feature selection, decision tree creation, and decision tree pruning (36, 37). They are used to properly allocate medical resources by evaluating AMR burden, for example, to evaluate healthcare utilization and cost for AMR (38).
Random forest (RF) is a decision tree ensemble technique that has been used to forecast antibiotic combination therapy using chemogenomic data and orthology (39).
SVM is a binary classification model that finds a partitioning hyperplane to split samples into distinct groups and is used to predict AMR phenotypes as resistant or susceptible, for example, Her et al. model for E. coli predictions (40).
Artificial neural networks (ANN) are abstract mathematical models that employ loosely modelled neurons to convey data through weighted, coupled computer units or ’neuron’ layers.
Table 3.
Comparison between various AI techniques on the basis of their learning speed and interpretability (41, 42).
Table 3.
Comparison between various AI techniques on the basis of their learning speed and interpretability (41, 42).
Alternatively, AI can also be employed for the facilitization of whole genome sequencing technique of AMR diagnosis (WST-AST). Given the deficient information provided about the mechanism of development and the quantification of AMR of conventional AST, AI use may be used to extract information. Yelin et al. examined a 10-year longitudinal dataset of over 0.7 million community-acquired UTIs and the subsequent correlation drawn between AMR and demographic variables, past history of urine cultures, and previous history of antibiotic usage by the patients to demonstrate said phenomenon (43). This led to the development of a ML-based AMR prediction system.
Figure 2.
Description of the performance of deep antibiotic resistant gene sequencing models (43).
Figure 2.
Description of the performance of deep antibiotic resistant gene sequencing models (43).
AI has also been studied for application in intensive care units (ICUs), where high-dimensional inputs such as images, numbers, text, and other data must be assessed rapidly and correctly, with the simultaneous construction of sophisticated, nonlinear connections between the data gathered (44). Deep learning speeds up the process by simultaneously recording and evaluating many inputs, allowing the building of prediction models such as recurrent, convoluted, or deep belief neural networks (45).
Despite its many benefits, the lack of standardisation and intermittent data updates make successful training of AMR prediction models impossible. While a comprehensive collection and exchange of AMR-related information might aid in understanding the patterns in AMR development, there is currently a data scarcity (46). As a result, small(er) datasets are used to train AI-based algorithms with good predicted accuracy (47). Due to insufficient data, this leads to prediction of incorrect high-dimensional characteristics, resulting in lesser accuracy. Moreover, an additional challenge in AI implementation in countering AMR include its limited generalizability of current AI-based applications by their ability to only process datasets with the same distribution (48). However, these disadvantages can be mitigated via transfer learning and few-shot learning, making automated annotation of unlabeled data using unsupervised learning a potential path for future research (49).
-
(iv)
National context
To curb the growing rise of AMR in India, the National Policy for AMR Containment was formed in 2011 including various objectives under a designated task force. These include, among other things, a review of the country’s current situation regarding the manufacture, use of antibiotics, design recommendations for and implementation of a monitoring system for AMR, and the beginning of investigations documenting prescription patterns.
One of the most significant measures taken to combat AMR in the country include the Pradhan Mantri Jan Arogya Yojana (PMJAY), a step towards reconciling the existing healthcare infrastructure with the aforementioned concept of UHC. This was accomplished by providing insurance to cover the costs of prevention, promotion, treatment, rehabilitation, and palliative care of the poorest 40% of the population.
In the context of AMR, this initiative posed a solution to reduce the out-of-pocket expenditure of the common Indian citizen, thereby facilitating equal healthcare access to all. It was also a step towards the standardization of allopathic clinical practices in the country, the tampering with which has facilitated AMR to evolve into the global threat it poses (50). The versatility of the program to cater to all fractions of the nation in its delivery of quality healthcare facilities has been attested to some extent, while some more study may required to confirm the fact (51).
In addition, the introduction of schedule H1 is one of the highlights emerging post-review phase to regulate sale of antibiotics and check unauthorized sale of antibiotics. This may be accompanied by surprise raids conducted by Drug Inspectors at zonal/sub-zonal levels. Furthermore, third-generation antibiotics and newer compounds such as carbapenems (ertapenem, imipenem, meropenem, etc.) may be color-coded, limiting their availability to tertiary institutions exclusively. Despite this, limiting the availability of fixed dosage combinations of antibiotics in the market, as well as providing motivation for retail pharmacies to market licensed medications and to universities/drug research institutions, may represent a baseline-level improvement against illicit drug sales.
