1. Introduction
Crohn's disease (CD) is a chronic inflammatory bowel disease with possible recurrences [
1,
2]. It is a subtype of inflammatory bowel disease (IBD), a chronic inflammatory disorder of the gastrointestinal tract. Treatment of this condition is lifelong [
3,
4]. The reason for its post-onset is a transbronchial inflammation, which can affect any part of the gastrointestinal tract. Its phenotype is usually variable and is determined either by the risk of progression or by the location of the disease [
5].
IBD are a group of diseases of unexplained etiology and chronic course, with periods of exacerbation and remission [
6]. Initially described in the 19th and early 20th centuries, individual cases from Great Britain and northern Europe grew in number and geographically expanded so that today they are recognized all over the world, and their number is not decreasing. A characteristic feature of this group of diseases is the continuity of inflammatory changes in the mucosa starting from the rectum, covering the ascending large intestine to the possible involvement of the final segment of the small intestine. The relationship between the disease and the immune system remains undeniable. Clinically, IBD carries many intestinal complications and systemic diseases, as well as dysplastic changes - the risk of developing colorectal cancer [
7].
IBD is most common in Scandinavia, Western Europe and North America. Currently, studies indicate that the incidence of IBD is lower in developing countries. In Asian populations, the incidence ranges from 5.3 to 63.6 per 100,000 people, while in North America it ranges from 37.5 to 238 per 100,000 people [
8]. It has been noted that in Europe, there is a geographical gradient in the prevalence of IBD with higher rates in the north and lower rates in the south. The same observation from the North American area was confirmed in Sonnenberg's work, in which the higher incidence of IBD occurred in the northern regions of the United States [
9].
Most IBD patients in Europe and North America are in the 30-40 age group at diagnosis. It has been observed that the average age at diagnosis tends to be slightly higher in Asian countries compared to Western countries, and a study by Souza in Southeast Brazil showed that there is a trend towards a second peak in new hospital admissions in the 60-69 age group because of IBD [
10].
The etiology of IBD remains unexplained until the end. There were genetic hypotheses, hypotheses of increased permeability of the mucosa for antigens from the intestinal lumen, hypotheses regarding the ongoing inflammation of small vessels of the mucosa leading to altered mucosal permeability. Certainly, the immune system plays the most important role in the pathogenesis of the disease [
11].
Genetic factors
Differences in the incidence of IBD by race and ethnicity indicated from the outset that genetic factors play an important role. An increased incidence of IBD is observed among family members of patients, especially among first-degree relatives. In this group, the incidence is 10-30% higher than in the general population, with no increased incidence among spouses, which argues against the involvement of infectious agents [
12]. A higher incidence of the disease has been described in identical twins. A higher prevalence of DR2HLA II, DR9 and DRB10 alleles has been described in patients with IBD. Predisposition to the development of the disease is also associated with the presence of certain regions of chromosomes 2 and 6 and regions located on chromosomes 3, 7 and 12 [
13].
Environmental factors
Nutritional deficiencies occur in 20–85% of IBD patients, with protein-energy malnutrition being the most common. There are also overweight or obese CU patients, they have a higher colectomy rate than eutrophic patients, increased need for permanent ileostomy, longer hospital stays, higher rate of incisional hernia after ileo-rectal anastomosis, increased risk of non-alcoholic fatty liver disease and thrombotic disease [
14]. Increased body weight is also associated with early loss of response to drugs in IBD. The formation of pathogenic microflora in genetically predisposed individuals is associated with changes in epithelial function, dysregulation of the immune function of the digestive tract and persistent inflammation of the intestines. [
15].
Symptoms
The symptoms of IBD range from rectal and anal irritation to frequent and profuse bloody diarrhea accompanied by colic pains in the middle and lower abdomen, especially on the left side [
16]. Profuse gastrointestinal bleeding may occur in 3% of patients. There are also cases of constipation.
In 1.6-6% of patients megacolon toxicum develops, in this form atony, intestinal dilatation and thinning of its wall are accompanied by a septic state resulting from the penetration of bacteria from the intestinal lumen into the blood. Complications of severe IBD include perforations, peritonitis, and massive gastrointestinal bleeding [
17]. Another type of complications are shortening and narrowing of the intestinal lumen, and developing dysplasia, which increases the risk of developing colorectal cancer. Colorectal cancer is most common in patients after the seventh year of the disease. The greatest risk concerns patients with pancolitis - 25% after 20 years. In comparison, patients with rectal and sigmoid colon involvement develop colorectal cancer in 3.7% after 20 years of disease [
18].
Patients with IBD, like patients with Crohn's disease, have a wide spectrum of extraintestinal symptoms. These include skin and mucosal symptoms - erythema nodosum and erythema multiforme, gangrenous dermatitis, skin infections, inflammation of the corners of the mouth and aphthae of the oral mucosa; eye symptoms - conjunctivitis, choroid and iris inflammation; joint symptoms - arthritis, ankylosing spondylitis, sacroiliitis; cardiovascular symptoms - pericarditis, vasculitis, aneurysms; anemia, vascular thrombosis; obstructive lung disease; sclerosing cholangitis, hepatitis, cirrhosis, pancreatic stenosis and cholangiocarcinoma, gallstones; urolithiasis, perirenal abscesses; amyloidosis. Extraintestinal symptoms have been shown to be associated with more advanced disease and poorer prognosis [
19].
Morphology
IBD is characterized by diffuse, continuous inflammation limited to the colonic mucosa. Macroscopically, the inflamed mucosa is swollen, granular, friable, reddened and bleeds easily, with or without ulcerations of various sizes [
20]. Mucosal changes include continuity from the rectum, proximal along a varying length of colon, with the distal colon likely to be more inflamed than the proximal. The regenerating mucosa shows signs of atrophy, the adjacent unchanged areas are thicker and protrude above the areas with atrophy, which gives the appearance of pseudopolyps. The histological picture is different in the active phase of inflammation and in remission [
21]. In the full-blown phase, the inflammation covers the mucous membrane and blood vessels. There is a profuse inflammatory infiltrate of lymphocytes, plasma cells, and macrophages with associated infiltration from neutrophils, less numerous eosinophils and mast cells [
22]. Granulocytes occur both in the lamina propria and penetrate into the crypts, creating microabscesses. Ulcers covered with granulocytic infiltration form above the inflamed crypts. In IBD, the mucosa may return to normal or remain atrophic during remission [
23]. Subsequent recurrences lead to disorders of the architecture of the glands in the form of shortening of the crypts and the presence of spaces between the lower part of the crypts and the upper edge of the muscular layer of the mucosa, as well as branching of the crypts, as well as persistent chronic inflammatory infiltration with hyperplasia of the intestinal lymphatic system and an increased number of plasma cells at the base of the membrane mucosa. The presence of Paneth cells in the mucosa of the left colon is a metaplastic process also associated with chronic damage to the crypt epithelium [
24]. Metaplasia of the pyloric glands is rare. In fulminant cases, necrosis and ulceration occur, which involve the submucosa and muscularis. In cases of long-term relapses and remissions, the intestinal wall may also be significantly shortened, with a dominant picture of fibrosis of the inner layers of the wall [
25].
