Submitted:
07 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Epigenetics and Gut Microbiota
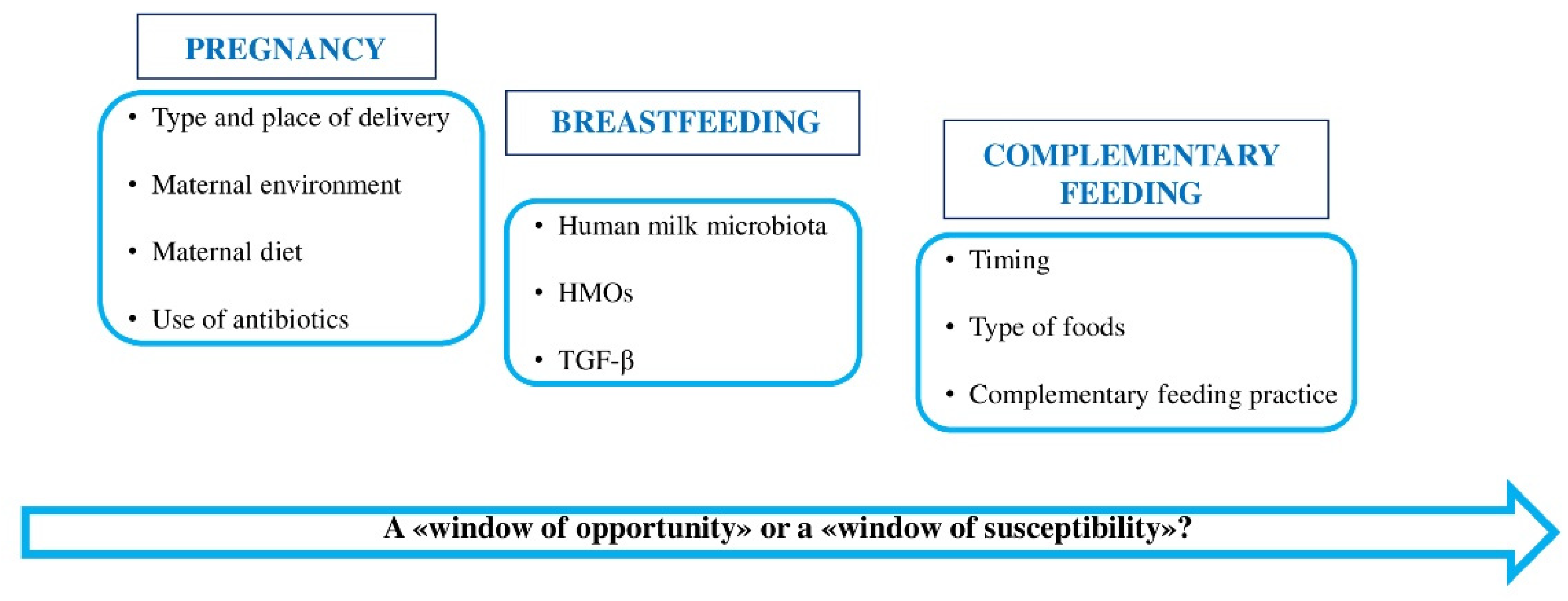
4. First 1000 days of life: “a window of opportunity” in gut microbiota
4.1. Gut Microbiota of pregnant women
4.2. Gut Microbiota in the first months of life
4.2.1. Role of Human Milk Microbiota
4.2.2. Role of Human Milk Oligosaccharides (HMOs)
4.3. Gut Microbiota and Complementary Feeding
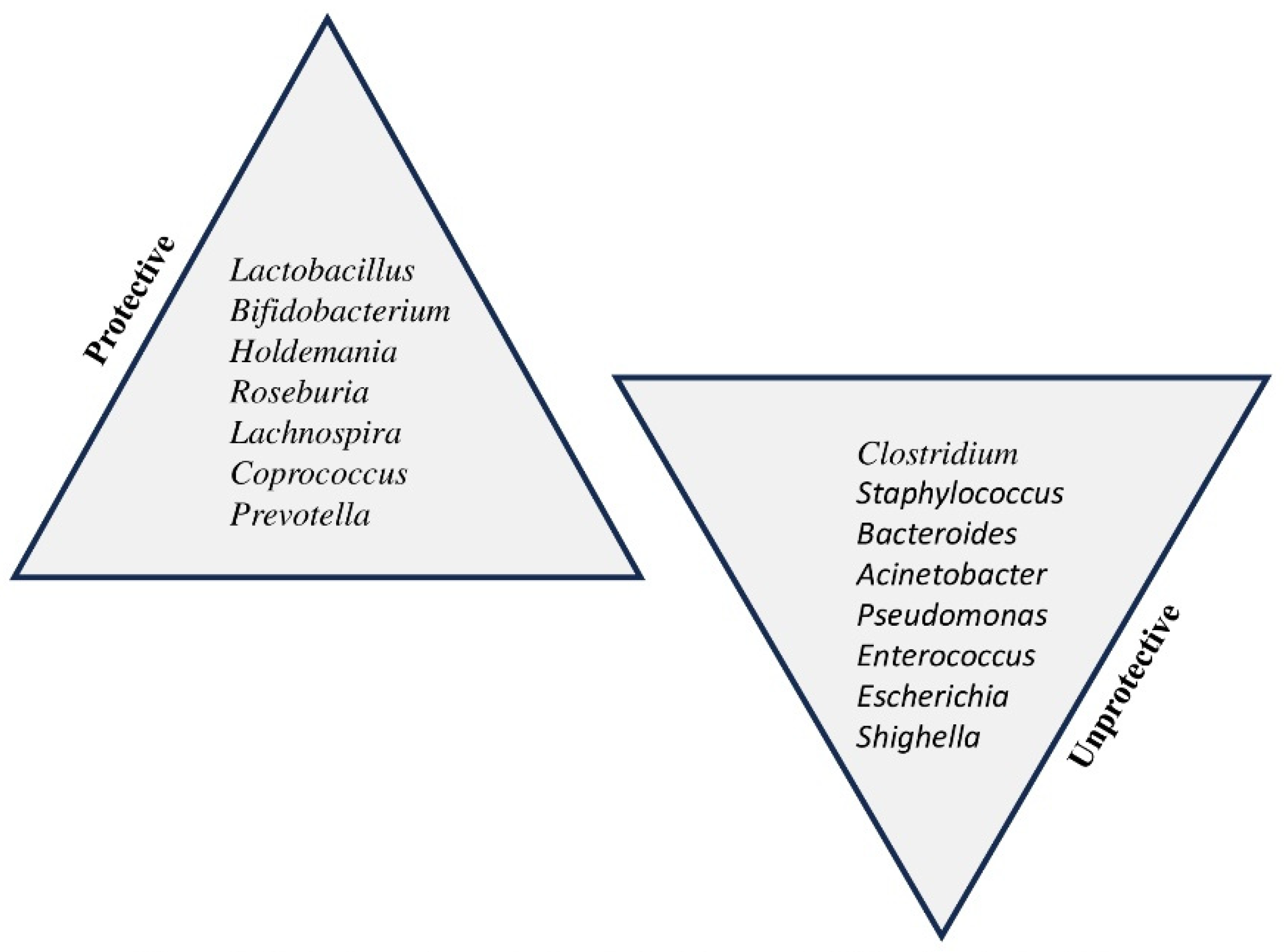
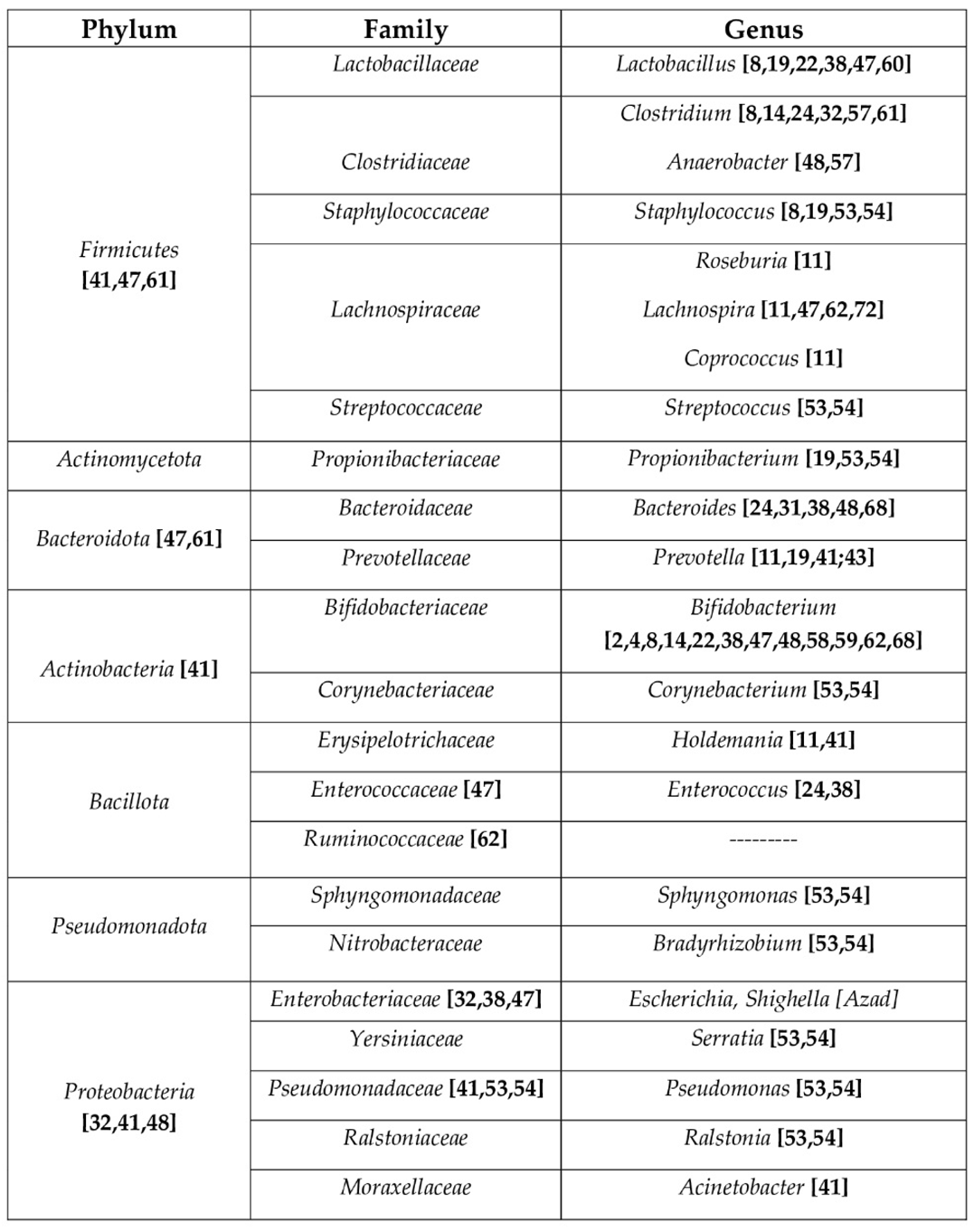
5. Microbiota’s dysbiosis: mechanisms associated with FA
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, T.; Dong, Y.; Yang, C.; Zhao, M.; He, Q. Pathogenesis of Children’s Allergic Diseases: Refocusing the Role of the Gut Microbiota. Front Physiol 2021, 12, 749544. [Google Scholar] [CrossRef]
- Molloy, J.; Allen, K.; Collier, F.; Tang, M.L.K.; Ward, A.C.; Vuillermin, P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int J Environ Res Public Health 2013, 10, 7235–7256. [Google Scholar] [CrossRef]
- Brzozowska, A.; Podlecka, D.; Jankowska, A.; Król, A.; Kaleta, D.; Trafalska, E.; Nowakowska-Swirta, E.; Kaluzny, P.; Hanke, W.; Bal-Gierańczyk, K.; Kowalska, M.; Polańska, K.; Jerzyńska, J. Maternal diet during pregnancy and risk of allergic diseases in children up to 7–9 years old from Polish Mother and Child Cohort study. Environ Res 2022, 208, 112682. [Google Scholar] [CrossRef]
- Davis, E.C.; Jackson, C.M.; Ting, T.; Harizaj, A.; Järvinen, K.M. Predictors and biomarkers of food allergy and sensitization in early childhood. Ann Allergy Asthma Immunol 2022, 129, 292–300. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G.B. Cellular and molecular mechanisms of vitamin D in food allergy. J Cell Mol Med 2018, 22, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Rackaityte, E.; Halkias, J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front Immunol 2020, 11, 588. [Google Scholar] [CrossRef]
