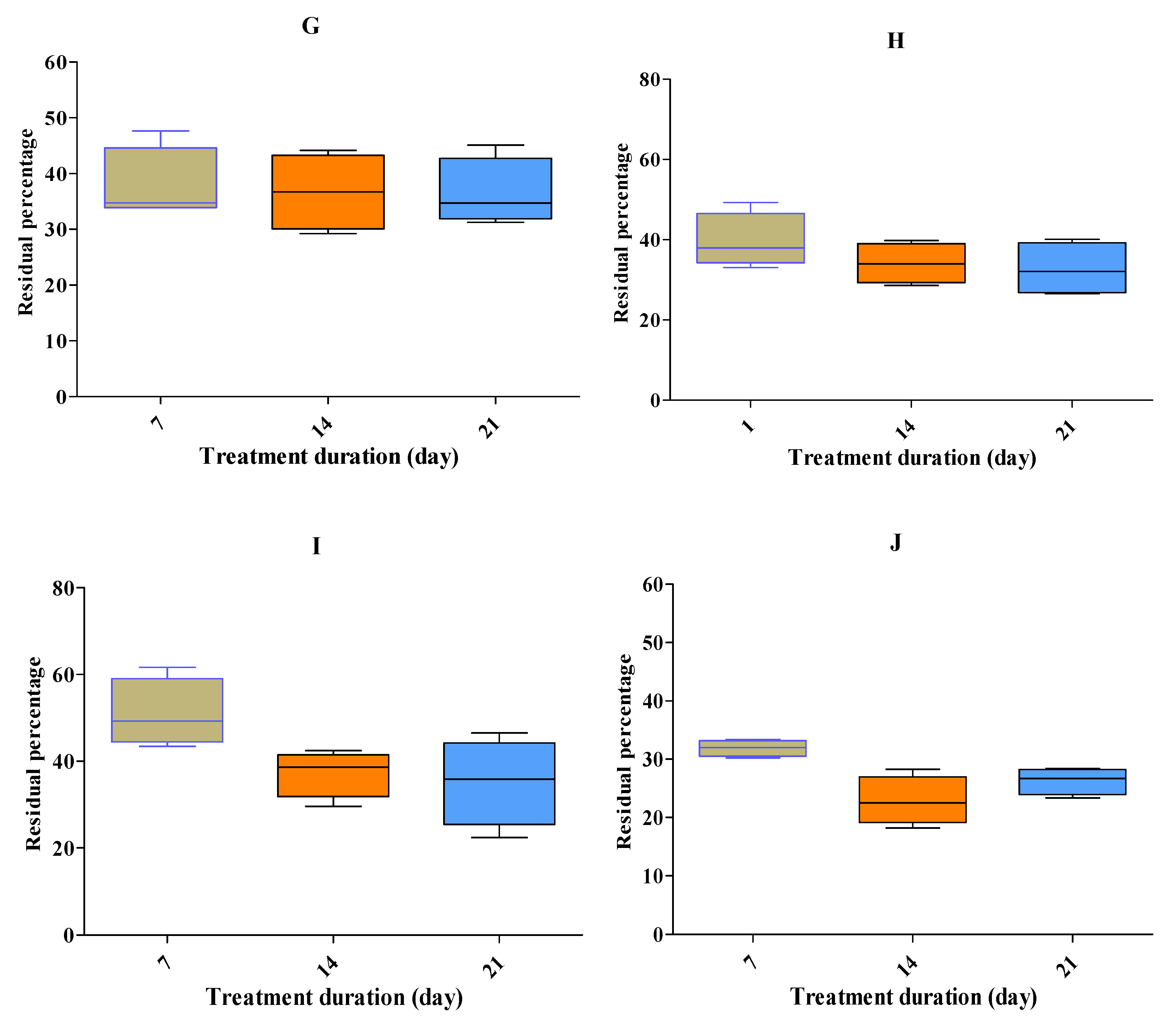1. Introduction
Global poultry meat consumption has been growing rapidly during the last two decades for various reasons, including the desire for healthier foods and calls for inexpensive sources of protein. Accordingly, a total of 280 million broilers were produced in Israel during 2021 via the implementation of intensive production methods involving reduced energy consumption and a 40 day growth period [
1]. During the broiler growth cycle, 0.3-0.85 kg of litter are produced per animal. In the same year, 336,000 tons of broiler litter (BL) were produced and used for agricultural soil fertilization, soil amendments and, in the form of dried dried broiler litter (DBL), as ruminant feed [
2,
3,
4]. BL contains more crude protein and less ash, as compared to layer hen or turkey litter.
Of the coccidiostats and antimicrobials utilized in poultry farming, 70-80 % are ex-creted and released into the environment [
5,
6]. Coccidiostats (ionophores, nicarbazine and clopidol) are compounds used in poultry farming for prophylactic and/or growth promotion purposes. In addition to coccidiostats, groups of therapeutic anti-microbial agents are also used for treatment and/or prevention of infection. According to the FDA, despite a decreasing trend, 6.1 million kg of antimicrobials and 4.2 million kg of ionophores were sold to US animal producers in 2019, of which 3% of the anti-microbials and 25 % of the ionophores were intended for use with poultry [
7]. Feeding animals with dried broiler litter thus involves the risk of spreading bacteria, anti-microbial resistance and toxins, as well as drug residues that can cause potential public health hazards, in addition to morbidity and/or even mortality in animals [
8,
9,
10].
The three major dried poultry litter producers/suppliers in Israel are compost north-west (stacking treatment), south industries (aerobic treatment) and north- east antimicrobial independent farms (aerobic treatment). Jointly, they supply 70,000-100,000 tons of dried poultry litter. The remaining 19% of broiler litter used as animal feed undergoes ensiling treatment by farmers or is used without any treatment. The most broiler litter using agents are pasture-based cattle. 48% were used for agriculture after composting treatment or as is.
The most widely used anti-microbial and decomposition treatments of broiler litter used as animal feed are aerobic (i.e., forced aeration), anaerobic (i.e., ensiling) and stacking. Stacking treatment is a semi-aerobic treatment involving passive pile windrows and in which turning is not required for aeration, as in composition. Good decomposition depends on litter composition, the litter environment, physical properties of the litter, the quality of the bedding and feed composition. Environmental conditions include moisture, aeration, pore space and flow of water for microbial activity, while physical factors include location (i.e., surface placement), insulated treatment space, litter volume and freshness, windrow height and width, and particle size. Litter hydrophobicity and the carbon: nitrogen ratio (20-30:1) also affect the ability to decompose litter quickly and completely. Specifically, a low carbon: nitrogen ratio reduces microbial activity and causes reduced decomposition, whereas high ratios also slow the decomposition process. The persistence and degradation of certain antimicrobials and ionophores in PL have been studied in terms of composting, aerobic digestion, and the impact of soil and aquatic environments [
11,
12,
13,
14,
15,
16]. However, the effect of stacking BL treatment on the degradation of pharmaceutical compounds is still largely unknown [
17,
18,
19].
