Submitted:
07 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and experimental methods
2.1. Surface pretreatment
2.2. Phosphate chemical conversion
2.3. Electrochemical measurements
2.4. Cell cultrue
2.5. Characterization of samples
3. Results
3.1. Phase composition
3.2. Microstructure
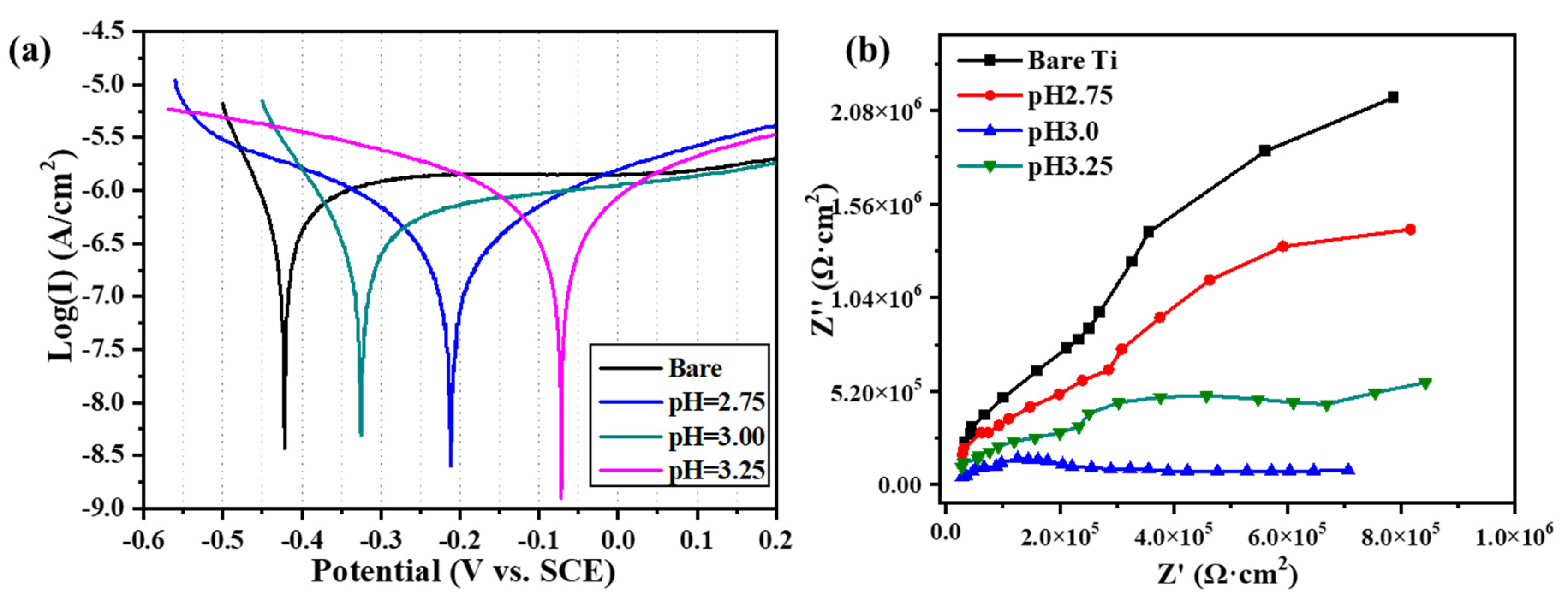
3.3. Corrosion Characteristics.
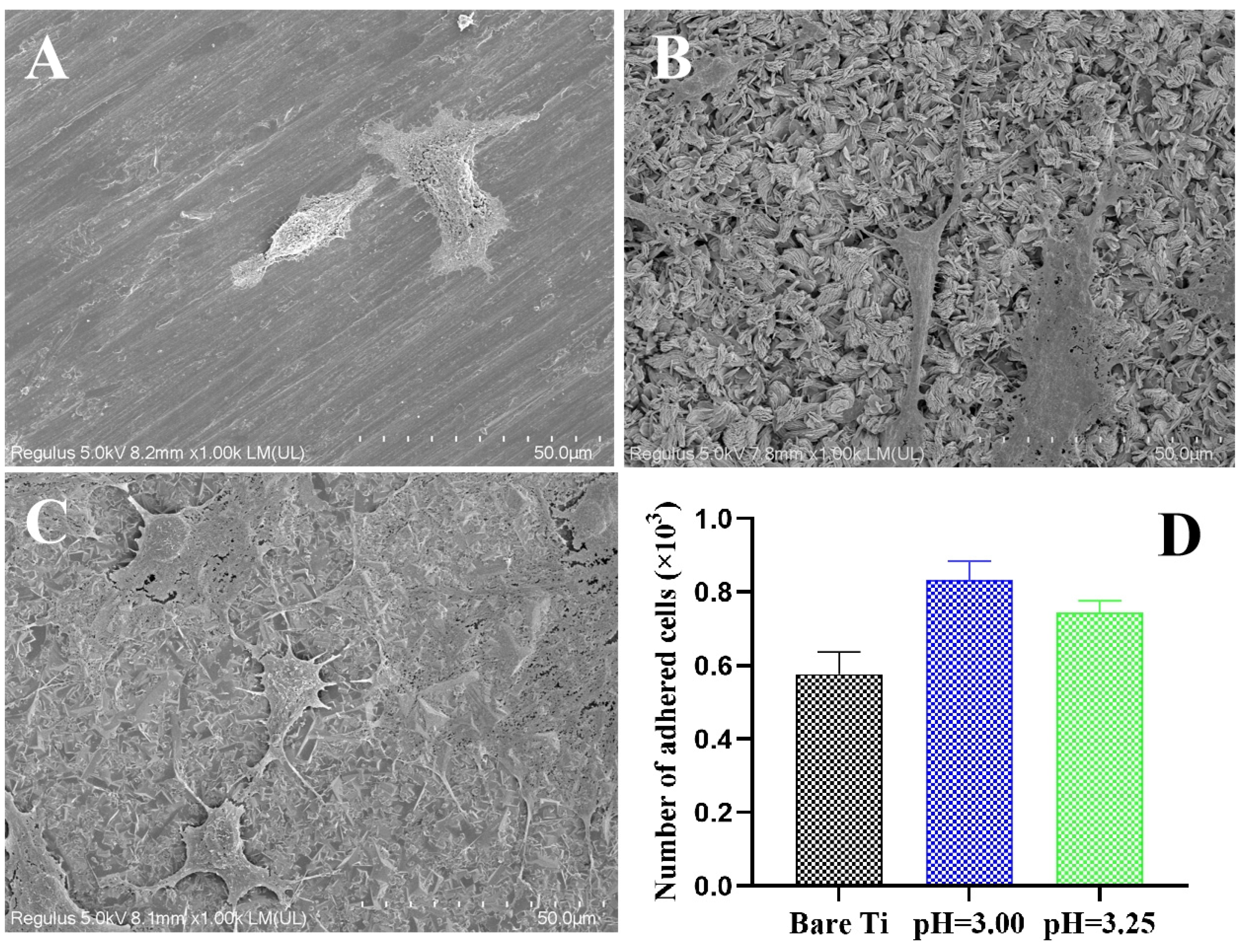
3.4. Cytocompatibility
4. Discussion
5. Conclusion
Acknowledgments
References
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement - a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; He, Y.; Lin, C.; Liu, P.; Cai, K. Antibacterial surface design of biomedical titanium materials for orthopedic applications. Journal of Materials Science & Technology 2021, 78, 51–67. [Google Scholar] [CrossRef]
- Shi, J.; Li, Y.; Gu, Y.; Qiao, S.; Zhang, X.; Lai, H. Effect of titanium implants with strontium incorporation on bone apposition in animal models: A systematic review and meta-analysis. Scientific Reports 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.T.; Xie, Y.W.; Xu, J.G.; Li, J.; Wang, H.; He, F.M. Effects of strontium-incorporated micro/nano rough titanium surfaces on osseointegration via modulating polarization of macrophages. Colloids Surf B Biointerfaces 2021, 207, 111992. [Google Scholar] [CrossRef]