- Tsabouri, S.; Priftis, K.N.; Chaliasos, N.; Siamopoulou, A. Modulation of gut microbiota downregulates the development of food allergy in infancy. Allergol Immunopathol (Madr) 2014, 42, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, P.J.; O’Hely, M.; Collier, F.; Allen, K.J.; Tang, M.L.K.; Harrison, L.C.; Carlin, J.B.; Saffery, R.; Ranganathan, S.; Sly, P.D.; et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat Commun 2020, 11, 1452. [Google Scholar] [CrossRef]
- Netting, M.J.; Middleton, P.F.; Makrides, M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition 2014, 30, 1225–1241. [Google Scholar] [CrossRef]
- Gao, Y.; Nanan, R.; Macia, L.; Tan, J.; Sominsky, L.; Quinn, T.P.; O’Hely, M.; Ponsonby, Al.; Tang, M.L.K.; Collier, F.; et al. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J Allergy Clin Immunol 2021, 148, 669–678. [Google Scholar] [CrossRef]
- Hirsch, A.G.; Pollak, J.; Glass, T.A.; Poulsen, M.N.; Bailey-Davis, L.; Mowery, J.; Schwartz, B.S. Early Life Antibiotic Use and Subsequent Diagnosis of Food Allergy and Allergic Diseases. Clin Exp Allergy 2017, 47, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Carta, M.; Montante, C.; Notarbartolo, V.; Corsello, G; Giuffrè, M. Current Insights on Early Life Nutrition and Prevention of Allergy. Front Pediatr 2020, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit Rev Food Sci Nutr 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Fernández, L.; Verhasselt, V. The Gut-Breast Axis: Programming Health for Life. Nutrients 2021, 13, 606. [Google Scholar] [CrossRef]
- Groer, M.W.; Morgan, K.H.; Louis-Jacques, A.; Miller, E.M. A scoping review of research on the human milk microbiome. J Hum Lact 2020, 36, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr Gastroenterol Hepatol Nutr 2022, 25, 194–210. [Google Scholar] [CrossRef]
- Renz, H.; Skevaki, C. Early life microbial exposures and allergy risks: Opportunities for prevention. Nat Rev Immunol 2021, 21, 177–191. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Bordoni, L.; Morano, S.; Calleja-Agius, J.; Lalor, J.G. Nutri-Epigenetics and Gut Microbiota: How Birth Care, Bonding and Breastfeeding Can Influence and Be Influenced? Int J Mol Sci 2020, 21, 5032. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front Microbiol 2022, 12, 796025. [Google Scholar] [CrossRef]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief Bioinform 2018, 19, 679–692. [Google Scholar] [CrossRef]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X. Epigenetics and Development of Food Allergy (FA) in Early Childhood. Curr Allergy Asthma Rep. 2014, 14, 460. [Google Scholar] [CrossRef]
