Submitted:
06 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Statistical analysis
3. Results
3.1. Pateints (forse meglio Population)
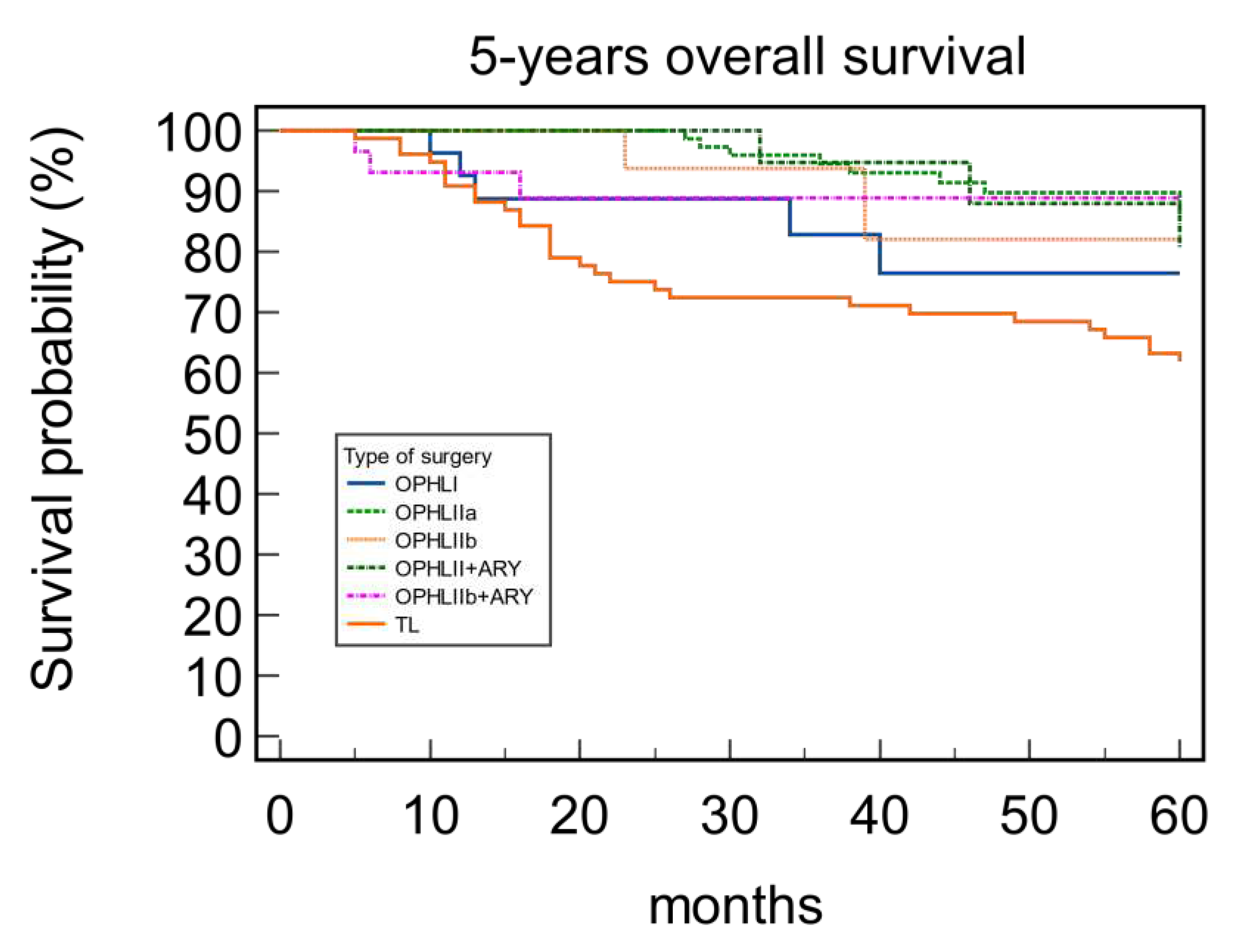
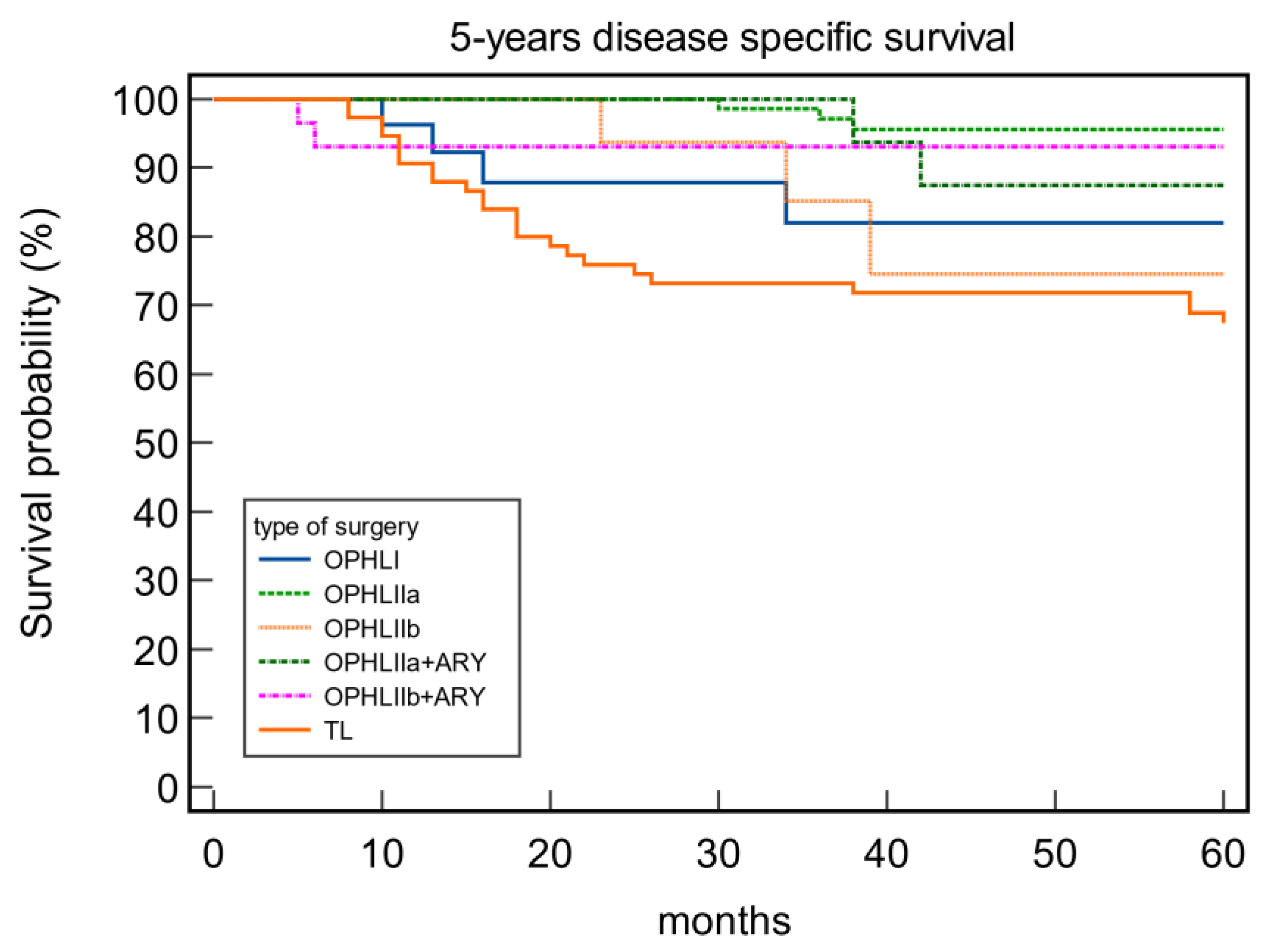
3.2. Survival rates and correlation between OPHLs and Total laryngectomy
3.3. Oncological Outcomes and Pattern of local failure of the OPHLs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; La Mantia, I.; Bianco, M.R.; Drago, G.D.; Le Fosse, M.C.; Azzolina, A.; Grillo, C.; Saita, V. Verbal performance of total laryngectomized patients rehabilitated with esophageal speech and tracheoesophageal speech: impacts on patient quality of life. Psychol Res Behav Manag. 2019, 12, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Allegra, E.; La Boria, A.; Lombardo, N. Modified Supracricoid Laryngectomy. Otolaryngol. Head Neck Surg. 2010, 142, 137–139. [Google Scholar] [CrossRef] [PubMed]
- de Vincentiis, M.; De Virgilio, A.; Bussu, F.; Gallus, R.; Gallo, A.; Bastanza, G.; Parrilla, C.; Greco, A.; Galli, J.; Turchetta, R.; Almadori, G.; Pagliuca, G.; Valentini, V.; Paludetti, G. Oncologic results of the surgical salvage of recurrent laryngeal squamous cell carcinoma in a multicentric retrospective series: emerging role of supracricoid partial laryngectomy. Head Neck. 2015, 37, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Saita,V. ; Azzolina, A.; De Natale, M.; Bianco, M.R.; Modica, DM.; Garozzo, A. Impact of the anterior commissure involvement on the survival of early glottic cancer treated with cricohyoidoepiglottopexy: a retrospective study. Cancer Manag Res. 2018, 8, 5553–5558. [Google Scholar] [CrossRef]
- Succo, G.; Peretti, G.; Piazza, C.; Remacle, M.; Eckel, HE.; Chevalier, D.; Simo, R.; Hantzakos, AG.; Rizzotto, G.; Lucioni, M.; Crosetti, E.; Antonelli, AR. Open partial horizontal laryngectomies: a proposal for classification by the working committee on nomenclature of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2014, 271, 2489–96. [Google Scholar] [CrossRef]