The challenge in determining antimicrobial and coccidiostat residues in broiler lit-ter is related to their physicochemical properties, as well as components of the broiler litter matrix that include moisture, organic and inorganic matter. Multiple antimicrobials and coccidostats have been identified by LC/MS/MS in matrices other than broiler litter [
20,
21]. However, traditional analytical methods for characterizing BL remain limited. Cleaning procedures based on solid phase extraction columns are specific to some antimicrobials, with sample preparation time being long and the amounts of solvents used being high. At the same time, fast multi-analyte detection methods using QuEChERS have been developed for use with food, vegetables, fruits, urine [
22,
23,
24,
25] and swine manure (not including polar antimicrobials and coccidiostat drugs [
26]).
In the present study, we report the first multi-residue analysis method for contain-ing five different anti-microbial classes, and both ionophores and synthetic cocccidiostats (together comprising 30 pharmaceutical compounds at the residue level). The method used to investigate the toxicity concentration of residues in BL and the degradation rate of 29 drug residues in lab scale stacking treatments. The target sensitivity of the method was set to closely correlate with the existing maximum residue limits (MRL) for these compounds in poultry liver. This was considered essential for the purpose of evaluating the potential for building resistant bacterial populations in the litter and the environmental spread thereof.
2. Results
2.1. Method validation
2.1.1. Suitability (repeatability)
The results for coccidiostats and tetracycline, sulfonamide, fluoroquinolone, macro-lide and beta-lactam groups of anti-microbials had a relative standard deviation (RSD) < 20%, except for oxytetracycline at high concentrations (
Table 1). Medium level recoveries at 500 µg•kg
-1 were 80-120% and RSD < 20% (shown in the S2 Table).
2.1.2. Inter-day recovery (reproducibility)
The results for coccidiostats and sulfonamide, fluoroquinolone, macrolide and beta-lactam groups of anti-microbials had an RSD < 20%, except for norfloxacin and chlortetracycline (
Table 2).
2.1.3. Specificity and selectivity
Good signal-to-noise ratios, selectivity, specificity and low interference at retention time (RT) were observed. The % coefficient of variation (CV) of blank sample areas for sulfachlorpyridazine and the limit of detection (LOQ) for erythromycin were 20.7 and 21.8%, respectively (
Table 3).
2.2. Identification and quantification of broiler litter samples
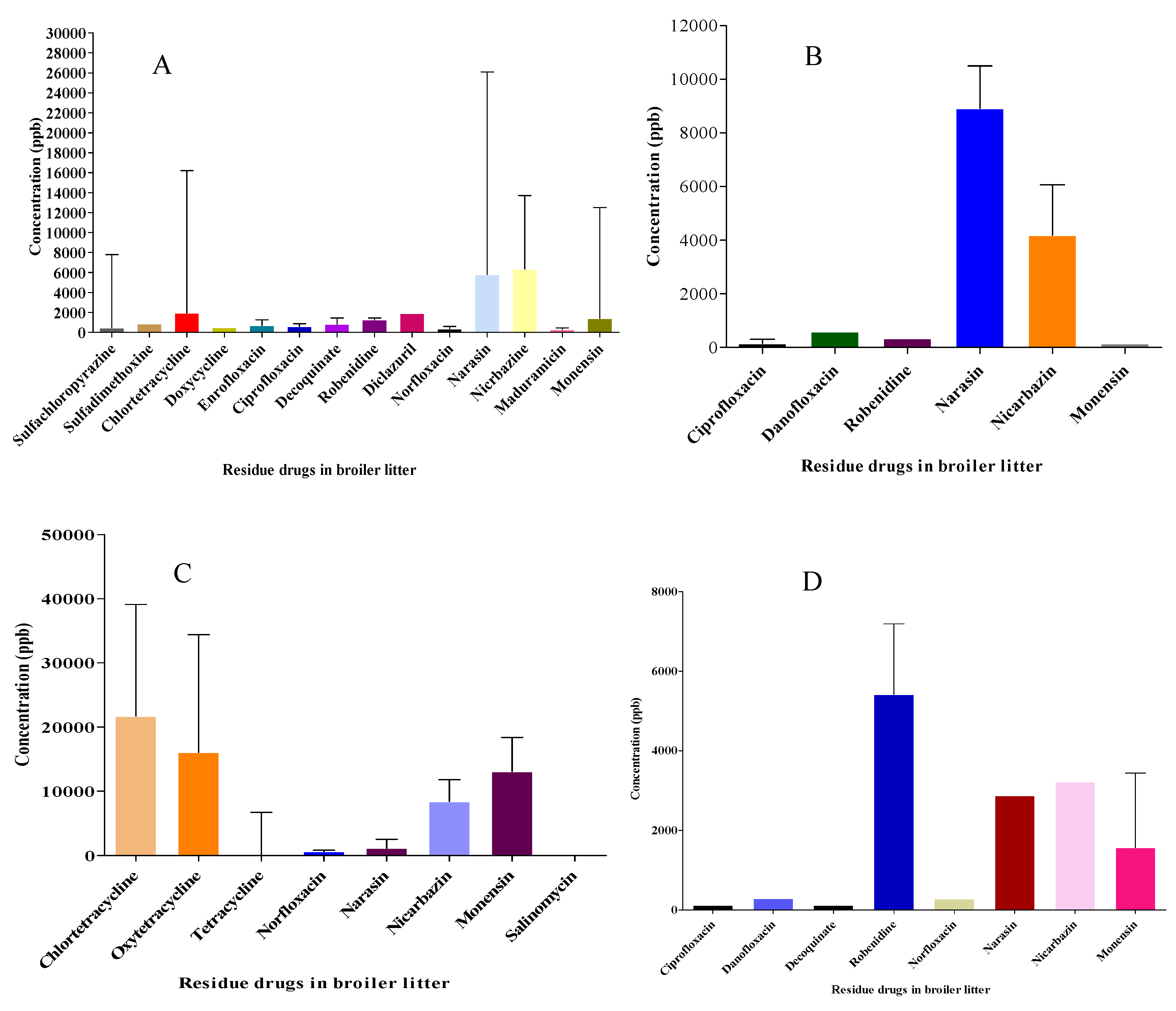
Broiler litter samples were collected at four different locations. Altogether, 18 anti-microbials and coccidiostats were detected in 42 batches of samples during 2019-2021. Most samples were positive for one or more of the analyzed compounds. Members of the tetracycline, fluoroquinolone and coccidiostat groups were detected more in the northwest and south of Israel, as compared to the northeast and other locations in the country (
Figure 1). Macrolides and beta-lactams were not detected at all. Indeed, beta-lactam anti-microbials are not stable in solution or in the environment.