- Di Costanzo, M.; De Paulis, N.; Capra, M.E.; Biasucci, G. Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy. Nutrients 2022, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Banderali, G.; Barberi, S.; Radaelli, G.; Lops, A.; Betti, F.; Riva, E.; Giovannini, M. Epigenetic Effects of Human Breast Milk. Nutrients 2014, 6, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Loret de Mola, C.; Davies, N.M.; Victora, C.G.; Relton, C.L. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS ONE 2017, 12, e0173070. [Google Scholar] [CrossRef]
- Van Esch, B.C.A.M.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; Joost van Neerven, R.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front Immunol 2020, 11, 2141. [Google Scholar] [CrossRef]
- Wang, M.; Yang, I.V.; Davidson, E.J.; Joetham, A.; Takeda, K.; O’Connor, B.P.; Gelfand, E.W. Forkhead Box Protein 3 (FoxP3) Demethylation is Associated with Tolerance Induction in Peanut-Induced Intestinal allergy. J Allergy Clin Immunol 2018, 141, 659–670. [Google Scholar] [CrossRef]
- Briollais, L.; Rustand, D.; Allard, C.; Wu, Y.; Xu, J.; Rajan, S.G.; Hivert, MF.; Doyon, M.; Bouchard, L.; O McGowan, P.; et al. DNA methylation mediates the association between breastfeeding and early-life growth trajectories. Clin Epigenetics 2021, 13, 231. [Google Scholar] [CrossRef]
- Barker, D.J.P. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public Health 2012, 126, 185–189. [Google Scholar] [CrossRef]
- Di Costanzo, M.; De Paulis, N.; Biasucci, G. Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy. Life (Basel) 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Fiedl, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin Exp Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Ehyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, H. Update on Early Nutrition and Food Allergy in Children. Yonsei Med J 2016, 57, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.M.; Bauer, T.; Geissler, S.; Hirche, F.; Thürmann, L.; Bauer, M.; Trump, S.; Bieg, M.; Weichenhan, D.; Gu, L.; et al. Increased vitamin D levels at birth and in early infancy increase offspring allergy risk-evidence for involvement of epigenetic mechanisms. J Allergy Clin Immunol. 