Submitted:
07 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Pancreatic cancer Fact Sheet
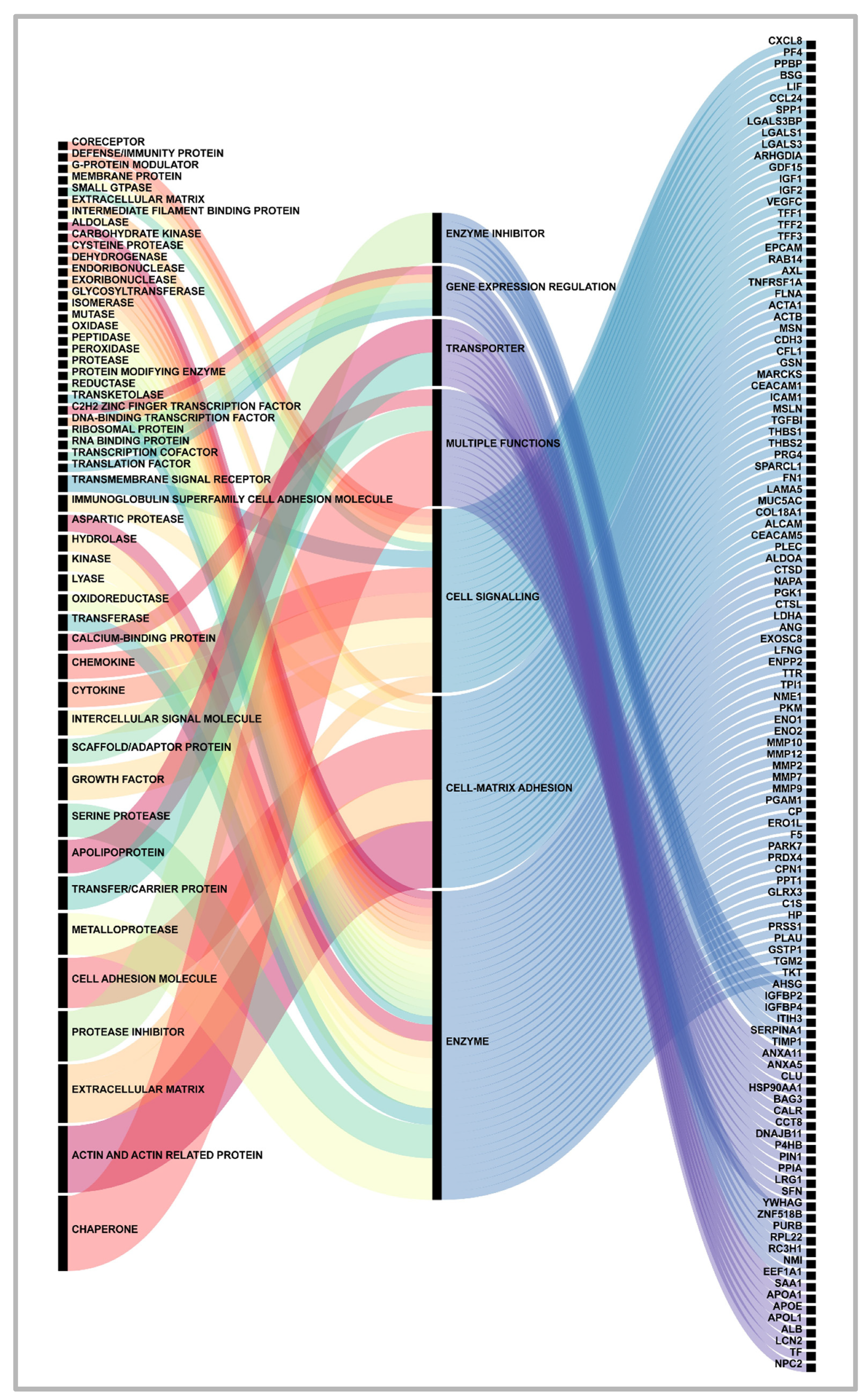
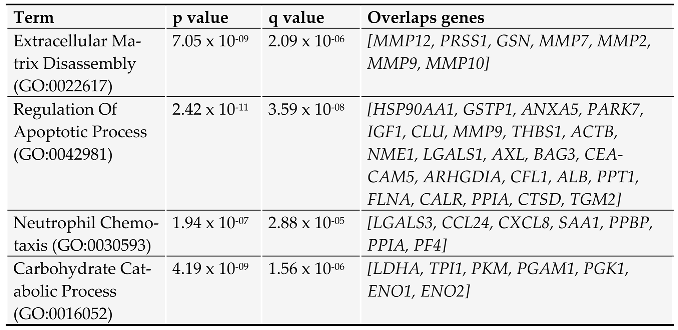
2. Proteins secreted by pancreatic cancer cells: messages sent to the neighborhood.
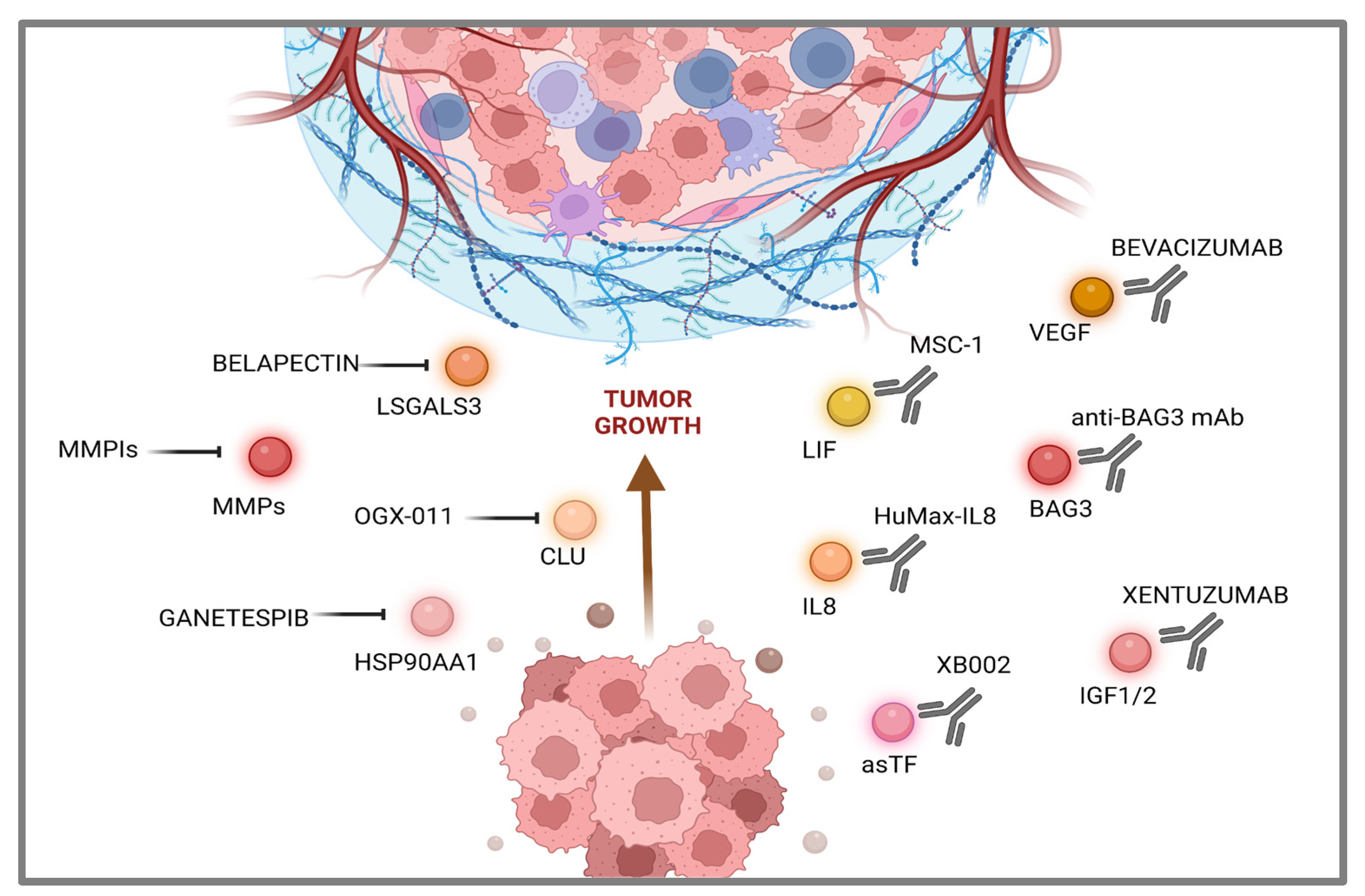
3. Communications breakdown operated by small drugs.
4. Communications breakdown operated by monoclonal antibodies.
5. Conclusion and Future directions
Funding
Conflicts of Interest
References
- Khalaf N, El-Serag HB, Abrams HR, Thrift AP. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clinical Gastroenterology and Hepatology. 2021;19(5):876-884. [CrossRef]
- Park W, Chawla A, O’Reilly EM. Pancreatic Cancer. JAMA. 2021;326(9):851. [CrossRef]
- Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Archives of Medical Science. 2020;16(4):820-824. [CrossRef]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer Journal for Clinicians. 2023;73(1):17-48. [CrossRef]
- Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Network Open. 2021;4(4):e214708. [CrossRef]
- Kindler HL. A Glimmer of Hope for Pancreatic Cancer. New England Journal of Medicine. 2018;379(25):2463-2464. [CrossRef]
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nature Reviews Drug Discovery. 2019;18(3):197-218. [CrossRef]
- Schepis T, De Lucia SS, Pellegrino A; et al. State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers. 2023;15(13):3423. [CrossRef]
- Morris VK, Kennedy EB, Baxter NN; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. Journal of Clinical Oncology. 2023;41(3):678-700. [CrossRef]
- Shah MA, Kennedy EB, Alarcon-Rozas AE; et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. Journal of Clinical Oncology. 2023;41(7):1470-1491. [CrossRef]
- Moy B, Rumble RB, Come SE; et al. Chemotherapy and Targeted Therapy for Patients With Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer That is Either Endocrine-Pretreated or Hormone Receptor–Negative: ASCO Guideline Update. Journal of Clinical Oncology. 2021;39(35):3938-3958. [CrossRef]
- Yilmaz E, Ismaila N, Bauman JE; et al. Immunotherapy and Biomarker Testing in Recurrent and Metastatic Head and Neck Cancers: ASCO Guideline. Journal of Clinical Oncology. 2023;41(5):1132-1146. [CrossRef]
- Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Frontiers in Immunology. 2022;13. [CrossRef]