In Figure 1, we show results for eighteen anti-microbial and coccidiostat drugs detected in BL. The prevalence (and medium concentration; mg•kg-1 (ppm)) of drug residues in BL were: Narasin 47% (2.88), nicarbazine 59.5% (6.06), monensin 57% (6.97), robendine 14% (1.35), salinomycin 4.2% (0.17), decoquinate 7.1% (0.19), diclazuril 4.2% (1.92), maduramycin (0.28 mg/kg), sulfachloropyrazine 9.5% (0.46), sulfadimethoxine 4.2% (0.85), norfloxacin 19% (0.7), danofloxacin 4.7% (0.43), enrofloxacin (9.5% (0.68), ciprofloxacin (14.3% (0.22), oxytetracycline 21.4% (8.92), tetracycline 23.8% (0.152), doxycycline 4.2% (0.49) and chlortetracycline 33% (8.92).
2.3. Anti-microbial and coccidiostat residue degradation upon stacking treatment
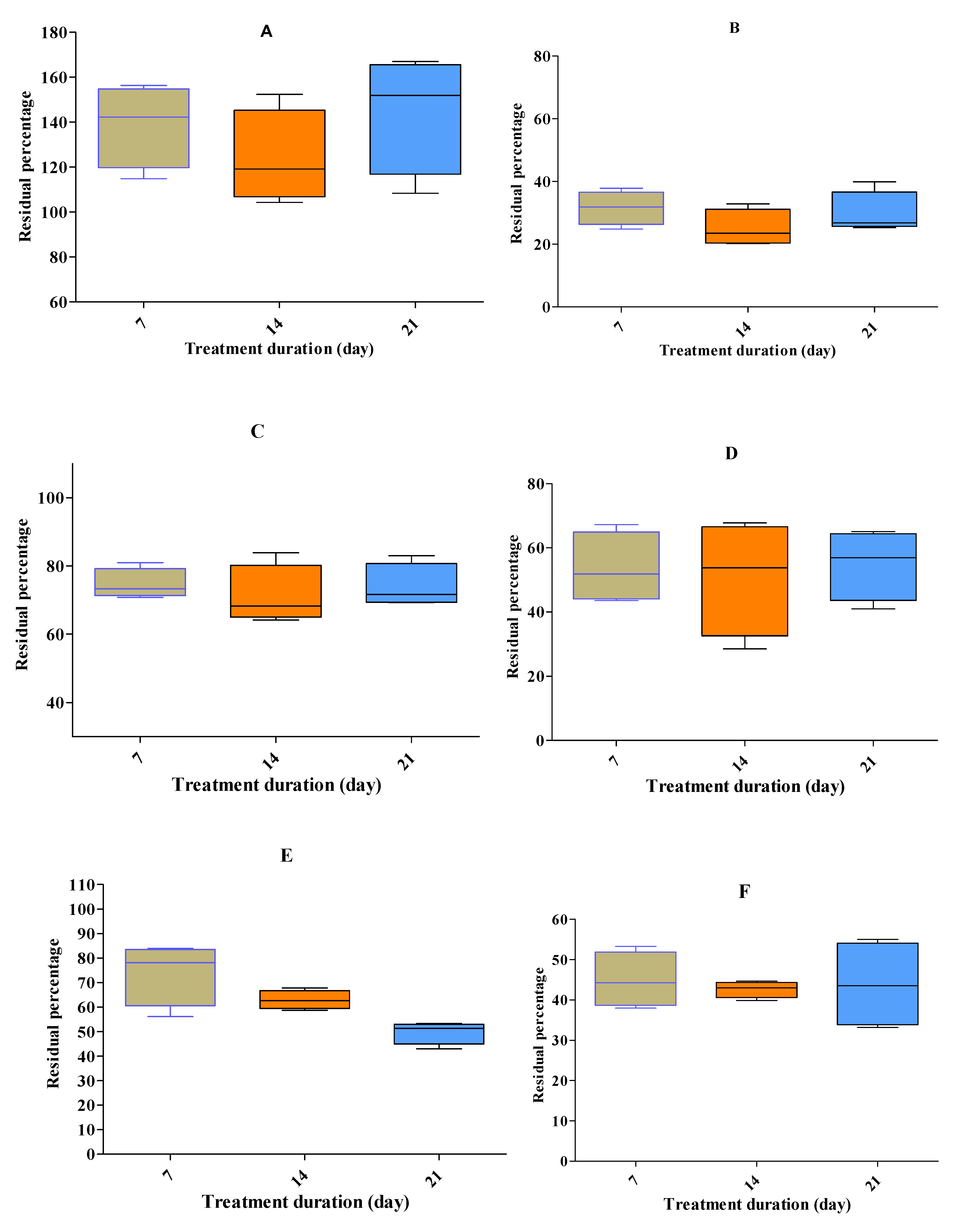
Four representative anti-microbials (tetracycline, ciprofloxacin, sulfisoxazole & amoxicillin) at high concentrations were degraded >95%, except for erythromycin (74%), by the stacking treatment (
Figure 2J). All five anti-microbial groups, namely, tetracycline, fluoroquinolones, macrolides, sulphonamides and beta-lactams (i.e., tetracycline, doxycycline, oxyteteracycline, chlortertracycline, amoxicillin, ciprofloxacin, danofloxacin, enrofloxacin, norfloxacin, sulfisoxazole, sulfachloropyrazine, sulfachloropyridazine, sulfadiazine, sulfadimidine, sulfadoxine, sulfadimethoxine, tylosine and erythromycin) at low residue concentrations were degraded >95%, except for tilmicosin (65%;
Figure 2I). Other than salinomycin, all eight coccidiostats persisted in BL (
Figure 2A-H). During treatment, the temperature and pH rose to 45-55°C and 7-8.8, respectively.
Residue percentages (±SEM) after three weeks of treatment at different spiked concentration of 0.5, 1, 1.5 and 2 ppm of dicoquinat (A), clopidol (B), maduramicin (C) narasin (D), monensin (E), diclazuril (F), nicarbazin (G) and robenidien (H), tilmicosin (I) and erythromycin (J). n = 20, 20, 15, 18, 15, 18, 15, 17, 20 and 20, respectively. The experimental data showed that degradation at a high rate was obtained during 7 days by the stacking treatments.