One of the disabling manifestations of CD is perianal fistulas, which in many cases are associated with rectal disease [
26]. The hallmarks of ulcerative colitis are bursting ulcers, granulomatous inflammation and submucosal fibrosis. Histologic features of CD include distortion of the crypts, lymphocyte infiltration and chronic inflammation of the rectum, mostly limited to the lamina propria [
27,
28]. Perianal fistulas are one of the most disabling manifestations of CD and are often associated with rectal disease [
26]. Additionally, the development of a fistula is a risk factor for more aggressive disease progression [
29]. Current therapeutic options for CD perianal fistulas are very limited and include antibiotics, immunomodulators, biologics and mesenchymal stem cells [
30]. However, these therapies are combined with chirurgical methods. Thus, surgical removal is the primary method of treating fistulas, although it is not a complete cure for the disease [
31] The etiopathogenesis of perianal fistula in CD is currently unknown. The diseased mucosa is destroyed and also ulcerated and then replaced by degranulation tissue. It can be assumed that inflammatory cells and myofibroblasts are interfered with, trying to repair the damaged tissue by depositing collagen or other components of the extracellular matrix (ECM). All this leads to remodeling of the tissue, which is characterized by high stiffness [
32,
33]. Fistulas in Crohn's disease are likely formed by epithelial-mesenchymal transition (EMT) of intestinal fibroblasts, which impairs the ability of the above cells to repair any damage to the mucosa [
34]. Under-going EMT, intestinal epithelial cells acquire properties of an invasive and migratory nature and above that lose both cell polarity and cell-to-cell contact. This leads to them becoming myofibroblasts [
35,
36]. However, the molecular mechanisms triggering EMT still remain to be investigated. Both CD and ulcerative colitis (UC) are two major types of non-specific inflammatory bowel disease that share common pathological and clinical features. However, they have several significant differences. The clinical diagnosis of IBD is usually established based on a collective evaluation of the clinical picture and findings on endoscopy, histopathology and radiography [
37,
38]. There is a need for objective clinical differentiation of CD and UC in patients who have inflammatory bowel disease in order to develop a treatment plan [
39,
40,
41,
42]. However, still the differential diagnosis of the above subtypes remains a major clinical challenge due to the fact that there is no single diagnostic modality for UC or Crohn's colitis [
35,
39,
41,
42,
43]. Based on the literature, about 5% - 15% of patients do not meet strict guidelines for the diagnosis of UC or CD [
36,
44]. And for about 14% of patients diagnoses change over time [
45,
46].
Tontini described miRNAs as biomarkers in the diagnosis of IBD in his study [
47]. At the time, however, a small number of studies showed that mRNAs could be directly used in the differential diagnosis of both CD and UC. Metalloproteinases (MMPs) are a large group of zinc-dependent proteolytic enzymes that are involved in the degradation and remodeling of the extracellular matrix (ECM) by cleaving specific elements [
48]. Impaired expression of matrix metalloproteinases (MMPs) as well as tissue inhibitors of metalloproteinases has been noted in CD fistula studies. In addition, it has been shown that attempts by myofibroblasts to repair damaged tissue, is a culmination of ECM remodeling. .(Abraham BP 2012). The important point is that the ECM, (highly hydrated) is composed of proteins and polysaccharides, mainly glycosaminoglycan hia-luronan (HA). As such, ECM provides essential physical support for cellular components and initiates important biochemical signals involved in tissue morphogenesis, differentiation and homeostasis [
49,
50]. HA binds to proteoglycan molecules and then binds to growth factors. They interact directly with receptors on cell surfaces, which in con-sequence induces intracellular signal transduction. In addition, they are involved in the regulation of gene transcription [
46].
The pathogenesis of CD-associated fistulas is poorly understood, in part because access to diseased tissues associated with fistulas is very limited. To date, there are also no accepted in vitro tissue models of pathogenic cellular alterations or in vivo models to study the disease [
51]. The literature refers to the transition of intestinal epithelial cells (IECs) to mesen-chymal cells. In addition, one can find entries referring to the regulation of matrix metalloproteinases and the overexpression of invasive molecules among the hypothesized pathways involved in the pathogenesis of CD with fistulas [
52]. MMPs are a product of many cell types, including leukocytes, mesenchymal cells and epithelial cells. Myofibroblasts are able to produce MMP1, -2, -3 and -9, while neutrophils and macrophages produce MMP8, -9, -10 and -12 [
53,
54,
55].
2. Materials and Methods
2.1. Large intestine tissue samples
The study included 31 patients diagnosed with Crohn's disease and ten patients with the healthy digestive tract, ranging in age from 23-70 years, with a mean age of 40.4. Patients were treated at the Department of General Surgery, Regional Clinical Hospital No. 2 in Rzeszow between 2018 and 2020. Bowel tissue samples were collected by resection of a selected bowel segment or during colectomy. A total of 41 samples were used.
The collected samples had a volume of 13 × 7 × 8 mm.
Patients diagnosed with Crohn's disease were treated with pharmacological agents (azatioprine, 5-ASA, infliximab, mercaptopurine, adalimumab, steroids, Ciprofloxacin, methipred).
2.2. Procedure of preparation samples
Sick tissues were catted out from the large intestine of the patients with Crohn's disease and healthy patients. The sampling process was carried out at the Frederic Chopin Clinical Hospital No. 2 in Rzeszow. This work was approved by RESOLUTION No. 2018/06/04 of the Bioethics Committee of the University of Rzeszow.
Those parts of tissues were treated with liquid nitrogen and immediately were frozen and stored at -80C until analyses were performed. 25 to 45 mg of the tissue piece was rinsed in ice-cold PBS buffer to remove the excess blood, dried with filter paper, and weighed again. Fragmented tissues were homogenized with Ripa Lysis buffer on the ice at 10:1 (10μL chilled RIPA Buffer per milligram of tissue). The lysis buffer contained 1 % sodium deoxycholate, 0.1 % SDS, and protease and phosphatase inhibitors added immediately before use.