- Di Costanzo F, Di Costanzo F, Antonuzzo L, Mazza E, Giommoni E. Optimizing First-Line Chemotherapy in Metastatic Pancreatic Cancer: Efficacy of FOLFIRINOX versus Nab-Paclitaxel Plus Gemcitabine. Cancers. 2023;15(2):416. [CrossRef]
- Principe DR, Underwood PW, Korc M, Trevino JG, Munshi HG, Rana A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Frontiers in Oncology. 2021;11. [CrossRef]
- Di Federico A, Mosca M, Pagani R; et al. Immunotherapy in Pancreatic Cancer: Why Do We Keep Failing? A Focus on Tumor Immune Microenvironment, Predictive Biomarkers and Treatment Outcomes. Cancers. 2022;14(10):2429. [CrossRef]
- Robinson JL, Feizi A, Uhlén M, Nielsen J. A Systematic Investigation of the Malignant Functions and Diagnostic Potential of the Cancer Secretome. Cell Reports. 2019;26(10):2622-2635.e5. [CrossRef]
- De Morais JA, Zelanis A. Bioinformatic reanalysis of public proteomics data reveals that nuclear proteins are recurrent in cancer secretomes. Traffic. 2021;23(2):98-108. [CrossRef]
- Padgaonkar M, Shendre S, Chatterjee P, Banerjee S. Cancer secretome: Finding out hidden messages in extracellular secretions. Clinical and Translational Oncology. 2022;25(5):1145-1155. [CrossRef]
- Sherman MH, Beatty GL. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annual Review of Pathology: Mechanisms of Disease. 2023;18(1):123-148. [CrossRef]
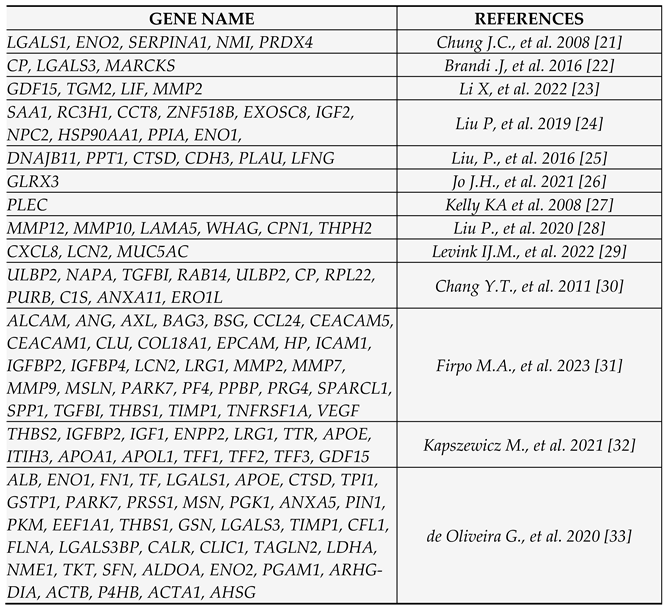
- Chung JC, Oh MJ, Choi SH, Bae CD. PROTEOMIC ANALYSIS TO IDENTIFY BIOMARKER PROTEINS IN PANCREATIC DUCTAL ADENOCARCINOMA. ANZ Journal of Surgery. 2008;78(4):245-251. [CrossRef]
- Brandi J, Pozza ED, Dando I; et al. Secretome protein signature of human pancreatic cancer stem-like cells. Journal of Proteomics. 2016;136:1-12. [CrossRef]
- Li X, Liu H, Dun MD; et al. Proteome and secretome analysis of pancreatic cancer cells. PROTEOMICS. 2022;22(13-14):2100320. [CrossRef]
- Liu P, Kong L, Jin H, Wu Y, Tan X, Song B. Differential secretome of pancreatic cancer cells in serum-containing conditioned medium reveals CCT8 as a new biomarker of pancreatic cancer invasion and metastasis. Cancer Cell International. 2019;19(1). [CrossRef]
- Liu P, Weng Y, Sui Z; et al. Quantitative secretomic analysis of pancreatic cancer cells in serum-containing conditioned medium. Scientific Reports. 2016;6(1). [CrossRef]