- Pellini, R.; Pichi, B.; Ruscito, P.; Ceroni, AR.; Caliceti, U.; Rizzotto, G.; Pazzaia, A.; Laudadio, P.; Piazza, C.; Peretti, G.; Giannarelli, D.; Spriano, G. Supracricoid partial laryngectomies after radiation failure: a multi-institutional series. Head Neck. 2008, 30, 372–9. [Google Scholar] [CrossRef]
- Mercante, G.; Grammatica, A.; Battaglia, P.; Cristalli, G.; Pellini, R.; Spriano, G. Supracricoid partial laryngectomy in the management of t3 laryngeal cancer. Otolaryngol Head Neck Surg 2013, 149, 714–720. [Google Scholar] [CrossRef]
- Piquet, JJ.; Desaulty, A.; Decroix, G. Crico-hyoido-epiglotto-pexy. Surgical technic and functional results. Ann Otolaryngol Chir Cervicofac. 1974, 91, 681–6. [Google Scholar]
- Guerrier, B.; Lallemant, JG.; Balmigere, G.; Bonnet, P.; Arnoux, B. Our experience in reconstructive surgery in glottic cancer. Ann Otolaryngol Chir Cervicofac. 1987, 104, 175–179. [Google Scholar]
- Garozzo. A.; Allegra, E.; La Boria, A.; Lombardo, N. Modified supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2010, 142, 137–139. [Google Scholar] [CrossRef]
- de Vincentiis, M.; Minni, A.; Gallo, A.; Di Nardo, A. Supracricoid partial laryngectomies: oncologic and functional results. Head Neck. 1998, 20, 504–9. [Google Scholar] [CrossRef]
- Allegra, E.; Lombardo, N.; La Boria, A.; Rotundo, G.; Bianco, MR.; Barrera, T.; Cuccunato, M.; Garozzo, A. Quality of voice evaluation in patients treated by supracricoid laryngectomy and modified supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2011, 145, 789–95. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Franco, T. , Trapasso, S., Domanico, R.; La Boria, A.; Garozzo, A. Modified supracricoid laryngectomy: oncological and functional outcomes in the elderly. Clin Interv Aging. 2012, 7, 475–80. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Araki, K.; Ogawa, K.; Shiotani, A. Laryngeal function after supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2009, 140, 487–492. [Google Scholar] [CrossRef]