The experimental data showed that high rates of degradation were obtained after seven days of stacking treatment. Tilmicosin persisted more than did macrolide groups (erythromycin and tylosin) at low spiked concentrations of 0.5-2 mg•kg (ppm). Residue percentages (±SEM) upon stacking treatment were 35 (±2.6) for tilmicosin. For clopidol, the value was 36.4 (±3.2). Tilmicosin and clopidol are drugs little used on Israel broiler farms. The four coccidiostats used in Israel at low frequencies are decoquinat, diclaziuril, maduramycin and robendien. The residue percentage of these coccidiostats were >100 (±9.9), 43.7 (±5.7), 73, and 32.7 (±3) %, re-spectively, after stacking treatment. For the most widely used coccidiostats, namely, narasin, monensin, and nicarbazine, degradation percentages upon stacking treatment were 54.9 (±5.4), 49 (±2.1) and 36.5 (±3), respectively
3. Discussion
The wide range of physical-chemical and biological properties of anti-microbials and coccidiostats in BL makes the development of multi-residue analytical methods very challenging. BL contains 54.8-64% natural organic matter and moisture [
27,
28]. Anti-microbials and coccidiostats in BL present variable chemical properties, such as protonated ionophores at low pH, the ionized forms of the anti-microbials (pKa), affinity to divalent elements, such as Mg++ and Ca++, and solubility. We found that ethyl acetate with methanol is the most suitable extraction solvent for beta-lactams, sulfonamide, fluoroquinolone, tetracycline, macrolide antibiotics and coccidiostats. EDTA solution was used to remove abiotic cations involved in and metallic reactions that can occur in BL. A combination of MgSO4 and PSA QuEChER mixture was utilized to clean the extracted BL matrix. MgSO4 reduces sorbent moisture content while PSA removes primary and secondary amino acids. Most other studies used acetonitrile as the extraction solvent and hydrophilic-lipophilic balanced (HLB) solid phase extraction cartridges [
16,
29,
30,
31,
32]. However, these methods are limited for some compounds and call for longer processing steps. The effect of composting treatment on the persistence of ionophores were also shown by Arikan [
33], who found that abiotic reactions with ionophores caused a reduction in recovery, more interference, and a lower sensitivity for their detection.
The traditional in-house extraction and cleaning procedures adopted at the start of this study involved the use of an extraction buffer comprising acetone: DDW (1:4) and SPE-plexa. This resulted in low interference, good recovery and selectivity for fluoroquinolone, tetracycline, and macrolide antimicrobials. However, this strategy incorporated a large number of sample preparation steps, higher cost and low recovery for other anti-microbial compounds, such as beta-lactams and sulfonamides.
Anti-microbial and coccidiostat residue concentrations varied at different locations across Israel. This might indicate differences among broiler farms using different drugs. Cocccidiostat, enrofloxacin, ciprofloxacin and tetracycline residue concentrations in broiler litter were low in Israel, relative to some other countries [
34,
35,
36,
37]. The presence of tetracycline, fluoroquinolone groups (important anti-microbials for humans) in broiler litter contributes to the increased rate of antibiotic-resistant pathogenic bacteria in environmental contaminations that affect human health. The low detection of sulphonamides reported here indicate a shifting away from sulphonamides to coccidiostats in poultry farming. However, the influence of coccidiostats on the development of resistant bacteria is still unclear.
Coccidiostat concentrations of maduramycin, monensin and narasin detected before any treatment in BL were considerably below toxic concentrations in cattle and sheep [
38,
39,
40,
41,
42]. During stacking treatment, the highest degradation rate was observed in the first week. This might reflect the functional activity and thus self-heating of microorganisms before termination of the fermentation process. The more biodegradable drugs originating from fermentation, as compared to synthetic compounds (logkow>2). Our stacking treatment results showed similarity to those from past composting treatment studies on degradation of some drug residues, like tetracycline, fluoroquinolone and some coccidiostats [
43,
44]. After 7 days of com-posting treatment of turkey litter, the chlortetracycline degradation ratio was low [
13], as in my study. However, there were large differences in the degradation ratios of mononsin, tylosin and sulfamethazine (54%, 76% and 0%, respectively) in the turkey composting study, as compare to our studies of BL degradation using stack-ing treatment. Degradation of sulfadiazine by Microbacterium lacus strain SDZm4 was investigated in previous studies of soil samples [
45]. Microbacterium are Gram-positive bacteria also found in broiler gut. Therefore, this may be a reason why sulfonamide degradation in broiler litter was > 95% more than in turkey litter. The low degradation of erythromycin after a high concentration spike may be due to the low activity of Pseudomonas bacteria in the litter. In a previous study, P. aerugiona was one of bacteria that biodegraded erythromycin drugs [
46].
In laboratory-scale study, All antimicrobials in the study were degradable <LOQ, except for tilmicosin, as seen with composting treatment for some drugs in broiler litter [
13,
47,
48]. However spiked samples of tilmicosin in BL during 40 days of composting treatment at 50-60% water content were <LOQ (11 µg•kg
-1) [
48]. Sun et al., explained the biodegradation of ionophores as depending on temperature and moisture [
6]. In our results, a rise of temperature upon oxygen supplying led to a higher degradation ratio than seen with anaerobic treatment systems. However, the piling and turning composting system in another study on the degradation of salinomycin and narasin showed the opposite trend [
49]. The Sun and Munaretto stud-ies also addressed the similarities of degradation of narasin and a synthetic of nara-sin with a methyl group salinomycin degradation ratio. However, salinomycin degradation in our study was >95% and that of narasin was 46-58% in the stacking treatment systems.
The activity of microorganisms on drug residue degradation was high in the first week. however the degradation percentage after one and two weeks for ten com-pounds were not significantly different due to low moisture, high ammonia levels, low nutrition and high temperature that eradicated microorganisms in the biotic treatment.
The treatment results with decoquinate at all three durations were unexpected. The decoquinate concentration increased with treatments. We investigate the effects of spiked concentration of a derivative of quinolone (decoquinate) and quinolones. However, the increased concentration of decoquinate were not derived from quinolones. A similar phenomenon were reported previously [51]. In that study, the stability of decoquinate and pre-mixtures (vitamin and minerals) stored at 25°C and humidity of 60% over 6 months were 87%. However, after 18 months, the decoquinate concentration increased to 106% [
50].
