Submitted:
07 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Sample Collection
2.4. Anthropometric and Biochemical Analysis
2.5. Circulating Total RNA Extraction
2.6. cDNA Synthesis
2.7. Circulating miRNA Real-Time Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
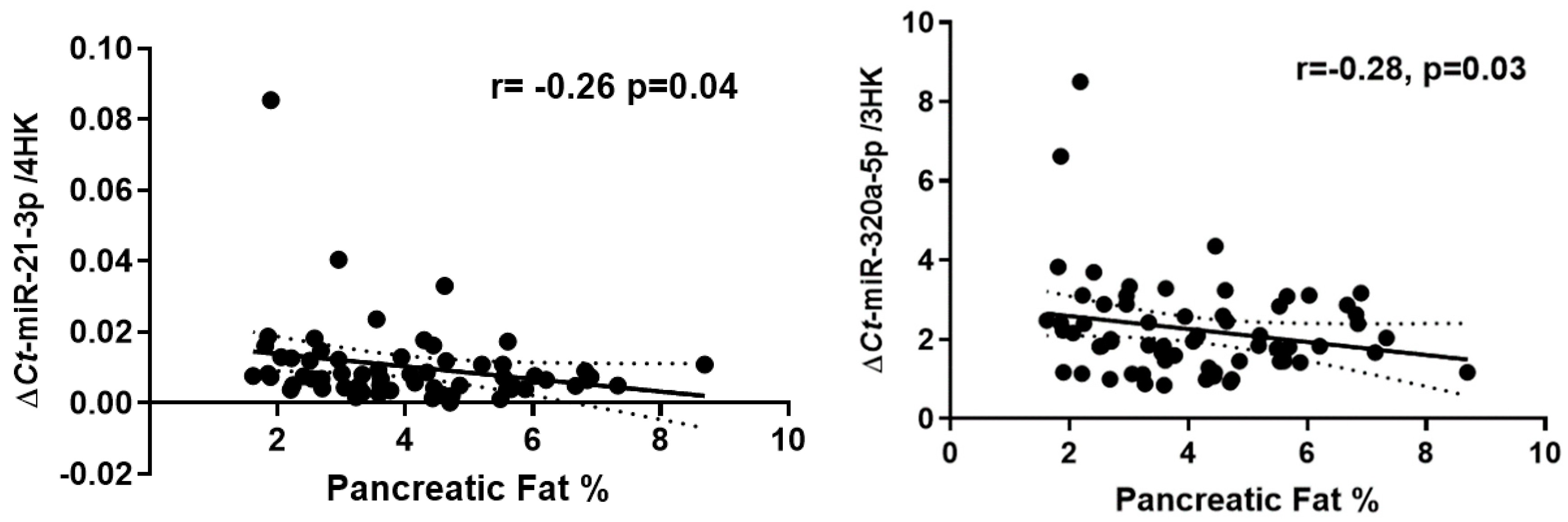
3.2. Expression of Circulating miRNAs Correlates with MR-%pancreas Fat But Not with MR-%liver Fat
3.3. Expression of Circulating miRNAs Correlates with HOMA2-IR, HbA1c and Fasting Plasma Insulin
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and Ectopic Fat, Atherosclerosis, and Cardiometabolic Disease: A Position Statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 1–20. [Google Scholar] [CrossRef]
- Catanzaro, R.; Cuffari, B.; Italia, A.; Marotta, F. Exploring the Metabolic Syndrome: Nonalcoholic Fatty Pancreas Disease. World J. Gastroenterol. 2016, 22, 7660. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Li, H. Nonalcoholic Fatty Liver Disease Pandemic Fuels the Upsurge in Cardiovascular Diseases. Circ. Res. 2020, 126, 679–704. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Marine, M.; Lu, D.; Swartz-Basile, D.A.; Saxena, R.; Zyromski, N.J.; Pitt, H.A. Nonalcoholic Fatty Pancreas Disease. HPB 2007, 9, 312–318. [Google Scholar] [CrossRef]
- Unger, R.H. Lipid Overload and Overflow: Metabolic Trauma and the Metabolic Syndrome. Trends Endocrinol. Metab. 2003, 14, 398–403. [Google Scholar] [CrossRef]
- Singh, R.G.; Yoon, H.D.; Poppitt, S.D.; Plank, L.D.; Petrov, M.S. Ectopic Fat Accumulation in the Pancreas and Its Biomarkers: A Systematic Review and Meta-Analysis. Diabetes. Metab. Res. Rev. 2017, 33, e2918. [Google Scholar] [CrossRef]
- Heber, S.D.; Hetterich, H.; Lorbeer, R.; Bayerl, C.; Machann, J.; Auweter, S.; Storz, C.; Schlett, C.L.; Nikolaou, K.; Reiser, M.; et al. Pancreatic Fat Content by Magnetic Resonance Imaging in Subjects with Prediabetes, Diabetes, and Controls from a General Population without Cardiovascular Disease. PLoS One 2017, 12, e0177154. [Google Scholar] [CrossRef]
- Al-Mrabeh, A.; Hollingsworth, K.G.; Steven, S.; Tiniakos, D.; Taylor, R. Quantification of Intrapancreatic Fat in Type 2 Diabetes by MRI. PLoS One 2017, 12, e0174660. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, Y.; Cai, H.; Zhang, Z.; Peng, Z.; Jiang, M.; Li, Y.; Li, C.; Li, Z.P.; Feng, S.T. Noninvasive Fat Quantification of the Liver and Pancreas May Provide Potential Biomarkers of Impaired Glucose Tolerance and Type 2 Diabetes. Medicine (Baltimore). 2016, 95, e3858. [Google Scholar] [CrossRef]
- LaPierre, M.P.; Stoffel, M. MicroRNAs as Stress Regulators in Pancreatic Beta Cells and Diabetes. Mol. Metab. 2017, 6, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Otgonsuren, M.; Younoszai, Z.; Allawi, H.; Raybuck, B.; Younossi, Z. Circulating MiRNA in Patients with Non-Alcoholic Fatty Liver Disease and Coronary Artery Disease. BMJ Open Gastroenterol. 2016, 3, e000096. [Google Scholar] [CrossRef]