- Yu, D.; Guo, S.; Yu, M.; Liu, W.; Li, X.; Chen, D.; Li, B.; Guo, Z.; Han, Y. Immunomodulation and osseointegration activities of Na2TiO3 nanorods-arrayed coatings doped with different Sr content. Bioact Mater 2022, 10, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, X.; Xiao, G.Y.; Lu, Y.P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Materials Science & Engineering C-Materials for Biological Applications 2015, 47, 97–104. [Google Scholar] [CrossRef]
- Rajabalizadeh, Z.; Seifzadeh, D. Strontium phosphate conversion coating as an economical and environmentally-friendly pretreatment for electroless plating on AM60B magnesium alloy. Surface and Coatings Technology 2016, 304, 450–458. [Google Scholar] [CrossRef]
- Zhao, D.-W.; Liu, C.; Zuo, K.-Q.; Su, P.; Li, L.-B.; Xiao, G.-Y.; Cheng, L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chemical Engineering Journal 2021, 408. [Google Scholar] [CrossRef]
- Li, Y.-B.; Lu, Y.-P.; Du, C.-M.; Zuo, K.-Q.; Wang, Y.-Y.; Tang, K.-L.; Xiao, G.-Y. Effect of Reaction Temperature on the Microstructure and Properties of Magnesium Phosphate Chemical Conversion Coatings on Titanium. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Du, C.; Zuo, K.; Ma, Z.; Zhao, M.; Li, Y.; Tian, S.; Lu, Y.; Xiao, G. Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Zhao, D.W.; Du, C.M.; Zuo, K.Q.; Zhao, Y.X.; Xu, X.Q.; Li, Y.B.; Tian, S.; Yang, H.R.; Lu, Y.P.; Cheng, L.; et al. Calcium–Zinc Phosphate Chemical Conversion Coating Facilitates the Osteointegration of Biodegradable Zinc Alloy Implants by Orchestrating Macrophage Phenotype. Advanced Healthcare Materials 2023, 12. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Luo, Z.; Hu, Y.; Yu, Y.; Tao, B.; Li, C.; Cai, K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fan, S.; Bai, X.; Ding, C. Strontium ranelate, a promising disease modifying osteoarthritis drug. Expert Opinion on Investigational Drugs 2017, 26, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, Q.; Li, Y.; Fan, J.; Wang, G.; Pan, H.; Ruan, C. Strontium incorporation improves the bone-forming ability of scaffolds derived from porcine bone. Colloids and surfaces. B, Biointerfaces 2017, 162, 279–287. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact Mater 2022, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta biomaterialia 2018, 65, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Beswick, A.D.; Peters, T.J.; Gooberman-Hill, R.; Whitehouse, M.R.; Blom, A.W.; Moore, A.J. Health Care Needs and Support for Patients Undergoing Treatment for Prosthetic Joint Infection following Hip or Knee Arthroplasty: A Systematic Review. PLOS ONE 2017, 12, e0169068. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V.; Fang, M.; Liu, Y. Discovery of New Solid Solution Phosphors via Cation Substitution-Dependent Phase Transition in M3(PO4)2:Eu2+ (M = Ca/Sr/Ba) Quasi-Binary Sets. The Journal of Physical Chemistry C 2015, 119, 2038–2045. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.-y.; Chen, C.-z.; Lu, Y.-p.; Geng, X.