2.3. Characteristics of Enzyme-linked Immunosorbent Assay
Then the homogenate was centrifuged for 10 min. at 10,000 × g. The supernatant was collected, and four metalloproteinases - MMP 3, MMP 7, MMP 8 and MMP 9 were analyzed by Enzyme-linked Immunosorbent Assay using SEA101HU, SEA102Hu, SEA103Hu and SEA553Hu kits (Cloud-Clone Corp., Kata, TX, USA).
2.4. Chemical analyses and statistica
All chemical analyses were performed in triplicate. The content of metalloproteinases was expressed as mean ± standard deviation. The significance of differences between mean levels of metalloproteinases within tissues of patient after and without recurrence was estimated using nonparametric the Mann-Whitney U test at p < 0.05.
The significance of metalloproteinases and occurrence place of Crohn within intestine on the recurrence of disease was modelled using a generalised linear mixed modelling (GLMM) procedure (Statistica ver. 13.3, TIBCO Software Inc., Palo Alto, CA, USA). Akaike’s information criterion (AIC) was used to evaluate with of the models was best supported by the data and only models with AIC < 2, as being equally supported, were analyzed.
3. Results
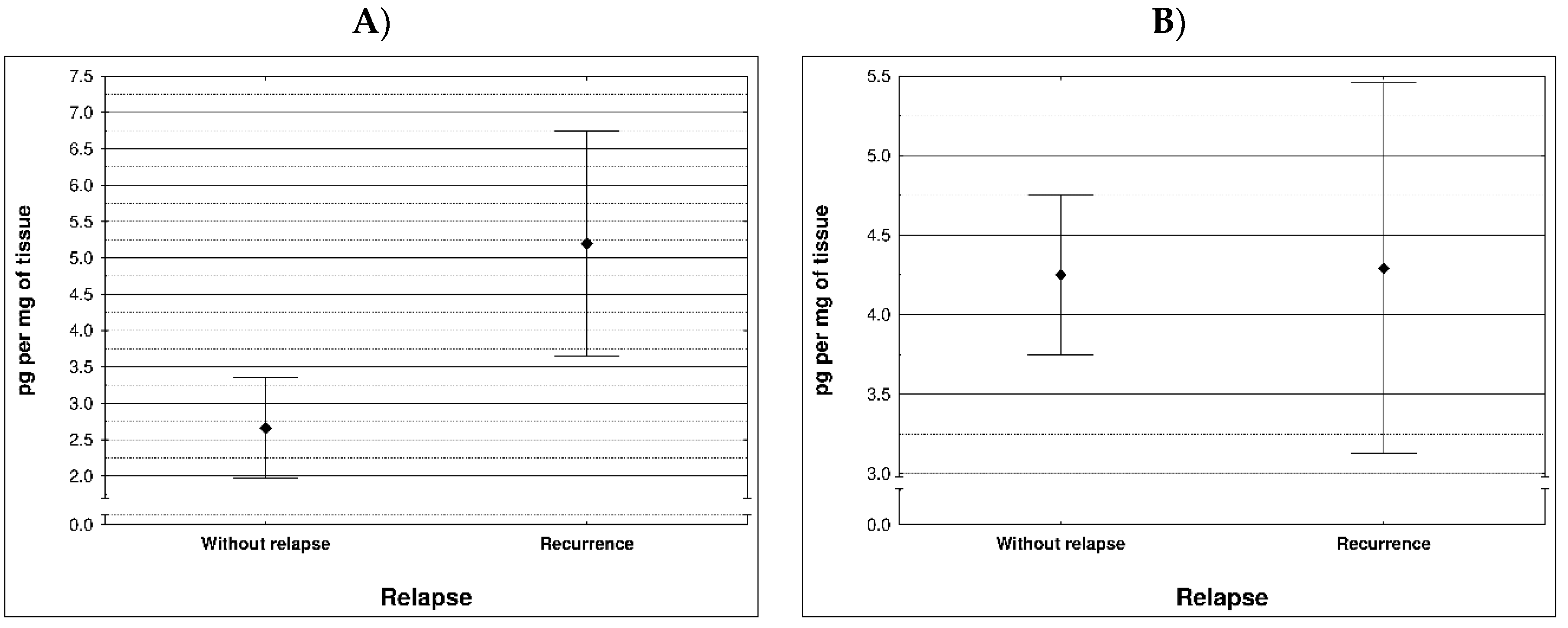
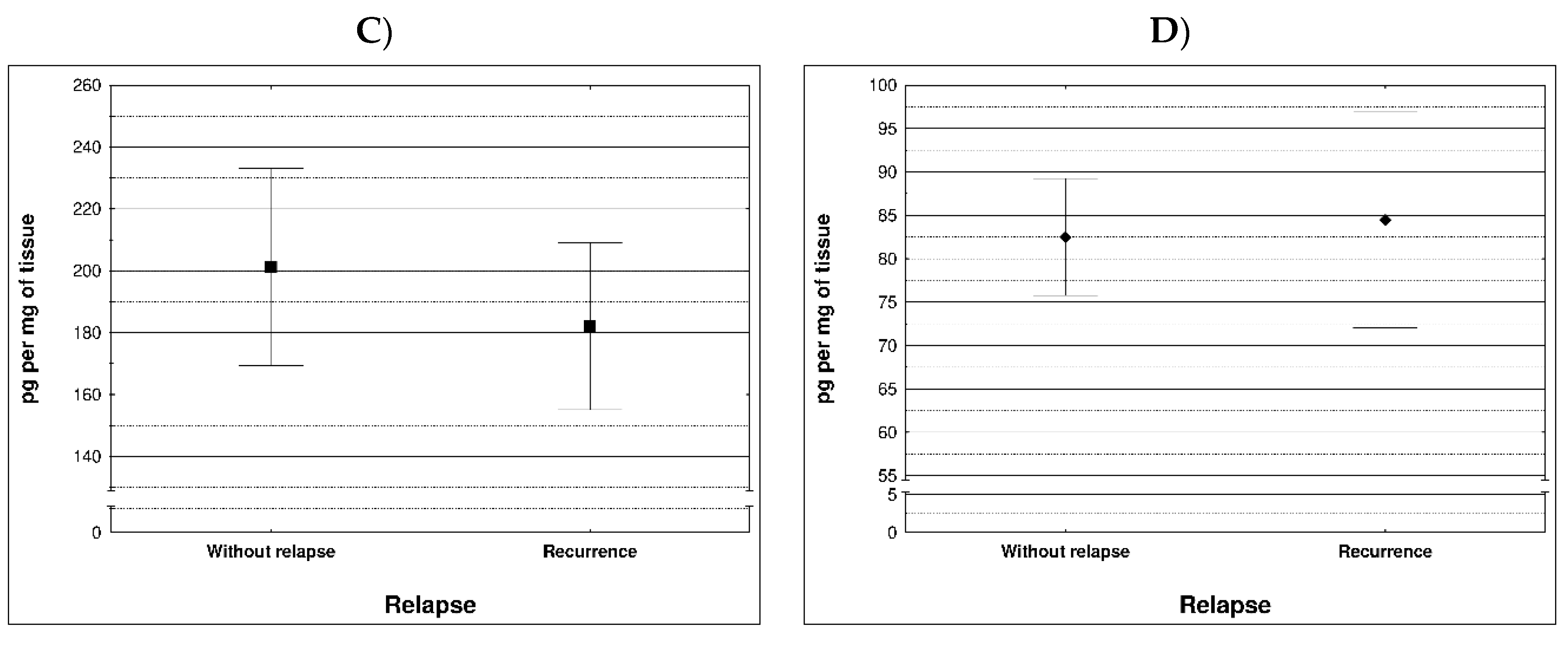
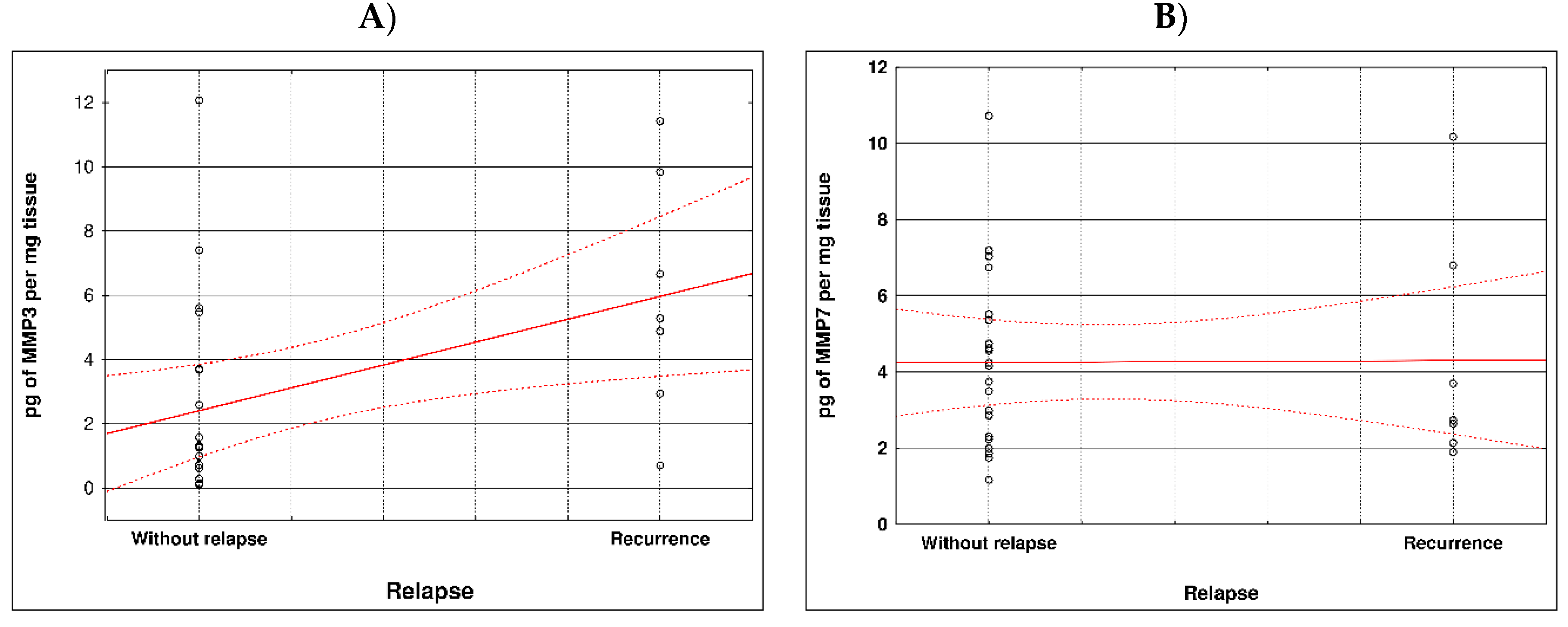
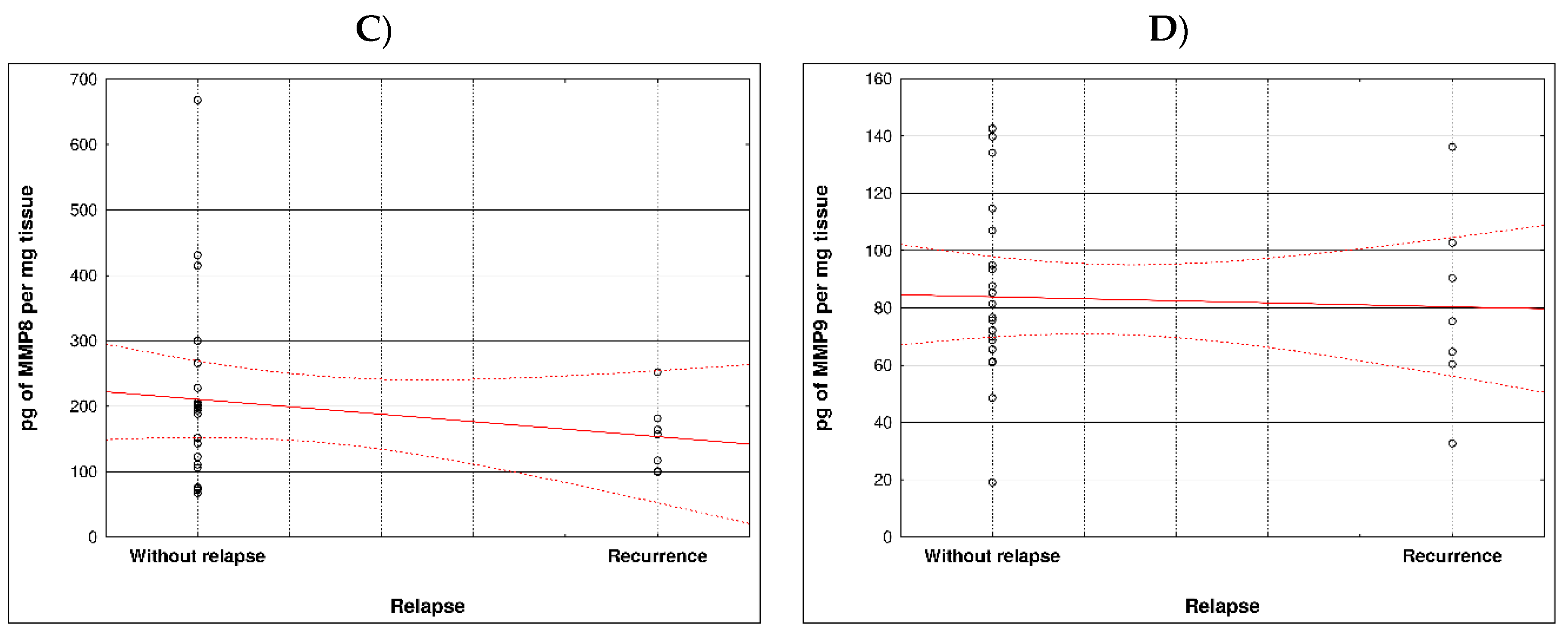
During examination in the large intestine, the presence of four metalloproteinases was found in the examined patients, the content of which was determined in the large intestine. (MMP 3, 7, 8 and 9). The statistical model constructed on the basis of chemical analyses demonstrated two variables (metalloproteinase 3 and metalloproteinase 8) significantly influenced the possibility of Crohn recurrence (
Figure 1 and
Table 1).