2016, 137, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Weisse, K.; Winkler, S.; Hirche, F.; Herberth, G.; Hinz, D.; Bauer, M.; Rӧder, S.; Rolle-Kampczyk, U.; von Bergern, M.; Olek, S.; et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 2013, 68, 220–228. [Google Scholar] [CrossRef]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr Allergy Immunol 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory Effects of Breast Milk on Food Allergy. Ann Allergy Asthma Immunol. 2019, 123, 133–143. [Google Scholar] [CrossRef]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Makrides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr 2016, 103, 128–143. [Google Scholar] [CrossRef]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr Allergy Immunol 2017, 28, 135–143. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.; Li, X.; Gao, Y.; Dai, N.; We, Y.; Liu, L.; Xing, Y.; Li, Z. Relationship between maternal-infant gut microbiota and infant food allergy. Front Microbiol 2022, 13, 933152. [Google Scholar] [CrossRef]
- Tuokkola, J.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Vaarala, O.; Kenward, M.G.; Virta, L.J.; Veijola, R.; Simell, O.; Ilonen, J.; et al. Maternal diet during pregnancy and lactation and cow’s milk allergy in offspring. Eur J Clin Nutr 2016, 70, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, P.J.; Macia, L.; Nanan, R.; Lk Tang, M.; Collier, F.; Brix, S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol 2017, 39, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Al Khatib, H.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Shen, X.; Wang, M.; Zhang, X.; He, M.; Li, M.; Cheng, G.; Wan, C.; He, F. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019, 19, 123. [Google Scholar] [CrossRef]