-w. Hopeite and scholzite coatings formation on titanium via wet-chemical conversion with controlled temperature. Surface and Coatings Technology 2020, 384, 125330. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.-y.; Jiang, C.-c.; Liu, B.; Li, N.-b.; Zhu, R.-f.; Lu, Y.-p. Influence of process parameters on microstructure and corrosion properties of hopeite coating on stainless steel. Corrosion Science 2015, 94, 428–437. [Google Scholar] [CrossRef]
- Wang, L.-l.; Xu, W.-h.; Xiao, G.-y.; Jiang, C.-c.; Zheng, Y.-z.; Lu, Y.-p. Chemical conversion of zinc–zinc phosphate composite coating on TC4 by galvanic coupling. New Journal of Chemistry 2017, 41, 14403–14408. [Google Scholar] [CrossRef]
- Phuong, N.V.; Lee, K.H.; Chang, D.; Moon, S. Effects of Zn2+ concentration and pH on the zinc phosphate conversion coatings on AZ31 magnesium alloy. Corrosion Science 2013, 74, 314–322. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.-y.; Lu, Y.-p. Effect of pH on the Phase Composition and Corrosion Characteristics of Calcium Zinc Phosphate Conversion Coatings on Titanium. Journal of The Electrochemical Society 2016, 163, C477–C485. [Google Scholar] [CrossRef]
- Zuo, K.-q.; Gong, Z.-y.; Xiao, G.-y.; Huang, S.-y.; Du, C.-m.; Liu, B.; Zhang, D.-s.; Lu, Y.-p. Microstructural evolution of strontium-zinc-phosphate coating on titanium via changing Zn2+ concentration in phosphate solution for enhanced osteogenic activity. Surface and Coatings Technology 2022, 433. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wei, Q.L.; Huang, Y.M. Preparation and adsorption properties of the biomimetic gama-alumina. Materials Letters 2015, 157, 67–69. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.Y.; Jiang, C.C.; Zheng, Y.Z.; Wang, L.L.; Lu, Y.P. Formation initiation and structural changes of phosphate conversion coating on titanium induced by galvanic coupling and Fe2+ ions. Rsc Advances 2016, 6, 75365–75375. [Google Scholar] [CrossRef]
- Akhtar, A.S.; Wong, K.C.; Mitchell, K.A.R. The effect of pH and role of Ni2+ in zinc phosphating of 2024-Al alloy. Part I: Macroscopic studies with XPS and SEM. Applied Surface Science 2006, 253, 493–501. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Hulliger, J. Growth of strontium hydrogen phosphate/gelatin composites: a biomimetic approach. New Journal of Chemistry 2016, 40, 5495–5500. [Google Scholar] [CrossRef]
- Scheel, H.J.; Fukuda, T. Crystal Growth Technology. J. Wiley, 2003; pp. 225–249. [Google Scholar]
- Zhi-Zhan, L.W.-J.S.E.-w.Z.Y.-q.Y.Z.-W.C. Nucleating Mechanism of Oxide Crystal and Its Particle Size. JOURNAL OF INORGANIC MATERIALS 2000, 15(5), 777–786. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Xu, J.; Li, X.; Li, X.; Yan, Y.; Qi, M.; Hu, M. Strontium coating by electrochemical deposition improves implant osseointegration in osteopenic models. Experimental and therapeutic medicine 2015, 9, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Hori, S.; Tani, T.; Ikeda-Fukazawa, T.; Aizawa, M. Hydrothermal synthesis of single-crystal α-tristrontium phosphate particles. Journal of the European Ceramic Society 2017, 37, 351–357. [Google Scholar] [CrossRef]
- Hulshof, F.F.B.; Papenburg, B.; Vasilevich, A.; Hulsman, M.; Zhao, Y.; Levers, M.; Fekete, N.; de Boer, M.; Yuan, H.; Singh, S.; et al. Mining for osteogenic surface topographies: In silico design to in vivo osseo-integration. Biomaterials 2017, 137, 49–60. [Google Scholar] [CrossRef] [PubMed]
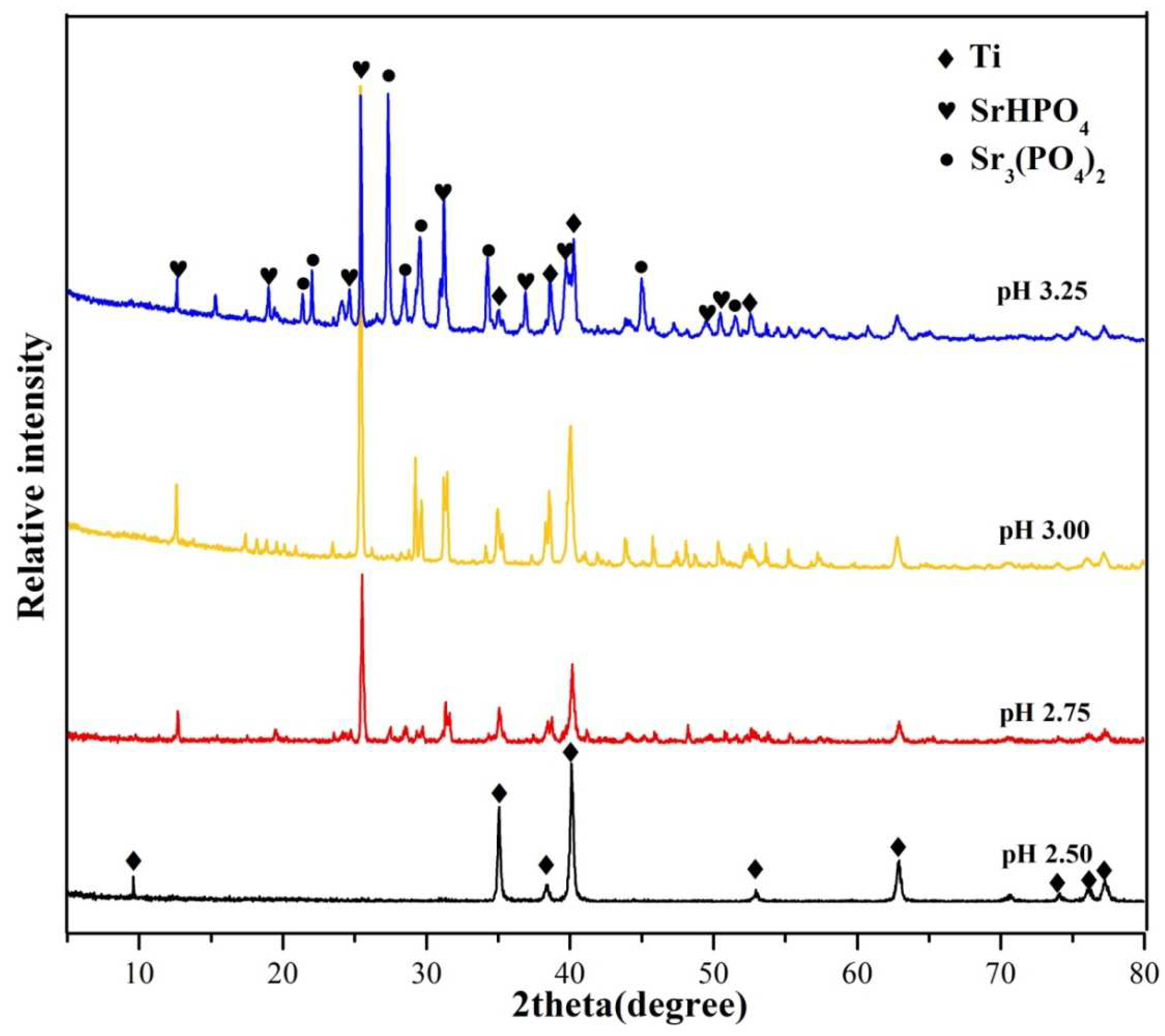
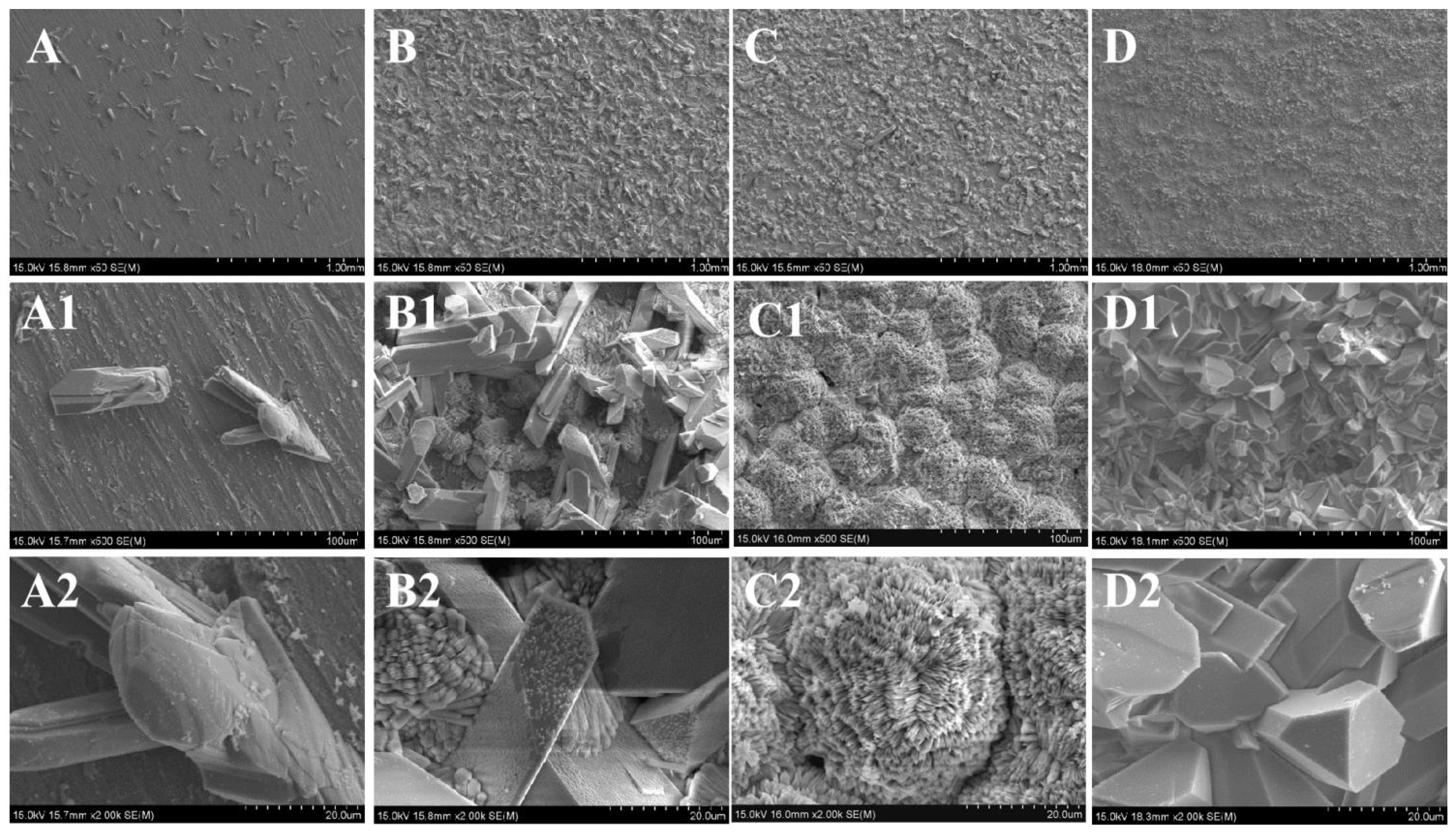
| pH value | O | P | Sr | Ti | C | Sr/P |
|---|---|---|---|---|---|---|
| 2.50 | 67.59 | 9.56 | 10.10 | 2.81 | 9.94 | 1.06 |
| 2.75 | 62.13 | 13.52 | 13.85 | ---- | 10.50 | 1.02 |
| 3.00 | 70.17 | 14.02 | 15.68 | 0.13 | ---- | 1.12 |
| 3.25 | 57.52 | 13.20 | 16.52 | ---- | 12.76 | 1.25 |
| Sample | Ecorr (V) | Icorr (× 10−8 A/cm2) | βa (V·dec−1) | −βc (V·dec−1) | Rp (× 104 Ω·cm2) |
|---|---|---|---|---|---|
| Bare Ti | −0.426 ± 0.006 | 12.67 ± 4.35 | 0.129 ± 0.007 | 0.107 ± 0.005 | 11.230 ± 0.675 |
| pH=2.75 | −0.212 ± 0.009 | 28.32 ± 6.24 | 0.221 ± 0.010 | 0.179 ± 0.019 | 15.163 ± 0.022 |
| pH=3.00 | −0.325 ± 0.011 | 30.04 ± 4.54 | 0.399 ± 0.006 | 0.088 ± 0.013 | 24.122 ± 0.286 |
| pH=3.25 | −0.072 ± 0.016 | 53.89 ± 3.46 | 0.445 ± 0.001 | 0.215 ± 0.002 | 16.026 ± 0.954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