4. Materials and Methods
4.1. Chemicals and reagents
Erythromycin, tilmicosin and oxytetracycline standards were purchased from Sig-ma-Aldrich Merck (St. Louis, MO). Tylosin, decoquinate, chlortetracycline and nicarbazin were obtained from A2S Analytical Standards Solutions (Saint Jean d’Illac, France). Ciprofloxacin, danofloxacin, norfloxacin, sulfadiazine, sulfisoxazole and ampicillin were obtained from Toronto Research Chemicals (Toronto, Canada). Enrofloxacin was obtained from Glentham (Corsham, Wiltshire, UK). Sulfadimidine, sulfachloropyridazine, sulfaquinoxaline, doxycycline, amoxicillin, maduramicin, diclazuril and clopidol were obtained from Dr. Ehrenstorfer (Augsburg, Germany), monensin from Acros (Geel, Belgium) and lasalocid from Santa Cruz (Huissen, The Netherlands). Semduramycin came from Phibro (Teaneck, NJ) and narasin from United States Pharmacopeia (Rockville, MD). Robendine, salinommycin, sulfachlo-ropyrazine and robendin-d8 were from HPC Standards (Atlanta, GA). All antimicrobial and coccidiostat standards were 97-98% pure, except for nicarbazine (90%) and clopidol (95%). HPLC-grade methanol, acetonitrile and formic acid were purchased from J. T. Baker (Deventer, The Netherlands) and EDTA was from Sig-ma-Aldrich Merck. Ammonium formate was obtained from Fisher Chemicals (Loughborough, UK). Analytical grade ethyl acetate, acetonitrile, and methanol were obtained from Bio-Lab (Jerusalem, Israel). UV/UF-ST de-ionized water (resistivity >18mΩ/cm) was produced with a Thermo Scientific apparatus (Long Branch, NJ).
Stock solutions containing 1000 mg•l-1 of the antimicrobials and coccidiostats was prepared in methanol, except for the beta-lactam solution (1000 mg•l-1(ppm)), which was prepared in double distilled water (DDW). Sulfonamides, fluoroquinolones and coccidiostats were stored at 4°C, the macrolide and tetracycline standards were stored at -20°C and the beta-lactam standard solution was kept at -80°C.
4.2. Sample Collection
Untreated BL samples were collected from treatment companies (aerobic and stack-ing process-based), with 590 poultry farms providing the litter to the companies after one cycle of production. Raw litter samples (100 g) were collected from broiler farms and treatment companies at nine locations utilizing a zigzag pattern. The samples were placed into a cold container and transferred to the laboratory within 12 h, where they were frozen at -20 °C pending analysis (European Commission (EC) Regulation No. 152/2009).
Pre-treatment of raw broiler litter: For multi-residue analytical method development, the litter was pre-treated as follows in the laboratory. Samples were freeze-dried by ly-ophilization and ground thoroughly to homogeneity. The moisture content of the litter at the time of collection was 30-40%. For high accuracy in multi-residue analysis (MRA), the sampling process, drying of the samples and sample homogeneity are critical. In the current study, drying the BL samples in an oven at 70°C for 72 h de-graded the majority of anti-microbials and coccidiostats (S7). Therefore, lyophilization was considered the preferred process for drying.
4.3. Sample extraction
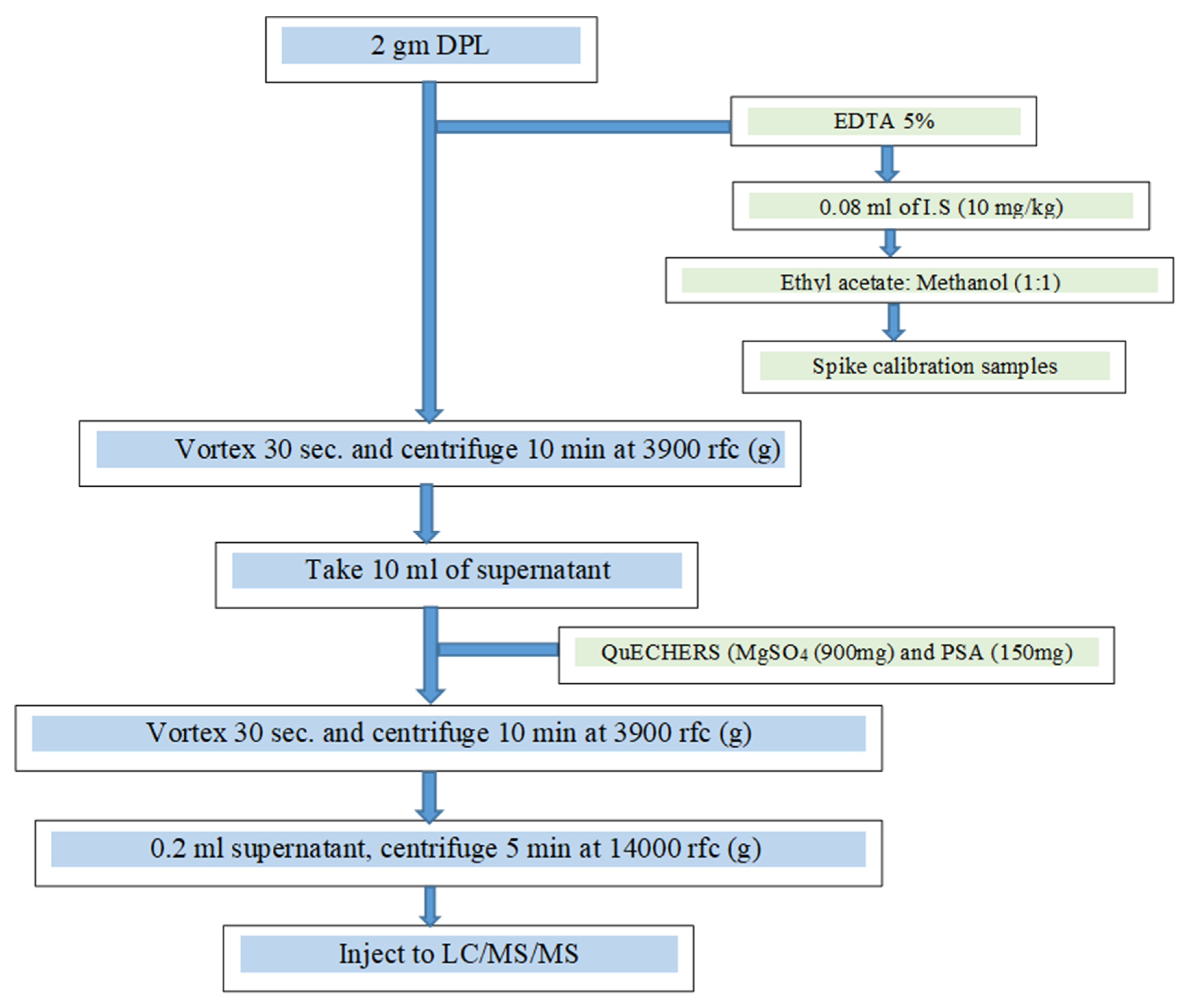
For sulfonamide, beta-lactam, tetracycline, fluoroquinolone, macrolide and coccidiostat analysis, 10 mL ethyl acetate:methanol (1:1) and 0.08 mL of a 10 mg kg-1 internal standard mixture of sulfisoxazole, roxithromycin (Toronto Re-search Chemicals), robenidine d8 (HPC Standards, Am Wieseneck, Germany), levofloxacin (Sigma-Aldrich), and minocycline (A2S Analytical Standards Solu-tions) were added to 2 g of BL, fortified with 30 compounds (S1) form the tetracycline, fluoroquinolone, sulfonamide, beta-lactam, macrolide and coccidiostat groups at a concentration of 10 ppm each and 0.2 ml 5% EDTA, vortexed and centrifuged at 6000 rfc (g) for 10 min. Ten mL of the supernatant were transferred to QuEChERS (990 mg MgSO4, 150 mg PSA (primary and secondary amines; Restek, Bellefonte, PA), vortexed and centrifuged at 3,900 rfc (g) for 10 min, filtered and transferred into a 0.25 mL vial and analyzed by LC/MS/MS.