- Jo JH, Kim SA, Lee JH; et al. GLRX3, a novel cancer stem cell-related secretory biomarker of pancreatic ductal adenocarcinoma. BMC Cancer. 2021;21(1). [CrossRef]
- Kelly KA, Bardeesy N, Anbazhagan R; et al. Targeted Nanoparticles for Imaging Incipient Pancreatic Ductal Adenocarcinoma. PLoS Medicine. 2008;5(4):e85. [CrossRef]
- Liu P, Kong L, Liang K; et al. Identification of dissociation factors in pancreatic Cancer using a mass spectrometry-based proteomic approach. BMC Cancer. 2020;20(1). [CrossRef]
- Levink IJM, Visser IJ, Koopmann BDM; et al. Protein biomarkers in pancreatic juice and serum for identification of pancreatic cancer. Gastrointestinal Endoscopy. 2022;96(5):801-813.e2. [CrossRef]
- Chang YT, Wu CC, Shyr YM; et al. Secretome-Based Identification of ULBP2 as a Novel Serum Marker for Pancreatic Cancer Detection. PLoS ONE. 2011;6(5):e20029. [CrossRef]
- Firpo MA, Boucher KM, Bleicher J; et al. Multianalyte Serum Biomarker Panel for Early Detection of Pancreatic Adenocarcinoma. JCO Clinical Cancer Informatics. 2023;(7). [CrossRef]
- Kapszewicz M, Małecka-Wojciesko E. Simple Serum Pancreatic Ductal Adenocarcinoma (PDAC) Protein Biomarkers—Is There Anything in Sight? Journal of Clinical Medicine. 2021;10(22):5463. [CrossRef]
- de Oliveira G, Paccielli Freire P, Santiloni Cury S; et al. An Integrated Meta-Analysis of Secretome and Proteome Identify Potential Biomarkers of Pancreatic Ductal Adenocarcinoma. Cancers. 2020;12(3):716. [CrossRef]
- Xu T, Xu X, Liu PC, Mao H, Ju S. Transcriptomic Analyses and Potential Therapeutic Targets of Pancreatic Cancer With Concomitant Diabetes. Frontiers in Oncology. 2020;10. [CrossRef]
- Slapak EJ, Duitman J, Tekin C, Bijlsma MF, Spek CA. Matrix Metalloproteases in Pancreatic Ductal Adenocarcinoma: Key Drivers of Disease Progression? Biology. 2020;9(4):80. [CrossRef]
- Jones LE, Humphreys MJ, Campbell F, Neoptolemos JP, Boyd MT. Comprehensive Analysis of Matrix Metalloproteinase and Tissue Inhibitor Expression in Pancreatic Cancer. Clinical Cancer Research. 2004;10(8):2832-2845. [CrossRef]
- Shoucair S, Chen J, Martinson JR; et al. Association of Matrix Metalloproteinase 7 Expression With Pathologic Response After Neoadjuvant Treatment in Patients With Resected Pancreatic Ductal Adenocarcinoma. JAMA Surgery. 2022;157(7):e221362. [CrossRef]
- Zhang JJ, Zhu Y, Xie KL; et al. Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism. Molecular Cancer. 2014;13(1). [CrossRef]