To combat AMR, three surveillance types—comprehensive, sentinel, and point prevalence—were taken into consideration. While comprehensive monitoring offers an accurate assessment of AMR burden by studying the entire population, it is not feasible because to the high number of laboratories required. Point prevalence studies are only effective for ensuring that surveillance data is representative. Sentinel surveillance, on the other hand, while just giving suggestive data, may be broadened in context to the rest of the population.
The sentinel surveillance method is most optimal for India as it allows for facile extrapolation from data at coordinating centers at the national level. This would include a robust system of classification of pathogens according to incidence alongside setting up a network of nationwide labs to facilitate the process of monitoring the various levels of healthcare. Development of a standardized methodology for the identification of microbes and multi-drug resistant strains of bacteria could also be ideally accompanied alongside a national repository of standard bacterial strains.
The initiation of studies documenting prescriptions patterns establishment of a monitoring system for the same could aid in tracing the course of AMR development. This situation, however, is exacerbated in India by the absence of prescription records in prescription records.
These steps can be combined with efforts to avoid the bioaccumulation of pharmacologically active chemicals in the body as well as the strengthening of regulations for the use of antibiotics across human, pastoral, and industrial settings. Furthermore, efforts to not exceed the specified tolerance limit of 0.1 mg/kg ppm: tetracycline, oxytetracycline, trimethoprim, oxalinic acid may eventually enable recommendations of special intervention methods such as antibiotic rationalization and antibiotic policies, particularly in hospitals.
The irrational use of antibiotics contributes to medical and economic inefficiency while also posing a major public health threat. Irrational drug use manifests in various forms including as unsafe drugs, ineffective drugs with doubtful efficacy, incorrect drug use, wrong drugs.
A three-tiered strategy may be employed to improve drug use: educative, managerial, and regulatory in combination with diagnostic methods pertaining to AMR (52).
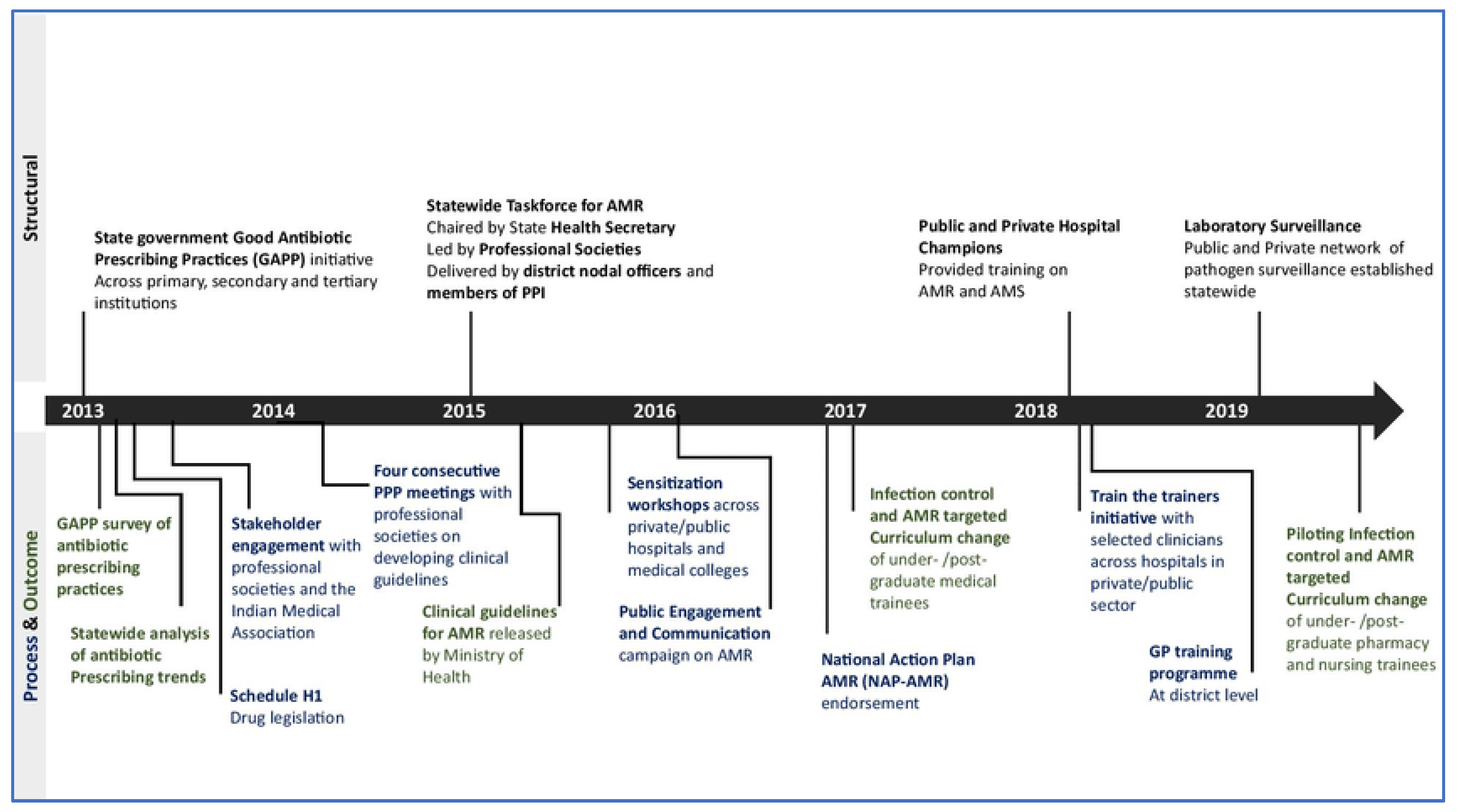
Figure 3.
Demonstrating timeline of implementation of national policies to counter AMR (53).
Figure 3.
Demonstrating timeline of implementation of national policies to counter AMR (53).
- (a)
State Action Plans for the containment of AMR
Three states have formally launched their SAPCAR: Kerala, Madhya Pradesh, and Delhi. The strategies of NAP-AMR form the framework for the development of SAPCAR. These can be summarized as: further AMR awareness through efficient communication and education, enhance available knowledge via advances in experimentation and surveillance, curtail the occurrence of infections via effective regulation, increase antibacterial effectiveness of food, animals, and human health, and lastly, garner financial support for AMR initiatives and research.