Correlation between recurrence of Crohn disease and metalloproteinases content within sick tissues of patients are presented at
Figure 2 and
Table 2.
4. Discussion
Under natural (physiological) conditions, MMPs are produced at very low levels and are responsible for normal tissue physiology. The process of production and activation of MMPs is strictly regulated to prevent excessive tissue degradation [
56] Warnaar et al., analyzed the expression of mRNA and the level of metalloproteinases (MMP-1, MMP-3) in tissue sections of the terminal ileum from patients with Crohn's disease and in the control group. In their results, they reported the following observations: MMP-1 and MMP-3 expression was increased in both pre-stenotic and stenotic tissue. MMP-1 content was elevated in the submucosal and muscle tissue of the prestenotic parts and in the muscle tissue of Crohn's stenosis samples. MMP-3 was significantly elevated in all tissue samples. Even in the submucosal layer of the proximal tissue of the resection margin, MMP-3 expression was significantly higher than in the control group [
57].
In a study conducted by Efsen et al., the proteolytic activity of MMPs in tissues was investigated and the effect of inhibitors (mainly drugs) as MMP inhibitors was investigated [
58]. Patients with Crohn's disease were qualified for the study. High-pressure liquid chromatography was used to measure MMP activity. The results unequivocally indicated that the mean total activity of MMPs was significantly higher in tissues diagnosed with Crohn's disease compared to fistulas of other disease entities and without signs of other ailments. Both MMP-3 and MMP-9 were elevated compared to non-Crohn's fistulas.
In turn, Matusiewicz, et al., conducted research to estimate MMP-9 concentrations in the sera of patients with Crohn's disease. 176 patients were qualified for the study group, who were divided into 3 groups (patients diagnosed with Crohn's disease, patients with ulcerative colitis, and a control group without signs of disease. The following observations were presented in the results: concentrations of MMP-9 in were significantly higher in active disease forms.For both CD and UC, serum MMP-9 positively correlated with disease activity.The authors concluded that the assessment of serum MMP-9 concentrations may help to differentiate between active disease form MMP-9 correlated better with inflammatory and angiogenic parameters in CD than in UC [
59].
Another determinant in the characteristics of Crohn's disease is MMP-19. Cervinková et al., analyzed the expression of MMP-19 in major forms of gastrointestinal diseases, including IBD, ulcerative colitis, Crohn's disease and colorectal cancer. The authors identified significant expression of MMP-19 in intact areas of the intestinal epithelium and macrophages. MMP-19 was also abundantly expressed in the endothelium of blood and lymphatic vessels of inflamed intestinal tissue. Significantly high MMP-19 immunoreactivity was also associated with macrophages in inflamed areas and myenteric plexuses [
60].
Barberio et al., attempted to test whether serum MMP-3 levels could be considered an early marker of treatment response in IBD patients. The study group consisted of 73 patients with IBD who were treated with infliximab. The results of the experiment confirmed that MMP3 levels were usually higher in patients who did not receive the drug than in patients who received drug therapy. The conclusion of the study was that serum MMP-3 determination may be one of the early markers of infliximab treatment [
61].
Shamseya et al., investigated the relationship between serum MMP-9 levels and disease activity in patients diagnosed with IBD. The research group consisted of 60 patients, 30 of whom were treated for Crohn's disease. ELISA was used for the study to determine serum MMP-9 levels. The Mayo scale was used to assess disease activity. The experiment confirmed that the concentration of MMP-9 in the serum was higher in patients with active Crohn's disease compared to patients with its inactive form. The lowest concentration was found in patients from the control group (i.e. without signs of disease). From the study, it can be concluded that serum MMP-9 can be used to differentiate between active and inactive disease forms [
62].
In turn, Gao et al. assessed the expression of MMP-2 and MMP-9 in the intestinal tissue of IBD patients. Tissue samples were collected from 47 patients and divided into three groups (group I – patients with IBD, group II – patients with CD, group III – patients without signs of disease, the so-called control group). An enzyme immunoassay (ELISA) was used to assess the activity of MMPs. The experiment showed that MMP-2 and MMP-9 were significantly elevated in IBD tissues, with significantly highest levels in severely inflamed tissues. The higher and worsening the inflammation, the higher the expression of MMP-9. Immunohistochemistry also showed that MMP-2 was present in the submucosa and MMP-9 in polymorphonuclear leukocytes [
63].