Figure 1 presents a schematic depiction of the protocol.
Figure 3.
Analytical scheme for drug determination in BL.
Figure 3.
Analytical scheme for drug determination in BL.
4.4. LC/MS/MS analysis
Anti-microbial and coccidiostat concentrations were measured using an Agilent HPLC (Palo Alto, CA) connected to 3200QTrap or 4000 mass spectrometer system (Sciex, Vaughan, Canada). Chromatographic separation was achieved with a Hypersil Gold C18, 2.1100 mm, 5 m reversed phase column (Thermo Electron Corporation, Bellefonte, PA) at 40C. Mobile phase consisted of 0.2% formic acid (Sigma) and acetonitrile (J.T. Baker). The acetonitrile gradient increased from 5% to 70% from 0 to 2 min, remained at 70% for 2 min and then returned to the initial level at 8 min.
For coccidiostat samples, the isocratic mobile phase consisted of 10% 0.01 M ammo-nium formate, pH 3.5 (Sigma):90% acetonitrile at a flow rate of 0.5 ml/min. The injection volume was 5 l, with a post-run period of 5 min. The mass spectrometer was operated in the positive / negative electrospray ionization mode (ESI+/-). The curtain gas was nitrogen (30 PSI) and the drying gas was heated to 350°C. For quantification, a multiple reaction monitoring (MRM) method was applied.
4.5. Method validation
The analytic method developed here was validated for recovery, accuracy, precision, suitability, specificity and selectivity. LLOQ and LLOD were determined ac-cording to the guidelines published by the European Commission (808/2021/EC and GL49/2015) for animal products except reproductivity and reproducibility that were 4 replication instead of 6. To the best of our knowledge, there are no published guidelines for validation with manure samples.
4.5.1. Calibration
Calibration curve and recovery: A calibration curve was prepared using fortified concentrations of 0, 100, 250, 500, 1000 and 1500 µg•kg-1. Drug-free poultry litter was spiked with a mix of anti-microbial and coccidiostat standards, extracted, cleaned, and analyzed by LC/MS/MS (S8).
4.5.2. Recovery
Recovery of each anti-microbial and coccidiostat was calculated according to Equation (1) and Equation (2).
where PABL is the peak area of the spiked BL sample and PAAS is the peak area of the clean analytical standard.
where SACON is the found sample concentration and CALCON is the added sample concentration. Linear regression analysis (y = mx + b, where m = slope, b = y intercept, y= analytical peak area) was applied. Here, x = analytical spiked concentration (ppb) was used to calculate sample values.
4.5.3. Suitability (repeatability) and inter-day recovery (reproducibility)
Injection of four replicates of known concentrations (100-1500 ppb) of fortified BL samples was performed for repeatability. Injection of four replicates of known concentrations (100-1500 ppb) of fortified broiler litter samples on four different days were analysed using two different LC/MS/MS instruments for reproducibility.
4.5.4. Specificity and selectivity
Twenty representative blank samples and five fortified samples at the limit of quantitation were assessed by LC/MS/MS to determine the specificity and selectivity of each compound.
4.6. Designing a method for stacking BL treatment processes in the laboratory
Broiler litter was removed from the facility over the course of a year. Stacking treatments were carried out in three separate jars containing 12 kg of BL over the course of three weeks. The initial moisture content balanced at 40%. The degrada-tion rate for the stacking treatment was determined every week. broiler litter free of drugs that originated from an antibiotic independent farm was spiked at initial concentration at concentrations of 5 mg•kg-1 (low level), 10 mg•kg-1 (medium level), 15 and 20 mg•kg-1 (high level) with a mix of five representative antimicrobial drugs, namely, sulfisoxazole. amoxicillin, erythromycin, tetracycline and ciprofloxa-cin. A mix of 29 antimicrobials used in poultry farming and coccidiostats were also spiked at low concentrations of 0.5 mg•kg-1 (low level), 1 mg•kg-1 (medium level), 1.5 and 2 mg•kg-1 (high level). Spiked BL samples were wrapped in cotton gauze fabric and mixed with BL in jars before each treatment and placed at the medium (n=4) and upper layers (n=4).
5. Conclusions
This study described a rapid method for simultaneous detection of a large number of anti-microbials and coccidiostats at residue concentrations. Effective extraction, cleaning, identification/quantification methods were essential to achieve good recovery and repeatability in a large number of samples containing low levels of pharmaceutical compounds in a complex matrix. The method offers an affordable tool for monitoring and understanding the presence, persistence, degradation and relation between residue drugs and resistant bacteria in BL. The method will thus help us to understand and mitigate animal and human health problems. As well as environmental effects, caused by antimicrobial and coccidiostat drug residues. The use of antimicrobials and coccidiostats in animal farming is of paramount concern with respect to human, animal and environmental contamination issues and as a source of pathogenic resistant bacteria. Still, most farmers use BL as ruminant feed and fertilizer without processing. Even though the effects of stacking treatment on degradation of anti-microbials were significantly high, the degradation of coccidiostats and tilmicosin were limited. At the same time, further study on any cross-resistance to coccidiostats In Gram-positive bacteria is needed. Clearly, poultry litter should be treated and supervised before use as feed supplement or for land application. Even though the degradation of antimicrobials was significantly high in the stacking treatments, the degradation of coccidiostats and tilmicosin was limited.