- Giampazolias E, Schulz O, Lim KHJ; et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell. 2021;184(15):4016-4031.e22. [CrossRef]
- Xue N, Du T, Lai F, Jin J, Ji M, Chen X. Secreted HSP90α-LRP1 Signaling Promotes Tumor Metastasis and Chemoresistance in Pancreatic Cancer. International Journal of Molecular Sciences. 2022;23(10):5532. [CrossRef]
- Chen Y, Kang M, Lu W; et al. DJ-1, a novel biomarker and a selected target gene for overcoming chemoresistance in pancreatic cancer. Journal of Cancer Research and Clinical Oncology. 2012;138(9):1463-1474. [CrossRef]
- Li M, Zhai Q, Bharadwaj U; et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106(10):2284-2294. [CrossRef]
- Han JM, Jung HJ. Cyclophilin A/CD147 Interaction: A Promising Target for Anticancer Therapy. Int J Mol Sci. 2022 Aug 19;23(16):9341. [CrossRef]
- Chen Q, Wang Z, Zhang K; et al. Erratum to: Clusterin confers gemcitabine resistance in pancreatic cancer. World Journal of Surgical Oncology. 2013;11(1). [CrossRef]
- Rosati A, Bersani S, Tavano F; et al. Expression of the Antiapoptotic Protein BAG3 Is a Feature of Pancreatic Adenocarcinoma and Its Overexpression Is Associated With Poorer Survival. The American Journal of Pathology. 2012;181(5):1524-1529. [CrossRef]
- Falco A, Rosati A, Festa M; et al. BAG3 Is a Novel Serum Biomarker for Pancreatic Adenocarcinomas. American Journal of Gastroenterology. 2013;108(7):1178-1180. [CrossRef]
- Rosati A, Basile A, D’Auria R; et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nature Communications. 2015;6(1). [CrossRef]
- Dufrusine B, Damiani V, Capone E; et al. BAG3 induces fibroblasts to release key cytokines involved in pancreatic cell migration. Journal of Cellular Biochemistry. 2021;123(1):65-76. [CrossRef]
- Singh RR, Mohammad J, Orr M, Reindl KM. Glutathione S-Transferase pi-1 Knockdown Reduces Pancreatic Ductal Adenocarcinoma Growth by Activating Oxidative Stress Response Pathways. Cancers. 2020;12(6):1501. [CrossRef]
- Zhang S, Yao HF, Li H; et al. Transglutaminases Are Oncogenic Biomarkers in Human Cancers and Therapeutic Targeting of TGM2 Blocks Chemoresistance and Macrophage Infiltration in Pancreatic Cancer. Research Square Platform LLC; 2023. Accessed August 2, 2023. [CrossRef]