- Goncalves, B.d.S.; Meadows, A.; Pereira, D.G.; Puri, R.; Pillai, S.S. Insight into the Inter-Organ Crosstalk and Prognostic Role of Liver-Derived MicroRNAs in Metabolic Disease Progression. Biomedicines 2023, 11, 1597. [Google Scholar] [CrossRef] [PubMed]
- López-Bermudo, L.; Luque-Sierra, A.; Maya-Miles, D.; Gallego-Durán, R.; Ampuero, J.; Romero-Gómez, M.; Berná, G.; Martín, F. Contribution of Liver and Pancreatic Islet Crosstalk to β-Cell Function/Dysfunction in the Presence of Fatty Liver. Front. Endocrinol. (Lausanne). 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Calderari, S.; Diawara, *!!! REPLACE !!!*; Garaud, A.; Gauguier, D. Biological Roles of MicroRNAs in the Control of Insulin Secretion and Action. Physiol. Genomics 2017, 49, 1–10. [Google Scholar] [CrossRef]
- Song, I.; Roels, S.; Martens, G.A.; Bouwens, L. Circulating MicroRNA-375 as Biomarker of Pancreatic Beta Cell Death and Protection of Beta Cell Mass by Cytoprotective Compounds. PLoS One 2017, 12, e0186480. [Google Scholar] [CrossRef]
- Lei, L.; Zhou, C.; Yang, X.; Li, L. Down-Regulation of MicroRNA-375 Regulates Adipokines and Inhibits Inflammatory Cytokines by Targeting AdipoR2 in Non-Alcoholic Fatty Liver Disease. Clin. Exp. Pharmacol. Physiol. 2018, 45, 819–831. [Google Scholar] [CrossRef]
- Gatfield, D.; Le Martelot, G.; Vejnar, C.E.; Gerlach, D.; Schaad, O.; Fleury-Olela, F.; Ruskeepää, A.-L.; Oresic, M.; Esau, C.C.; Zdobnov, E.M.; et al. Integration of MicroRNA MiR-122 in Hepatic Circadian Gene Expression. Genes Dev. 2009, 23, 1313–1326. [Google Scholar] [CrossRef]
- Lynn, F.C. Meta-Regulation: MicroRNA Regulation of Glucose and Lipid Metabolism. Trends Endocrinol. Metab. 2009, 20, 452–459. [Google Scholar] [CrossRef]
- Qu, Y.; Ding, Y.; Lu, J.; Jia, Y.; Bian, C.; Guo, Y.; Zheng, Z.; Mei, W.; Cao, F.; Li, F. Identification of Key MicroRNAs in Exosomes Derived from Patients with the Severe Acute Pancreatitis. Asian J. Surg. 2023, 46, 337–347. [Google Scholar] [CrossRef]
- Sequeira, I.R.; Yip, W.C.; Lu, L.W.W.; Jiang, Y.; Murphy, R.; Plank, L.D.; Cooper, G.J.S.; Peters, C.N.; Lu, J.; Hollingsworth, K.G.; et al. Pancreas Fat, an Early Marker of Metabolic Risk? A Magnetic Resonance Study of Chinese and Caucasian Women: TOFI_Asia Study. Front. Physiol. 2022, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [CrossRef] [PubMed]