Supplementary Materials
Table S1: Antibiotics and coccidiosats approved for use in poultry by national regulatory authorities in the USA, Brazil, China, Poland, United Kingdom, Germany, France, Israel and Spain, based on national reports as described in
Section 1; Table S2: Intra-day analytical recovery (in %) of 30 anti-microbials and coccidiostats in poultry litter as described in
Section 2.1.1, Figure S3: Antimicrobial and coccidiostat residues in Israeli broiler litter (2019-2021) as described in
Section 2.2, Table S4: Average poultry litter pH (n=4) as described in
Section 2.3, Table S5: Reference to some drug residue degradation related to temperature and pH in turkey and broiler litter composting methods as described in
Section 3, Table S6: Reference and case report on intoxication of drug residues in ruminants as described in section 3, Table S7:Comparison of antimicrobial and coccidiostat degradation in poultry litter upon drying by oven or lyophilzation described in
Section 4.2, Figure S8: Calibration curves for some anti-microbials and coccidiostats in fortified PL (n=5) as described in Section 4.4.1.
Author Contributions
Conceptualization, S.E., M.B. and S.J.M.; methodology, S.E., C.S., S.J.M. and M.B.; software, X.X.; validation, S.E., S.J.M. and M.B.; formal analysis, S.E. and C.S.; investigation, S.E.; resources, S.E.; data curation, S.E.; writing—original draft preparation, S.E.; writing—review and editing, S.J.M. and M.B.; visualization, S.E.; supervision, S.J.M. and M.B; project administration, S.J.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Chief Scientist, Ministry of Agriculture and Rural development, grant number 33-09-0001.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available on request from S.J.M.
Acknowledgments
We acknowledge the contribution of BL treatment companies in Israel. The authors thank the chief scientist of the Ministry of Agriculture for partial support of the project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rocha, AG.; Dilkin, P.; Montanhini Neto, R.; Schaefer, C.; Mallmann, CA. Growth performance of broiler chickens fed on feeds with varying mixing homogeneity. Vet Anim Sci. 2022, 17, 1–10. [CrossRef]
- Bishop, E.J.B.; Wilke, P.I.; Nash, W.J.; Nell, J.A.G. Macdonald D.A. Compaan J.P, Grobler J. Kingman E.R. Poultry manure as a livestock feed (Part 2). Fmg S Afr. 1971, 46, 49.
- Fontenot, J P. Recycling animal waste by feeding to enhance environmental quality. Prof. Anim. Sci. 1991, 7, 1-8. [CrossRef]
- Hansen, M.; Björklund, E.; Krogh, KA.; Halling-Sørensen, B. Analytical strategies for assessing ionophores in the environment. TrAC - Trends Anal Chem. 2009, 28, 521–33. [CrossRef]
- Sun, P.; Cabrera, ML.; Huang, CH.; Pavlostathis, SG. Biodegradation of veterinary ionophore antibiotics in broiler litter and soil microcosms. Environ Sci Technol. 2014, 48, 2724–31. [CrossRef]
- Yudhistira, S. Summary report on antimicrobial sold or distributed for use in food producing animals. FDA. 2019.
- Furtula, V.; Farrell, EG.; Diarrassouba, F.; Rempel, H.; Pritchard, J.; Diarra, MS. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult Sci. 2010, 89, 180–8. [CrossRef]
- Hribar, C. Understanding Concentrated Animal Feeding Operations and Their Impact on Communities. Natl Assoc Local Boards Heal [Internet]. 2010, 1–22. Available from: http://www.cdc.gov/nceh/ehs/docs/understanding_cafos_nalboh.pdf.
- Oehme, F W and Pickrell, J A. An Analysis of the Chronic Oral Toxicity of Polyether Ionophore Antibiotics in Animals. Vet. Hum. Toxicol. 1999, 41: 251.
- Sarmah, AK.; Meyer, MT.; Boxall, ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006, 65, 725–59. [CrossRef]
- Kim, SC.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007, 41, 50-57. [CrossRef]
- Dolliver, H.; Gupta, S.; Noll, S. Antibiotic Degradation during Manure Composting. J. Environ. Qual. 2008, 37, 1245. [CrossRef]
- Balc, A. Science of the Total Environment Investigation of the tetracycline , sulfonamide , and fl uoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. 2009, 407, 4652–64. [CrossRef]
- Bohn, P.; Bak, SA.; Björklund, E.; Krogh, KA.; Hansen, M. Abiotic degradation of antibiotic ionophores. Environ Pollut [Internet]. 2013, 182, 177–183. [CrossRef]
- Gorissen, B.; Reyns, T.; Devreese, M. Determination of selected veterinary antimicrobials in poultry excreta by UHPLC-MS / MS, for application in Salmonella control programs. 2015, 4447–57. [CrossRef]
- Capleton, AC.; Courage, C.; Rumsby, P.; Holmes, P.; Stutt, E.; Boxall, ABA. Prioritising veterinary medicines according to their potential indirect human exposure and toxicity profile. Toxicol Lett. 2006, 163, 213–23. [CrossRef]
- Hao, C.; Lissemore, L.; Nguyen, B.; Kleywegt, S.; Yang, P.; Solomon, K. Determination of Pharmaceuticals in Environmental Waters by Liquid Chromatography/Electrospray Ionization/Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2006, 384, 505-513. [CrossRef]
- Watanabe, N.; Harter, TH.; Bergamaschi, B A. Environmental Occurrence and Shallow Ground Water Detection of the Antibiotic Monensin from Dairy Farms. J Environ Qual. 2008, 37, 78-85. [CrossRef]
- Hu, XG., Luo, Y.; Zhou, QX.; Xu, L. Determination of thirteen antibiotics residues in manure by solid phase extraction and high performance liquid chromatography. Fenxi Huaxue/ Chinese J Anal Chem. 2008, 36, 1162–1166. [CrossRef]
- Rashid, A.; Mazhar, SH.; Zeng, Q.; Kiki, C.; Yu, CP.; Sun, Q. Simultaneous analysis of multiclass antibiotic residues in complex environmental matrices by liquid chromatography with tandem quadrupole mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci [Internet]. 2020, 1145, 122103. [CrossRef]
- Filigenzi, MS.; Ehrke, N.; Aston, L S.; Poppenga, R H. Evaluation of a Rapid Screening Method for Chemical Contaminants of Concern in Four Food-Related Matrices using QuEChERS Extraction, UHPLC and High Resolution Mass Spectrometry. Food Additives & Contaminants Part A, Chemistry, Anal. Control, Expo. Risk Assess. 2011, 28, 1324-1339. [CrossRef]
- Poudel, U.; Dahal, U.; Dhakal, S. Review of Poultry Production and Poultry Vaccine Manufacture in Nepal. 2021, 1–7. [CrossRef]
- Svahn, O.; Björklund, E. Thermal stability assessment of antibiotics in moderate temperature and subcriticalwater using a pressurized dynamic flow-through system. Int J Innov Appl Stud [Internet]. 2015, 11, 872–80. Available from: http://www.diva-portal.org/smash/record.