- Prieto-Fernández L, Menéndez ST, Otero-Rosales M; et al. Pathobiological functions and clinical implications of annexin dysregulation in human cancers. Frontiers in Cell and Developmental Biology. 2022;10. [CrossRef]
- Bouter A, Gounou C, Bérat R; et al. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nature Communications. 2011;2(1). [CrossRef]
- Bouvet F, Ros M, Bonedeau E; et al. Defective Membrane Repair Machinery Impairs Survival of Invasive Cancer Cells. Cold Spring Harbor Laboratory; 2020. Accessed August 2, 2023. [CrossRef]
- Dong X, Javle M, Hess KR, Shroff R, Abbruzzese JL, Li D. Insulin-Like Growth Factor Axis Gene Polymorphisms and Clinical Outcomes in Pancreatic Cancer. Gastroenterology. 2010;139(2):464-473.e3. [CrossRef]
- Martínez-Bosch N, Cristóbal H, Iglesias M; et al. Soluble AXL is a novel blood marker for early detection of pancreatic ductal adenocarcinoma and differential diagnosis from chronic pancreatitis. eBioMedicine. 2022;75:103797. [CrossRef]
- Miller MA, Sullivan RJ, Lauffenburger DA. Molecular Pathways: Receptor Ectodomain Shedding in Treatment, Resistance, and Monitoring of Cancer. Clinical Cancer Research. 2017;23(3):623-629. [CrossRef]
- Jiang W, Li X, Xiang C, Zhou W. Neutrophils in pancreatic cancer: Potential therapeutic targets. Frontiers in Oncology. 2022;12. [CrossRef]
- Jin W, Xu HX, Zhang SR; et al. Tumor-Infiltrating NETs Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Annals of Surgical Oncology. 2018;26(2):635-643. [CrossRef]
- Yi N, Zhao X, Ji J; et al. Serum galectin-3 as a biomarker for screening, early diagnosis, prognosis and therapeutic effect evaluation of pancreatic cancer. Journal of Cellular and Molecular Medicine. 2020;24(19):11583-11591. [CrossRef]
- Guo Y, Shen R, Yu L; et al. Roles of galectin-3 in the tumor microenvironment and tumor metabolism (Review). Oncology Reports. Published online September 22, 2020. [CrossRef]
- Teijeira A, Garasa S, Ochoa MC; et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clinical Cancer Research. 2020;27(9):2383-2393. [CrossRef]
- Menzies-Gow A, Ying S, Sabroe I; et al. Eotaxin (CCL11) and Eotaxin-2 (CCL24) Induce Recruitment of Eosinophils, Basophils, Neutrophils, and Macrophages As Well As Features of Early- and Late-Phase Allergic Reactions Following Cutaneous Injection in Human Atopic and Nonatopic Volunteers. The Journal of Immunology. 2002;169(5):2712-2718. [CrossRef]