- Méndez, C.S.; Bueno, S.M.; Kalergis, A.M. Contribution of Gut Microbiota to Immune Tolerance in Infants. J immunol Res. 2021, 2021, 7823316. [Google Scholar] [CrossRef]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition for food allergy in infants. Appl Environ Microbiol 2014, 80, 2546–2554. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin.Immunol. 2012, 129, 434–440. [Google Scholar] [CrossRef]
- Kielleniva, K.; Ainonen, S.; Vänni, P.; Paalanne, N.; Renko, M.; Salo, J.; Tejesvi, M.V.; Pokka, T.; Pirttilä, A.M.; Tapiainen, T. Microbiota of the first-pass meconium and subsequent atopic and allergic disorders in children. Clin Exp Allergy 2022, 52, 684–696. [Google Scholar] [CrossRef]
- Shu, S.A.; Yuen, A.W.T.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, GX.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin Rev Allergy Immunol 2019, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Leyva, L.L.; Brereton, N.J.B.; Koski, K.G. Emerging frontiers in human milk microbiome research and suggested primers for 16S rRNA gene analysis. Comput Struct Biotechnol J 2020, 19, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Moubareck, C.A. Human Milk Microbiota and Oligosaccharides: A Glimpse into Benefits, Diversity, and Correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr Dev Nutr 2020, 4, nzaa027. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cesarani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Y.; Liu, L.; Li, Z. Association Between Breastmilk Microbiota and Food Allergy in Infants. Front Cell Infect Microbiol 2022, 11, 770913. [Google Scholar] [CrossRef]
- Rey-Mariňo, A.; Pilar Francino, M. Nutrition, Gut Microbiota, and Allergy Development in Infants. Nutrients 2022, 14, 4316. [Google Scholar] [CrossRef]
- Dai, D.L.Y.; Petersen, C.; Hoskinson, C.; Del Bel, K.L.; Becker, A.B.; Moraes, T.J.; Mandhane, P.J.; Finlay, B.B.; Simons, E.; Kozyrskyj, A.L.; et al. Breastfeeding enrichment of B. longum subsp. infantis mitigates the effect of antibiotics on the microbiota and childhood asthma risk. Med 2023, 4, 92–112. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrion. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Yokanovich, L.T.; Newberry, R.D.; Knoop, K.A. Regulation of oral antigen delivery early in life: Implications for oral tolerance and food allergy. Clin Exp Allergy 2021, 51, 518–526. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann Nutr Metab 2021, 30, 1–14. [Google Scholar] [CrossRef]