- Sequeira, I.R.; Yip, W.; Lu, L.; Jiang, Y.; Murphy, R.; Plank, L.; Zhang, S.; Liu, H.; Chuang, C.L.; Vazhoor-Amarsingh, G.; et al. Visceral Adiposity and Glucoregulatory Peptides Are Associated with Susceptibility to Type 2 Diabetes: The TOFI_Asia Study. Obesity 2020, 28, 2368–2378. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.E.; Fraser, K.; Kruger, M.C.; Sequeira, I.R.; Yip, W.; Lu, L.W.; Plank, L.D.; Murphy, R.; Cooper, G.J.S.; Martin, J.C.; et al. Untargeted Metabolomics Reveals Plasma Metabolites Predictive of Ectopic Fat in Pancreas and Liver as Assessed by Magnetic Resonance Imaging: The TOFI_Asia Study. Int. J. Obes. 2021, 45, 1844–1854. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Markworth, J.F.; Aasen, K.M.M.; Zeng, N.; Cameron-Smith, D.; Mitchell, C.J. Acute Resistance Exercise Modulates MicroRNA Expression Profiles: Combined Tissue and Circulatory Targeted Analyses. PLoS One 2017, 12, e0181594. [Google Scholar] [CrossRef]
- Ramzan, F.; D’Souza, R.F.; Durainayagam, B.R.; Milan, A.M.; Markworth, J.F.; Miranda-Soberanis, V.; Sequeira, I.R.; Roy, N.C.; Poppitt, S.D.; Mitchell, C.J.; et al. Circulatory MiRNA Biomarkers of Metabolic Syndrome. Acta Diabetol. 2020, 57, 203–214. [Google Scholar] [CrossRef]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum MicroRNAs. PLoS One 2016, 11, e0153200. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Singh, R.G.; Yoon, H.D.; Wu, L.M.; Lu, J.; Plank, L.D.; Petrov, M.S. Ectopic Fat Accumulation in the Pancreas and Its Clinical Relevance: A Systematic Review, Meta-Analysis, and Meta-Regression. Metabolism 2017, 69, 1–13. [Google Scholar] [CrossRef]
- Petäjä, E.M.; Yki-Järvinen, H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, L.; Wei, X.; Liu, B.; Zhao, J.; Xie, P.; Yang, B.; Wang, L. MiR-21-3p Aggravates Injury in Rats with Acute Hemorrhagic Necrotizing Pancreatitis by Activating TRP Signaling Pathway. Biomed. Pharmacother. 2018, 107, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuźnicka, M. NGS Reveals Molecular Pathways Affected by Obesity and Weight Loss-Related Changes in MiRNA Levels in Adipose Tissue. Int. J. Mol. Sci. 2018, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.; Gburcik, V.; Petrovic, N.; Gallagher, I.J.; Nedergaard, J.; Cannon, B.; Timmons, J.A. Gene-Chip Studies of Adipogenesis-Regulated MicroRNAs in Mouse Primary Adipocytes and Human Obesity. BMC Endocr. Disord. 2011, 11, 7. [Google Scholar] [CrossRef]
- Androsavich, J.R.; Chau, B.N.; Bhat, B.; Linsley, P.S.; Walter, N.G. Disease-Linked MicroRNA-21 Exhibits Drastically Reduced MRNA Binding and Silencing Activity in Healthy Mouse Liver. RNA 2012, 18, 1510–1526. [Google Scholar] [CrossRef]
- Calo, N.; Ramadori, P.; Sobolewski, C.; Romero, Y.; Maeder, C.; Fournier, M.; Rantakari, P.; Zhang, F.-P.; Poutanen, M.; Dufour, J.-F.; et al. Stress-Activated MiR-21/MiR-21* in Hepatocytes Promotes Lipid and Glucose Metabolic Disorders Associated with High-Fat Diet Consumption. Gut 2016, 65, 1871–1881. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Wang, D.; Lin, J.H.; Liao, Z.; Ji, J.T.; Du, T.T.; Jiang, F.; Hu, L.H.; Li, Z.S. Novel Blood-Based MicroRNA Biomarker Panel for Early Diagnosis of Chronic Pancreatitis. Sci. Rep. 2017, 7, 40019. [Google Scholar] [CrossRef]