Our study showed that two variables (metalloproteinase 3 and metalloproteinase 8) is-totally affected the possibility of Crohn's disease relapse compared to the other metalloprotinases, namely MMPs 7 MM9. According to the literature, up-regulated expression of MMPs plays different functions in pathogenesis, cycles of acute inflammation and resolution, and chronic processes such as fibrosis and fistula forms of IBD, including CD. The function of both beneficial and non-beneficial MMPs has not yet been well studied. However, this knowledge has begun to be established for about 6 years [
64]. But in terms of regenerative role, MMPs have not yet been sufficiently studied in inflammatory bowel disease [
65]. The incidence of IBD has been increasing worldwide for about 20 years. Some Western countries, such as Canada, predict that there will be an increase of nearly 33.4% between 2015 and 2025 [
66]. As we know, the etiology of the disease is still unexplored at an adequate level, but many studies in this area have identified MMPs as risk factors for the development and progression of IBD due to either proteolytic regulation or modulation of transcription factors [
67,
68]. MMPs are activated after interactions in the cell-cell and cell-ECM areas or in the mechanism of response to pro-inflammatory cytokines widely expressed in IBD [
68,
69]. MMPs are involved in the modulation of IBD pathogenesis and cytokines, which are involved in inflammatory processes, when developing in the intestine have the property of increasing MMP levels. As an example, TNFα and bradykinin are able to induce MMP3 expression through a signaling cascade that includes PKC, PKD1 and MEK [
70]. Another example is interleukin 17A (IL17A) and IL17F, which can increase the expression of both MMP1 and -3 through the myofibroblast. Both IL17 cytokines increased the expression of MMP1 and -3 [
71]. In contrast, other studies suggest a protective function of MMPs in IBD. This is because Mmp2-/- mice have an acute pro-inflammatory response and also a greater susceptibility to disease progression [
72]. MMPs are important in many human diseases, but no synthetic MMP inhibitor with a broad spectrum of action has properly completed clinical trials through both pro-cancer or anti-tumor effects of MMPs in cancer [
73]. MMPs (MMP-2, MMP-9, MMP-14) are able to damage the capillary layer and at the same time promote exosmosis of cancer cells. MMP -9 can reduce the level of IL receptor which is located on the surface of T lymphocytes, and besides, it can suppress immunity and promote development on cancer [
74,
75,
76]. In the case of MMP-8, it can directly inhibit tumor metastasis. Side effects of MMP inhibitors, having a broad spectrum of action usually interfere with MMP-8 tumor inhibition [
77].
5. Conclusions
MMP proteins, through complex mechanisms that include induction of multiple molecule-lar signaling pathways including the EMT process, are very important in the pathway from any precancerous lesion or polyp to advanced CRC (including CD). Further studies are needed to understand the detailed action of MMPs.
Most MMPs are increased in CAC I CD colitis. However, based on our study, it was metalloproteinase 3 and metalloproteinase 8 that had a significant effect on the possibility of Crohn's disease recurrence Therefore, lowering the levels of these MMPs may effectively prevent the development of the disease. Based on clinical studies, the condition of CRC/CD patients could be stabilized to some extent by inhibiting MMP levels. Therefore, the levels of MMPs can be used to predict the status and development of inflammatory diseases, CAC and CD.
Author Contributions
Conceptualization, G. Ch., G.P., D.A, K.D, A.M., D.B-A., R.F.; investigation, G. Ch., G.P., D.A, K.D, A.M., D.B-A R.F; sample collection, G. Ch., G.P., D.A, R.F., writing—original draft preparation, G.P., D.A, R.F.; writing—review and editing G.P., D.A, R.F.; visualization, G. Ch., G.P., D.A, R.F.; project administration, G. Ch., G.P., D.A, K.D, A.M., D.B-A R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee of University of Rzeszów (protocol code 2018/06/04) and date of approval: 14 June 2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torres, J., Mehandru, S., Colombel, .JF. Crohn‘s disease. Lancet. 2017, 389, 1741–1755.
- de Souza, H.S., Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol 2016, 13, 13–27.
- Mak, W.Y; et al. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020, 35, 380–389. [Google Scholar] [PubMed]
- Ng, S.C., Shi, H.Y., Hamidi, N., Underwood, F.E., Tang, W., Benchimol, E.I., Panaccione, R., Ghosh, S., Wu, J.C.Y., Chan, F.K.L.; et al.Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778.
- Roda, G., Chien, Ng. S., Kotze, P.G.; et al. Crohn‘s disease. Nat Rev Dis Primers. 2020, 6–22.
- Actis GC, Pellicano R, Fagoonee S, Ribaldone DG. History of Inflammatory Bowel Diseases. J Clin Med. 2019, 8, 1970. [CrossRef]
- Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol. 2011, 17, 578–93. [CrossRef]
- da Silva BC, Lyra AC, Rocha R, Santana GO. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol. 2014, 20, 9458–67. [CrossRef]
- Borowitz, SM. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front Pediatr. 2023, 10, 1103713. [Google Scholar] [CrossRef]
- Aniwan S, Santiago P, Loftus EV Jr, Park SH. The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United European Gastro-enterol J. 2022, 10, 1063–1076. [CrossRef]
- Qin, X. Etiology of inflammatory bowel disease: A unified hypothesis. World J Gastroenterol. 2012, 18, 1708–22. [Google Scholar] [CrossRef]
- Aniwan S, Harmsen WS, Tremaine WJ, Loftus EV Jr. Incidence of inflammatory bowel disease by race and ethnicity in a population-based inception cohort from 1970 through 2010. Therap Adv Gastroenterol. 2019, 12, 1756284819827692. [CrossRef]
- Ek WE, D'Amato M, Halfvarson J.The history of genetics in inflammatory bowel dis-ease. Ann Gastroenterol. 2014, 27, 294–303.
- Balestrieri P, Ribolsi M, Guarino MPL, Emerenziani S, Altomare A, Cicala M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients. 2020, 12, 372. [CrossRef]
- Pazmandi J, Kalinichenko A, Ardy RC, Boztug K. Early-onset inflammatory bowel disease as a model disease to identify key regulators of immune homeostasis mechanisms. Immunol Rev. 2019, 287, 162–185. [CrossRef]
- Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019, 12, 113–122. [CrossRef]
- Fontana T, Falco N, Torchia M, Tutino R, Gulotta G. Bowel perforation in Crohn's Disease: Correlation between CDAI and Clavien-Dindo scores. G Chir. 2017, 38, 303–312. [CrossRef]
- Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J Gastroenterol. 2008, 14, 3937–47. [CrossRef]
- Huang BL, Chandra S, Shih DQ. Skin manifestations of inflammatory bowel disease. Front Physiol. 2012, 3, 13. [CrossRef]
- Villanacci V, Reggiani-Bonetti L, Salviato T, Leoncini G, Cadei M, Albarello L, Caputo A, Aquilano MC, Battista S, Parente P. Histopathology of IBD Colitis. A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD). Pathologica. 2021, 113, 39–53. [CrossRef]
- Fabián O, Kamaradová K. Morphology of inflammatory bowel diseases (IBD). Cesk Patol. 2022, 58, 27–37. [PubMed]
- Ansar W, Ghosh S. Inflammation and Inflammatory Diseases, Markers, and Mediators: Role of CRP in Some Inflammatory Diseases. Biology of C Reactive Protein in Health and Disease. 2016, 67–107. [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Singh R, Balasubramanian I, Zhang L, Gao N. Metaplastic Paneth Cells in Ex-tra-Intestinal Mucosal Niche Indicate a Link to Microbiome and Inflammation. Front Physiol. 2020, 11, 280. [CrossRef]
- Bamias G, Pizarro TT, Cominelli F. Immunological Regulation of Intestinal Fibrosis in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2022, 28, 337–349. [CrossRef]
- Gecse, K.B., Bemelman, W., Kamm M.A.; et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn‘s disease. Gut. 2014, 63, 1381–1392. [CrossRef]
- Bouma, G., Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol, 2003, 3, 521–33. [CrossRef] [PubMed]
- Hendrickson, B.A., Gokhale, R., Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev, 2002, 15, 79–94. [CrossRef] [PubMed]
- Beaugerie, L., Seksik P., Nion-Larmurier, I.; et al. Predictors of Crohn‘s disease. Gastroenterology. 2006, 130, 650–656.