- Di Profio, E.; Magenes, V.C.; Fiore, G.; Agostinelli, M.; La Mendola, A.; Acunzo, M.; Francavilla, R.; Indrio, F.; Bosetti, A.; D’Auria, E.; et al. Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients 2022, 14, 3198. [Google Scholar] [CrossRef]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Alvarez-Quintero, R.; Velasquez-Mejia, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and MetaAnalysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Comberiati, P.; Costagliola, G.; D’Elios, S.; Peroni, D. Prevention of Food Allergy: The Significance of Early Introduction. Medicina (Kaunas) 2019, 55, 323. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015, 372, 803–813. [Google Scholar] [CrossRef]
- Grimshaw, K.E.C.; Maskell, J.; Oliver, E.M.; Morris, R.C.G.; Foote, K.D.; Clare Mills, E.N.; Roberts, G.; Margetts, B.M. Introduction of complementary foods and the relationship to food allergy. Pediatrics 2013, 132, e1529–e1538. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Haszard, J.J.; Lawley, B.; Otal, A.; Taylor, R.W.; Szymlek-Gay, E.A.; Fleming, E.A.; Daniels, L.; Fangupo, L.J.; Tannock, G.W.; et al. Mediation Analysis as a Means of Identifying Dietary Components That Differentially Affect The Fecal Microbiota of Infants Weaned by Modified Baby-Led and Traditional Approaches. Appl Environ Microbiol. 2018, 84, e00914–e00918. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life gut microbiome and egg allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Wang, J.; Hao, M.; Che, H. Antibiotic-Induced Gut Microbiota Dysbiosis Damages the Intestinal Barrier, Increasing Food Allergy in Adult Mice. Nutrients 2021, 13, 3315. [Google Scholar] [CrossRef]
- Nance, C.L.; Deniskin, R.; Diaz, V.C.; Paul, M.; Anvari, S.; Anagnostou, A. The Role of the Microbiome in Food Allergy: A Review. Children (Basel) 2020, 7, 50. [Google Scholar] [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