- Sabrkhany S, Kuijpers MJE, van Kuijk SMJ; et al. A combination of platelet features allows detection of early-stage cancer. European Journal of Cancer. 2017;80:5-13. [CrossRef]
- Lecot P, Ardin M, Dussurgey S; et al. Gene Signature of Circulating Platelet-Bound Neutrophils Is Associated with Poor Prognosis in Cancer Patients. Cold Spring Harbor Laboratory; 2021. Accessed August 2, 2023. [CrossRef]
- Takehara M, Sato Y, Kimura T; et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Science. 2020;111(8):2883-2894. [CrossRef]
- Niu X, Yin L, Yang X; et al. Serum amyloid A 1 induces suppressive neutrophils through the Toll-like receptor 2–mediated signaling pathway to promote progression of breast cancer. Cancer Science. 2022;113(4):1140-1153. [CrossRef]
- Liu YH, Hu CM, Hsu YS, Lee WH. Interplays of glucose metabolism and KRAS mutation in pancreatic ductal adenocarcinoma. Cell Death & Disease. 2022;13(9). [CrossRef]
- Yang P, Li Z, Wang Y, Zhang L, Wu H, Li Z. Secreted pyruvate kinase M2 facilitates cell migration via PI3K/Akt and Wnt/β-catenin pathway in colon cancer cells. Biochemical and Biophysical Research Communications. 2015;459(2):327-332. [CrossRef]
- Lay AJ, Jiang XM, Kisker O; et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408(6814):869-873. [CrossRef]
- Principe M, Borgoni S, Novelli F. Commentary: “Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis.” Journal of Rare Diseases Research & Treatment. 2017;2(5):18-21. [CrossRef]
- Zhao Z, Zhang J, Yin L; et al. Upregulated GDF-15 expression facilitates pancreatic ductal adenocarcinoma progression through orphan receptor GFRAL. Aging. Published online November 17, 2020. [CrossRef]
- Albrengues J, Bourget I, Pons C; et al. LIF Mediates Proinvasive Activation of Stromal Fibroblasts in Cancer. Cell Reports. 2014;7(5):1664-1678. [CrossRef]
- Xelwa N, Candy GP, Devar J, Omoshoro-Jones J, Smith M, Nweke EE. Targeting Growth Factor Signaling Pathways in Pancreatic Cancer: Towards Inhibiting Chemoresistance. Frontiers in Oncology. 2021;11. [CrossRef]
- van den Berg YW, van den Hengel LG, Myers HR; et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proceedings of the National Academy of Sciences. 2009;106(46):19497-19502. [CrossRef]
- Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Molecular Cancer Therapeutics. 2018;17(6):1147-1155. [CrossRef]
- Praharaj PP, Patra S, Panigrahi DP, Patra SK, Bhutia SK. Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2021;1875(2):188500. [CrossRef]