The SAPCAR establishment consists of four phases: mapping of AMR stakeholders, documenting AMR and its containment, a state workshop for drafting the SAPCAR, and lastly, the actualization of State governance mechanisms for AMR containment.
The first step in SAPCAR implementation is to conduct background searches to identify key officials/AMR champions in the state, such as state government departments, administrators and senior experts at medical colleges and hospitals, medical research centers alongside pharmacy, veterinary, agriculture, science colleges/research institutes, key NGOs working on AMR containment in the state, and diagnostics and pharmaceutical companies. This will be followed by a peer evaluation of compiled data by a core group of AMR specialists in the state.
The background document on AMR and its containment in the state provides an analysis of AMR in the state as well as summarizing existing methods for its containment. It forms the basis for SAPCAR development by capturing key information sector-wise on the following aspects of AMR: general awareness and understanding, regulations on drug use and stewardship, ongoing research and innovations, and collaborations between various entities to develop new drugs or novel therapeutic methods.
State workshop to draft the SAPCAR streamlines AMR containment activities in accordance with the NAPAMR. This is achieved by ongoing technical sessions to address the strategic priorities of the state action plan. Consolidating existing information via behavioral studies across the general population, professionals (healthcare, AYUSH, veterinary, environment), and industry employees (food processing, pharmaceutical) facilitates the establishment of baseline trends across segments of the State’s population. Incorporating AMR topics within the framework of professional education creates opportunities for development and implementation of AMR training modules across sectors. Moreover, monitoring AMR in human, pastoral and, environmental sectors by increasing antimicrobial susceptibility testing (AST) capacity serves to contribute to awareness stakeholder ideas and alerting clinicians to the lapses in management that have led to emergence of resistant strains. This notwithstanding, operationalization of state networks for AMR surveillance in all sectors with annual consultations would AMR surveillance database/reports available on key AMR agents. Securing sustainable funds for SAPCAR while encouraging basic and operational research for AMR containment eases the implementation of State AMR operational plan alongside AMR research projects.
Intrastate AMR collaborations, involving partnerships with not only the commercial sector and but also civil society organizations for AMR containment, would further enhance SAPCAR drafting based on constructive outputs.. Multisectoral collaboration and cooperation are critical for effective governance mechanisms for AMR containment and action across sectors.