- Usui, K.; Hayashizaki, Y.; Minagawa, T.; Hashiyada, M.; Nakano, A.; Funayama, M. Rapid determination of disulfoton and its oxidative metabolites in human whole blood and urine using QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Leg Med [Internet]. 2012, 14, 309–316. [CrossRef]
- Guo, C.; Wang, M.; Xiao, H.; Huai, B.; Wang, F.; Pan, G. Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J Chromatogr B [Internet]. 2016, 1027, 110–8. [CrossRef]
- Dias, BO.; Silva, CA, Higashikawa FS, Roig A, Sánchez-Monedero MA. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour Technol [Internet]. 2010, 101, 1239–1246. [CrossRef]
- Kiss, NÉ.; Tamás, J.; Szőllősi, N.; Gorliczay, E.; Nagy, A. Assessment of composted pelletized poultry litter as an alternative to chemical fertilizers based on the environmental impact of their production. Agric. 2021, 11, 1130. [CrossRef]
- Hurst, JJ.;Wallace, JS.; Aga, DS. Method development for the analysis of ionophore antimicrobials in dairy manure to assess removal within a membrane-based treatment system. Chemosphere [Internet]. 2018, 197, 271–279. [CrossRef]
- Pugazhendhi, A.; Dhanarani, S.; Manogari, G. Saudi Journal of Biological Sciences Impact on degradation of antibiotics from poultry litter using Autothermal Thermophilic Aerobic Digestion (ATAD ). Saudi J Biol Sci [Internet]. 2021, 28, 988–992. [CrossRef]
- Yévenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martín, BS. Detection of antimicrobial residues in poultry litter: Monitoring a risk through a selective and sensitive hplc–ms/ms method. Animals. 2021, 11, 1–12. [CrossRef]
- Arikan OA, Mulbry W, Rice C. The effect of composting on the persistence of four ionophores in dairy manure and poultry litter. Waste Management. 2016; 54: 110–117. [CrossRef]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Vol. 148, Environmental Pollution. 2007, 570–579. [CrossRef]
- Zhao, L.; Dong, YH.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Vol. 408, Science of the Total Environment. 2010, 1069–1075. [CrossRef]
- Leal, RMP.; Figueira, RF.; Tornisielo, VL.; Regitano, JB. Occurrence and sorption of fluoroquinolones in poultry litters and soils from São Paulo State, Brazil. Vol. 432, Science of the Total Environment. 2012, 344–349. [CrossRef]
- Van Epps, A.; Blaney, L. Antibiotic Residues in Animal Waste: Occurrence and Degradation in Conventional Agricultural Waste Management Practices. Curr Pollut Reports [Internet]. 2016, 2, 135–155. [CrossRef]
- Anderson, TD.; Van Alstine, W. G.; Ficken, M. D.; Miskimins, D. W.; Carson, T. L.; Osweiler, G. D. Acute Monensin Toxicosis in Sheep: Light and Electron Microscopic Changes. Am J Vet Res. 1984, 6, 1142–1147.
- Confer, AW.; Reavis, DU.; Panciera, RJ. Light and electron microscopic changes in cardiac and skeletal muscle of sheep with experimental monensin toxicosis. Vet Pathol. 1983, 20, 590–602. [CrossRef]
- Bastianello, SS.; Fourie, N.; Prozesky, L.; Nel, PW.; Kellermann, TS. Cardiomyopathy of ruminants induced by the litter of poultry fed on rations containing the ionophore antibiotic, maduramicin. II. Macropathology and histopathology. Onderstepoort J Vet Res. 1995, 62, 5–18.
- Huyben, MWC.; Sol, J.; Counotte, GHM.; Roumen, MPHM.; Borst, GHA. Salinomycin poisoning in veal calves. Vet Rec. 2001, 149: 183–184. [CrossRef]
- Svahn, O.; Björklund, E. Thermal stability assessment of antibiotics in moderate temperature and subcritical water using a pressurized dynamic flow-through system. 2015, 11, 872–880.
- Gonzalez, M.; Barkema, HW.; Keefe, GP. Monensin toxicosis in a dairy herd. 2005, 46, 910–912.
- Esperón, F.; Albero, B.; Ugarte-ruíz, M.; Domínguez, L.; Carballo, M.; Tadeo, JL. Assessing the benefits of composting poultry manure in reducing antimicrobial residues , pathogenic bacteria , and antimicrobial resistance genes. A field-scale study. 2020, 27738–27749. [CrossRef]
- Subirats, J.; Murray, R.; Scott, A.; Lau, CHF.; Topp, E. Composting of chicken litter from commercial broiler farms reduces the abundance of viable enteric bacteria, Firmicutes, and selected antibiotic resistance genes. Sci Total Environ [Internet]. 2020, 746, 141113. [CrossRef]
- Tappe, W., Herbst, M.; Hofmann, D.; Koeppchen, S.; Kummer, S.; Thiele, B. Degradation of sulfadiazine by Microbacterium lacus strain SDZm4, isolated from lysimeters previously manured with slurry from sulfadiazine-medicated pigs. Appl Environ Microbiol. 2013, 79, 2572–2577. [CrossRef]
- Šabic, M.; Cižmek, L.; Domanovac, MV.; Meštrovic, E. Biodegradation of erythromycin with environmental microorganism Pseudomonas aeruginosa 3011. Chem Biochem Eng Q. 2015, 29, 367–373. [CrossRef]
- Ho, Y.; Bin, Zakaria MP.; Latif, PA.; Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour Technol [Internet]. 2013, 131, 476–484. [CrossRef]
- Ramaswamy, J.; Prasher, SO.; Patel, RM.; Hussain, SA.; Barrington, SF. The effect of composting on the degradation of a veterinary pharmaceutical. Bioresour Technol [Internet]. 2010, 101, 2294–2299. [CrossRef]
- Munaretto, JS.; Yonkos, L.; Aga, DS. Transformation of ionophore antimicrobials in poultry litter during pilot-scale composting. Environ Pollut [Internet]. 2016, 212, 392–400. [CrossRef]
- Bampidis, V.; Azimonti, G.; De Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kouba, M.; Durjava, M. K.; Lopez-Alonso, M.; Puente, S. L.; Marcon, F.; Mayo, B.; Pechova, A.; Petkova, M.; Ramos, F.; Sanz, Y.; Villa, R. E.
- Woutersen R, Aquilina G, Bories G, Gropp J. Safety and efficacy of Deccox (decoquinate) for chickens for fattening. EFSA Journal.2019, 17, 1–29. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).