- Zhang Y, Lu L, Song F; et al. Research progress on non-protein-targeted drugs for cancer therapy. Journal of Experimental & Clinical Cancer Research. 2023;42(1). [CrossRef]
- Cardin DB, Thota R, Goff LW; et al. A Phase II Study of Ganetespib as Second-line or Third-line Therapy for Metastatic Pancreatic Cancer. American Journal of Clinical Oncology. 2018;41(8):772-776. [CrossRef]
- Lang JE, Forero-Torres A, Yee D; et al. Safety and efficacy of HSP90 inhibitor ganetespib for neoadjuvant treatment of stage II/III breast cancer. npj Breast Cancer. 2022;8(1). [CrossRef]
- Zhang L, Wang P, Qin Y; et al. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene. 2016;36(9):1297-1308. [CrossRef]
- Kram M. Galectin-3 inhibition as a potential therapeutic target in non-alcoholic steatohepatitis liver fibrosis. World Journal of Hepatology. 2023;15(2):201-207. [CrossRef]
- Friedbichler K, Hofmann MH, Kroez M; et al. Data from Pharmacodynamic and Antineoplastic Activity of BI 836845, a Fully Human IGF Ligand-Neutralizing Antibody, and Mechanistic Rationale for Combination with Rapamycin. American Association for Cancer Research (AACR); 2023. Accessed August 3, 2023. [CrossRef]
- Weyer-Czernilofsky U, Hofmann MH, Friedbichler K; et al. Data from Antitumor Activity of the IGF-1/IGF-2–Neutralizing Antibody Xentuzumab (BI 836845) in Combination with Enzalutamide in Prostate Cancer Models. American Association for Cancer Research (AACR); 2023. Accessed August 3, 2023. [CrossRef]
- Schmid P, Cortes J, Joaquim A; et al. XENERA-1: A randomised double-blind Phase II trial of xentuzumab in combination with everolimus and exemestane versus everolimus and exemestane in patients with hormone receptor-positive/HER2-negative metastatic breast cancer and non-visceral disease. Breast Cancer Research. 2023;25(1). [CrossRef]
- Sullivan LA, Carbon JG, Roland CL; et al. r84, a Novel Therapeutic Antibody against Mouse and Human VEGF with Potent Anti-Tumor Activity and Limited Toxicity Induction. PLoS ONE. 2010;5(8):e12031. [CrossRef]
- Kindler HL, Niedzwiecki D, Hollis D; et al. Gemcitabine Plus Bevacizumab Compared With Gemcitabine Plus Placebo in Patients With Advanced Pancreatic Cancer: Phase III Trial of the Cancer and Leukemia Group B (CALGB 80303). Journal of Clinical Oncology. 2010;28(22):3617-3622. [CrossRef]