Two key governance mechanisms have been highlighted for tackling AMR: multisectoral steering committee (MSC) and a technical working group (TWG) for AMR containment.
The MSC will manage SAPCAR’s mandate and objectives, which include collaboration within the healthcare sector and with allied industries to progress towards AMR control..
Furthermore, the body would enable collaboration for AMR-related activities between the government, commercial sector, civil society, and funders to guarantee a network of information exchange across all sectors, creating synergy between new and current AMR programs. Moreover, the committee will assess and support policy level recommendations from the Technical Working Group on AMR, with the goal of eventually endorsing SAPCAR and overseeing its development and attainment of milestones.
The TWG for AMR containment, on the other hand, provides technical and operational oversight for activities in the state to combat AMR. By ensuring periodic data collection and collaborative effort amongst stakeholders, the TWG aids in SAPCAR development by engaging all key stakeholders and evaluating the progress of its implementation (54).
- (b)
State-wise analysis of SAPCAR
- -
Kerala
The SAPCAR aligns with the aims of both the National and Global Action Plans on AMR, incorporating intersectoral collaboration and a One Health approach.
The assessment of baseline load in government teaching institutions and several tertiary care private hospitals has improved AMR surveillance. Nurses from throughout the state’s government medical colleges have been identified to help establish infection monitoring in the state, with the Department of Microbiology at Government Medical College Thiruvananthapuram (GMCT) serving as the nodal center.
GMCs and hospitals (GMC/H) are involved in the monitoring of diseases that are prevalent in the public health domain, particularly infections of the bloodstream, skin and soft tissue. At the Medical College Hospital (TVM), HAI rates for ventilator-associated pneumonia, catheter-associated UTIs, and bloodstream infection are being calculated in 20 ICUs and 8 high dependency units (HDUs).
These involvements have occurred in tandem with the tracking of six pathogens: E. coli, Klebsiella spp., Acinetobacter spp., Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus spp.).
GMC Thiruvananthapuram is compiling data from AMR surveillance from all teaching hospitals to study AMR trends over time, with plans to expand to district, secondary, and primary level hospitals in order to map AMR trends over time along a community/population level. Private healthcare institutions are asked to join the data gathering system following the introduction of government-directed AMR surveillance.
Application of rigorous IPC strategies throughout human and animal health and agriculture has proved to prevent infections and restrict antibiotic usage, manifesting in the SAPCAR as a scaling up of infection control programs to combat the spread of antibiotic resistant organisms.
In addition to major tertiary health care institutions and high end laboratotaries AMR suveillance has been extended to secondary hospitals using a hub and spoke approach. Samples collected from secondary hospitals in government sector and sent to the micro biology department for testing and the results reported to the state coordinator.
Improving biosecurity recommendations for farm implementation and promoting antibiotic alternatives (such as immunization). Kerala’s status as a consumer State for pharmaceuticals with a total consumption of drugs at around 20000 crores per annum, with antibiotics comprising 20% of the total drugs consumed annually in the state. The Drugs Control Department plays an important role in implementing regulatory action by optimising antibiotic usage, limiting the sale of not-of-standard quality (NSQ) medications and over the counter (OTC), and adhering to the Government of India’s (GoI) Red Line program. Furthermore, the GMCT has launched an antibiotic stewardship program to guarantee that appropriate therapy is provided to the right patient in a timely manner. Finally, the SAPCAR aspires to enable research into alternative tactics and novel compounds to tackle rising drug resistance, as demonstrated by a current study at the Rajiv Gandhi Centre for Biotechnology on the establishment of role of phytochemicals and natural antimicrobial substances to fight AMR. In consequence of these changes, the General Hospital Ernakulam has shown a considerable reduction in the use of high-end antibiotics, as well as a reduction in length of stay, mortality, and cost of treatment.
However, SAPCAR implementation in Kerala has been accompanied by unique challenges including lack of human resources in terms of skill and number and lack of coordination between the major consumers of antibiotics including human health, veterinary and aquaculture. This is compounded to by the lack of standard practices in allopathic medicine practices and experimentation techniques across healthcare institutions and microbiology laboratories. Amelioration of existing SAPCAR can be achieved by identifying and curbing the roles anthropogenic activities play in contamination of natural water bodies and reducing OTC antibiotic sale (55).
- -
Madhya Pradesh
As in Kerala, AMR containment in MP is in accordance with the NAP-AMR.