- Marzo, M., Felice, C., Pugliese, D.; et al. Management of perianal fistulas in Crohn‘s disease: An up-to-date review. World J Gastroenterol. 2015, 21, 1394–1403. [CrossRef]
- Wasmann, K.A., de Groof, E.J., Stellingwerf,0\ M.E.; et al. Treatment of perianal fistulas in Crohn‘s disease, seton versus anti-TNF versus surgical closure following anti-TNF [PISA]: A randomised controlled trial. J Crohns Colitis. 2020, 14, 1049–1056. [CrossRef]
- Scharl, M., Rogler, G. Pathophysiology of fistula formation in Crohn‘s disease. World J Gastrointest Pathophysiol. 2014, 5, 205–212. [CrossRef]
- Dongre, A. Dongre, A., Weinberg, R.A., New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Magro, F, Langner, C., Driessen, A., Ensari, A., Geboes, K., Mantzaris, G.J.; et al.. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis, 2013, 7, 827–51. [CrossRef]
- Annese, V., Daperno, M., Rutter, M.D., Amiot, A., Bossuyt, P., East, J.; et al.. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis, 2013, 7, 982–1018. [CrossRef]
- Burisch, J., Pedersen, N., Čuković-Čavka, S., Brinar, M., Kaimakliotis, I., Duricova, D.; et al.. East-west gradient in the incidence of inflammatory bowel disease in europe: The ECCO-EpiCom inception cohort. Gut, 2014, 63, 588–97. [CrossRef]
- Seyedian, S.S., Nokhostin, F., Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life, 2019, 12, 113–22. [CrossRef]
- Feakins, R.M. Ulcerative colitis or crohn's disease? pitfalls and problems. Histopathology, 2014, 64, 317–35. [Google Scholar] [CrossRef]
- Dignass, A., Lindsay, J.O., Sturm, A., Windsor, A., Colombel, J.F., Allez, M.; et al.. Second european evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. J Crohns Colitis 2012, 6, 991–1030. [CrossRef] [PubMed]
- Dignass, A., Van Assche, G., Lindsay, J.O., Lémann, M., Söderholm, J., Colombel, J.F.; et al.. The second european evidence-based consensus on the diagnosis and management of crohn's disease: Current management. J Crohns Colitis, 2010, 4, 28–62. [CrossRef] [PubMed]
- Tun, G.S., Cripps, S., Lobo, A.J. Crohn's disease: Management in adults, children and young people - concise guidance. Clin Med (Lond), 2018, 18, 231–6. [CrossRef]
- Kornbluth, A., Sachar, D.B., Ulcerative colitis practice guidelines in adults (update): American college of gastroenterology, practice parameters committee. Am J Gastroenterol, 2004, 99, 1371–85. [CrossRef]
- LeBleu, V.S., Taduri, G., O’Connell, J., Teng, Y., Cooke, V.G., Woda, C., Sugimoto, H., Kalluri. R., Origin and function of myofibroblasts in kidney fibrosis. Nat Med 2013, 19, 1047–1053.
- Nuij, V.J., Zelinkova, Z., Rijk, M.C., Beukers, R., Ouwendijk, R.J., Quispel, R.; et al.. Phenotype of inflammatory bowel disease at diagnosis in the netherlands: A population-based inception cohort study (the delta cohort). Inflammation Bowel Dis, 2013, 19, 2215–22. [CrossRef]
- Abraham B.P., Mehta S., El-Serag H.B. Natural history of pediatric-onset inflammatory bowel disease: A systematic review. J Clin Gastroenterol, 2012, 46, 581–9. [CrossRef]
- Henriksen, M., Jahnsen, J., Lygren, I., Sauar, J., Schulz, T., Stray, N.; et al.. Change of diagnosis during the first five years after onset of inflammatory bowel disease: Results of a prospective follow-up study (the IBSEN study). Scand J Gastroenterol 2006, 41, 1037–43. [CrossRef] [PubMed]
- Tontini, G.E., Vecchi, M., Pastorelli, L., Neurath, M.F., Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: State of the art and future perspectives. World J Gastroenterol, 2015, 21, 21–46. [CrossRef] [PubMed]
- Derkacz, A., Olczyk, P., Olczyk, K., Komosinska-Vassev, K. The role of extracellular matrix components in inflammatory bowel diseases. J Clin Med 2021, 10. [CrossRef]
- Marcello, P.W., Schoetz, D.J., Jr., Roberts, P.L., Murray, J.J., Coller, J.A., Rusin, L.C; et al.. Evolutionary changes in the pathologic diagnosis after the ileoanal pouch procedure. Dis Colon Rectum, 1997, 40, 263–9. [CrossRef] [PubMed]
- Siegmund, B., Feakins, R.M., Barmias, G., Ludvig, J.C., Teixeira, F.V., Rogler, G., Scharl, M., Results of the Fifth Scientific Workshop of the ECCO (II): Pathophysiology of perianal fistulizing disease. J Crohns Colitis 2016, 10, 377–386. [CrossRef] [PubMed]
- McGregor, C.G.C., Tandon, R., Simmons, A. Pathogenesis of Fistulating Crohn's Disease: A Review. Cell Mol Gastroenterol Hepatol. 2023, 15, 1–11. [CrossRef] [PubMed]
- Schuppan, D., Freitag, T. Fistulising Crohn's disease: MMPs gone awry. Gut. 2004, 53, 622–624. [CrossRef] [PubMed]
- Andoh, A., Bamba, S., Brittan, M., Fujiyama, Y., Wright, N.A. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther, 2007, 114, 94–106. [CrossRef] [PubMed]
- Yoo, J., Rodriguez, Perez, C.E., Nie, W., Sinnett-Smith, J., Rozengurt, E. Protein kinase D1 mediates synergistic MMP-3 expression induced by TNF-α and bradykinin in human colonic myofibroblasts. Biochem Biophys Res Commun, 2007, 413, 30–35.