- Van Cutsem E, Vervenne WL, Bennouna J; et al. Phase III Trial of Bevacizumab in Combination With Gemcitabine and Erlotinib in Patients With Metastatic Pancreatic Cancer. Journal of Clinical Oncology. 2009;27(13):2231-2237. [CrossRef]
- Wang MT, Fer N, Galeas J; et al. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nature Communications. 2019;10(1). [CrossRef]
- Borazanci E, Schram AM, Garralda E; et al. Phase I, first-in-human study of MSC-1 (AZD0171), a humanized anti-leukemia inhibitory factor monoclonal antibody, for advanced solid tumors. ESMO Open. 2022;7(4):100530. [CrossRef]
- Basile A, De Marco M, Festa M; et al. Development of an anti-BAG3 humanized antibody for treatment of pancreatic cancer. Molecular Oncology. 2019;13(6):1388-1399. [CrossRef]
- Iorio V, Rosati A, D’Auria R; et al. Combined effect of anti-BAG3 and anti-PD-1 treatment on macrophage infiltrate, CD8+ Tcell number and tumour growth in pancreatic cancer. Gut. Published online August 11, 2017:gutjnl-2017-314225. [CrossRef]
- De Marco M, Gauttier V, Pengam S; et al. Concerted BAG3 and SIRPα blockade impairs pancreatic tumor growth. Cell Death Discovery. 2022;8(1). [CrossRef]
- Iorio V, De Marco M, Basile A; et al. CAF-Derived IL6 and GM-CSF Cooperate to Induce M2-like TAMs–Letter. Clinical Cancer Research. 2019;25(2):892-893. [CrossRef]
- Rosati A, Marzullo L, De Marco M, De Laurenzi V, D’Amico MF, Turco MC. Toxicity in combined therapies for tumours treatments: A lesson from BAG3 in the TME? Frontiers in Immunology. 2023;14. [CrossRef]
- Li P, Rozich N, Wang J; et al. Anti-IL-8 antibody activates myeloid cells and potentiates the anti-tumor activity of anti-PD-1 antibody in the humanized pancreatic cancer murine model. Cancer Letters. 2022; 539:215722. [CrossRef]
- Bilusic M, Heery CR, Collins JM; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. Journal for ImmunoTherapy of Cancer. 2019;7(1). [CrossRef]
- .Lewis CS, Karve A, Matiash K; et al. A First-In-Class, Humanized Antibody Targeting Alternatively Spliced Tissue Factor: Preclinical Evaluation in an Orthotopic Model of Pancreatic Ductal Adenocarcinoma. Frontiers in Oncology. 2021;11. [CrossRef]
- Ganguly K, Kimmelman AC. Reprogramming of tissue metabolism during cancer metastasis. Trends in Cancer. 2023;9(6):461-471. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