- Ling, H.-Y.; Ou, H.-S.; Feng, S.-D.; Zhang, X.-Y.; Tuo, Q.-H.; Chen, L.-X.; Zhu, B.-Y.; Gao, Z.-P.; Tang, C.-K.; Yin, W.-D.; et al. Changes in MicroRNA (MiR) Profile and Effects of Mir-320 in Insulin-Resistant 3t3-L1 Adipocytes. Clin. Exp. Pharmacol. Physiol. 2009, 36, e32–e39. [Google Scholar] [CrossRef]
- Lê, K.A.; Ventura, E.E.; Fisher, J.Q.; Davis, J.N.; Weigensberg, M.J.; Punyanitya, M.; Hu, H.H.; Nayak, K.S.; Goran, M.I. Ethnic Differences in Pancreatic Fat Accumulation and Its Relationship with Other Fat Depots and Inflammatory Markers. Diabetes Care 2011, 34, 485–490. [Google Scholar] [CrossRef]
- Huang, R.S.; Gamazon, E.R.; Ziliak, D.; Wen, Y.; Im, H.K.; Zhang, W.; Wing, C.; Duan, S.; Bleibel, W.K.; Cox, N.J.; et al. Population Differences in MicroRNA Expression and Biological Implications. RNA Biol. 2011, 8, 692–701. [Google Scholar] [CrossRef]
- Tiscornia, O.M.; Cresta, M.A.; de Lehmann, E.S.; Celener, D.; Dreiling, D.A. Effects of Sex and Age on Pancreatic Secretion. Int. J. Pancreatol. 1986, 1, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genomics. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]


| Participant Characteristics | Asian Chinese (n=34) | European Caucasian (n=34) |
| Age (years) | 41.0 ± 13.0 | 47.8 ± 15.4 |
| BMI (kg/m2) | 26.7 ± 4.2 | 28.0 ± 4.5 |
| Waist Circumference (cm) | 85.6 ± 11.1 | 91.7± 13.9 |
| BP-Systolic (mmHg) | 120 ± 22 | 120 ± 19 |
| BP-Diastolic (mmHg) | 65 ± 12 | 64 ± 8 |
| HbA1c (%NGSP) | 5.4 | 5.3 |
| Fasting Plasma Glucose (FPG) (mmol/L) | 5.2 ± 0.5 | 5.1 ± 0.7 |
| Total Cholesterol (mmol/L) | 4.5 ± 0.9 | 5.2 ± 0.9 |
| LDL-C (mmol/L) | 2.5 ± 0.7 | 2.9 ± 0.9 |
| HDL-C (mmol/L) | 1.4 ± 0.4 | 1.8 ± 0.4 |
| Triglycerides (mmol/L) | 1.3 ± 0.7 | 1.0 ± 0.5 |
| HOMA2-IR | 1.8 ± 1.0 | 1.6 ± 1.2 |
| MR-%pancreas fat | 4.3 ± 2.0 | 4.1 ± 1.9 |
| MR-%liver fat | 4.6 ± 0.8 | 3.8 ± 0.8 |
| Model | B | SEM | Expected (B) | P-value | |
|---|---|---|---|---|---|
| (Constant) | -0.29 | 0.19 | -1.48 | 0.14 | |
| miR-21-3p | -3.87 | 1.63 | -2.37 | 0.02 | |
| miR-320a-5p | -0.02 | 0.01 | -1.53 | 0.13 | |
| BMI (kg/m2) | 0.01 | 0.00 | 3.07 | 0.00 | |
| FPG (mmol/L) | 0.11 | 0.03 | 3.60 | 0.00 | |
| Insulin (pg/ml) | -0.00 | 0.00 | -0.48 | 0.62 | |
| Ethnicity | 0.00 | 0.03 | 0.23 | 0.81 | |
| Model | B | SEM | Expected (B) | P-value |
|---|---|---|---|---|
| (Constant) | -1.04 | 0.47 | -2.21 | 0.03 |
| miR-21-3p | -4.92 | 3.82 | -1.28 | 0.20 |
| miR-320a-5p | -0.02 | 0.04 | -0.50 | 0.61 |
| BMI (kg/m2) | 0.02 | 0.01 | 2.16 | 0.03 |
| FPG (mmol/L) | 0.12 | 0.08 | 1.52 | 0.13 |
| Insulin (pg/ml) | 0.00 | 0.00 | 1.86 | 0.06 |
| Ethnicity | 0.05 | 0.09 | 0.56 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