- Drygiannakis, I., Valatas, V., Sfakianaki, O., Bourikas, L., Manousou, P., Kambas, K., Ritis, K., Kolios, G., Kouroumalis, E. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: Implication in intestinal fibrosis. J Crohn’s Colitis, 2013, 7, 286–300.
- Marônek M, Marafini I, Gardlík R, Link R, Troncone E, Monteleone G. Metalloprotein-ases in Inflammatory Bowel Diseases. J Inflamm Res. 2021, 14, 1029–1041. [CrossRef]
- Warnaar N, Hofker HS, Maathuis MH, Niesing J, Bruggink AH, Dijkstra G, Ploeg RJ, Schuurs TA. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn's disease. Inflamm Bowel Dis. 2006, 12, 863–9. [CrossRef]
- Efsen E, Saermark T, Hansen A, Bruun E, Brynskov J. Ramiprilate inhibits functional matrix metalloproteinase activity in Crohn's disease fistulas. Basic Clin Pharmacol Toxicol. 2011, 109, 208–16. [CrossRef]
- Matusiewicz M, Neubauer K, Mierzchala-Pasierb M, Gamian A, Krzystek-Korpacka M. Matrix metalloproteinase-9: Its interplay with angiogenic factors in inflammatory bowel diseases. Dis Markers. 2014, 2014, 643645. [CrossRef]
- Cervinková M, Horák P, Kanchev I, Matěj R, Fanta J, Sequens R, Kašpárek P, Sarnová L, Turečková J, Sedláček R. Differential expression and processing of matrix metalloproteinase 19 marks progression of gastrointestinal diseases. Folia Biol (Praha). 2014, 60, 113–22.
- Barberio B, D'Incà R, Facchin S, Dalla Gasperina M, Fohom Tagne CA, Cardin R, Ghisa M, Lorenzon G, Marinelli C, Savarino EV, Zingone F. Matrix Metalloproteinase 3 Predicts Therapeutic Response in Inflammatory Bowel Disease Patients Treated With Infliximab. Inflamm Bowel Dis. 2020, 26, 756–763. [CrossRef] [PubMed]
- Shamseya AM, Hussein WM, Elnely DA, Adel F, Header DA. Serum matrix metalloproteinase-9 concentration as a marker of disease activity in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2021, 33 Suppl. S1, e803–e809. [CrossRef] [PubMed]
- Gao Q, Meijer MJ, Kubben FJ, Sier CF, Kruidenier L, van Duijn W, van den Berg M, van Hogezand RA, Lamers CB, Verspaget HW. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis. 2005, 37, 584–92. [CrossRef] [PubMed]
- de Bruyn, M., Vandooren, J., Ugarte-Berzal, E., Arijs, I., Vermeire, S., Opdenakker, G. B The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit Rev Biochem Mol Biol, 2016, 51, 295–358. [CrossRef] [PubMed]
- de Almeida, L.G.N., Thode, H., Eslambolchi, Y., Chopra, S., Young, D., Gill, S. at al. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol Rev, 2022, 74, 712–68. [CrossRef]
- Coward, S., Clement, F., Williamson, T., Hazlewood, G., Ng, S., Heitman, S., Seow, C., Panaccione, R., Ghosh, S., Kaplan, G.G. The rising burden of inflammatory bowel disease in North America from 2015 to 2025: A predictive model. Am J Gastroenterol, 2015, 110, S829. [CrossRef]
- Sagi, I. ., Gaffney, J.P., Dufour, A., Overall, C.M.Subtracting matrix out of the equation: New key roles of matrix metalloproteinases in innate immunity and disease, in Matrix Metalloproteinase Biology (Sagi I and Gaffney JP, eds), 2015, 131–152, John Wiley & Sons, Inc, Hoboken NJ.
- O’Sullivan, S., Gilmer, J.F., Medina, C. Matrix metalloproteinases in inflammatory bowel disease: An update. Mediators Inflamm 2015, 964131.
- Hu, J., Van den Steen, P.E., Sang, Q-XA, Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov, 2007, 6, 480–498. [CrossRef]
- Yoo, J., Rodriguez, Perez, C.E., Nie, W., Sinnett-Smith, J., Rozengurt, E. Protein kinase D1 mediates synergistic MMP-3 expression induced by TNF-α and bradykinin in human colonic myofibroblasts. Biochem Biophys Res Commun, 2011, 413, 30–35. [CrossRef]
- Yagi, Y., Andoh, A., Inatomi, O., Tsujikawa, T., Fujiyama, Y. Inflammatory responses induced by interleukin-17 family members in human colonic subepithelial myofibroblasts. J Gastroenterol, 2007, 42, 746–753. [CrossRef]
- Garg, P., Rojas, M., Ravi, A., Bockbrader. K., Epstein, S., Vijay-Kumar, M., Gewirtz, A.T., Merlin, D., Sitaraman, S.V. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: Contrasting role of gelatinases in the pathogenesis of colitis. J Immunol, 2006, 177, 4103–4112.
- Levin, M. , Udi, Y., Solomonov, I., Sagi, I. Next generation matrix metalloproteinase inhibitors - novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res, 2017, 1864 (11 Pt A), 1927–39. [CrossRef]
- Zucker, S., Cao, J., Chen, W.T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene, 2000, 19, 6642–50. [CrossRef] [PubMed]
- Shi, Y., Mam X., Fang, G., Tian, X., Ge, C. Matrix metalloproteinase inhibitors (MMPIS) as attractive therapeutic targets: Recent progress and current challenges. NanoImpact, 2021, 21, 100293. [CrossRef] [PubMed]
- Winer, A., Adams, S., Mignatti, P,. Matrix metalloproteinase inhibitors in cancer therapy: Turning past failures into future successes. Mol Cancer Ther, 2018, 17, 1147–55. [CrossRef] [PubMed]
- Kessenbrock, K., Plaks, V., Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell, 2010, 141, 52–67. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).