The Indian Initiative for Management of Antibiotic Resistance (IIMAR, MP) has been involved since 2008 for promotion of prudent use of antibiotics to reduce the spread of antibiotic resistance. These efforts have been added to by the Indo-Swedish collaborative research project between RD Gardi Medical College, Ujjain and Karolinska Institutet, Stockholm, Sweden, APRIAM for the institution of an antibiotic stewardship program including infection prevention and control and wastewater treatment. In addition, the Hand Hygiene Team has been involved in implementation research at a hospital and community level in India leading the Swachh Bharat – Swastha Bharat campaign since 2011 to improve hygiene practices among healthcare providers
Integrated Disease Surveillance Program (IDSP) in associated with Mahatma Gandhi Memorial Medical College under National Centre for Disease Control (NCDC) aims to upgrade health laboratories at all levels, from peripheral to national reference labs. This function extends to the establishment of quality assurance system to improve quality of lab data thereby assessing baseline AMR burden and providing evidence-based information for action.
Given the importance of AMR monitoring in animals, Nanaji Deshmukh Veterinary Science University (NDVSU) conducts antibiotic residue research in animal products. Furthermore, the implementation of IPC measures in animal husbandry is critical to reducing antimicrobial residues in the environment, such as the adoption of a demand-based approach to Water, Sanitation, and Hygiene (WASH), Nirmal Bharat Abhiyan, National Rural Drinking Water Programme, and the Swachh Bharat Abhiyan, launched by the Indian government to meet the Clean India target of eliminating open defecation by 2019. The Antibiotic Stewardship Program fosters the launch of annual antibiogram reports by compiling the antibiotic susceptibility profile of clinically significant pathogens headed by AIIMS Bhopal. The institution has initiated the execution of Hospital Antibiotic Policy on an annual basis, based on the prevalent antibiogram and the ‘National Treatment Guidelines for Antimicrobial Use in Infectious Diseases’ alongside working with Department of Public Health and Family Welfare to launch the State Antibiotic Policy in 2018
Research and innovations on newer antimicrobials have been headlined by National Institute for Research in Tribal Health (NIRTH) Jabalpur via conductance of research activities to address health problems within the tribal population. NDVSU, Amity Institute of Biotechnology, and Pinnacle Biomedical Research Institute are other entities actively involved in projects to explore alternative approaches to fight bacterial infections. SAPCAR MP recognizes that fostering intersectoral collaborations for better engagement on AMR activities is crucial to cross sectoral working.
However, some challenges have impeded the full-fledged implementation of SAPCAR including communication gaps, untrained personnel, weak laboratory systems, low quality surveillance data, regulatory issues, and poor inter-sectoral collaboration. However, strong support from the policy makers and dedicated stakeholders as well as ongoing efforts at hospitals and research institutes cast optimism on successful future outcomes for AMR containment in MP (56).
-
(v)
Overall progress overview and future direction
In 2013, the Indian Council of Medical Research established the AMR monitoring and research network (AMRSN). To advance medical research into AMR, the ICMR and the Research Council of Norway (RCN) launched a joint call for antimicrobial resistance research in 2017, in addition to the existing Indo-German collaboration for AMR research between the ICMR and the German Federal Ministry of Education and Research (BMBF). The ICMR launched the Antibiotic Stewardship Programme (AMSP) as a pilot project in 20 tertiary care hospitals across India to prevent antibiotic abuse and overuse in hospital wards and intensive care units, following the DCGI’s prohibition of 40 fixed dosage combinations (FDCs) deemed improper by the ICMR (57).
A four-tiered strategy for increasing infection control measures would be most suited for future endeavors: lowering illness burden, supporting fundamental and operational research, continous surveillance of AMR in environment, human and animal health and forming a multi-sectoral national steering council to direct national efforts. AMR is no longer a clinician’s concern alone. Policy makers, civil society and industrial associations need to come together to manage AMR.
The emergence of superbugs underscore the significance of continued AMR-control monitoring and implementation at the community, municipal, and national levels (58). There is an undeniable need for collaboration among those active in academia, hospitals and other health care settings, industry, and government. The AMR problem necessitates the proprietorship and participation of multiple stakeholders, as well as a tactical solution including the goals of the formation of a national alliance for AMR prevention and control, the establishment of a surveillance network capturing resistance emergence as well as the patterns of its spread.