- Schindler, A.; Favero, E.; Nudo, S.; Albera, R.; Schindler, O.; Cavalot, AL. Long-term voice and swallowing modifications after supracricoid laryngectomy: objective, subjective and self-assessment data. Am J Otolaryngol. 2006, 27, 378–383. [Google Scholar] [CrossRef]
- Schindler, A.; Pizzorni, N.; Mozzanica, F.; Fantini, M.; Ginocchio, D.; Bertolin, A.; Crosetti, E.; Succo, G. Functional outcomes after supracricoid laryngectomy: what do we not know and what do we need to know? Eur Arch Otorhinolaryngol. 2016, 273, 3459–3475. [Google Scholar] [CrossRef]
- Lips, M.; Speyer, R.; Zumach, A.; Kross, KW.; Kremer, B. Supracricoid laryngectomy and dysphagia: A systematic literature review. Laryngoscope. 2015, 125, 2143–56. [Google Scholar] [CrossRef]
- Campo, F.; Mazzola, F. : Bianchi, G.; Manciocco, V.; Ralli, M.; Greco, A.; Sperduti, I.; de Vincentiis, M.; Pellini, R. Partial laryngectomy for naïve pT3N0 laryngeal cancer: Systematic review on oncological outcomes. Head & Neck. 2023, 45, 243–250. [Google Scholar]
- Allegra, E.; Bianco, MR.; Ralli, M.; Greco, A.; Angeletti, D.; de Vincentiis, M. Role of Clinical-Demographic Data in Survival Rates of Advanced Laryngeal Cancer. Medicina. 2021, 57, 267. [Google Scholar] [CrossRef]
- Saraniti, C.; Verro, B.; Ciodaro, F.; Galletti, F. Oncological Outcomes of Primary vs. Salvage OPHL Type II: A Systematic Review. Int J Environ Res Public Health. 2022, 19, 1837. [Google Scholar] [CrossRef] [PubMed]
- Laccourreye, O.; Muscatello, L.; Laccourreye, L.; Naudo, P.; Brasnu, D.; Weinstein, G. Supracricoid partial laryngectomy with cricohyoidoepiglottopexy for "early" glottic carcinoma classified as T1-T2N0 invading the anterior commissure. Am J Otolaryngol. 1997, 18, 385–90. [Google Scholar] [CrossRef] [PubMed]
- Atallah, I.; Berta, E.; Coffre, A.; Villa, J.; Reyt, E.; Righini, CA. Supracricoid partial laryngectomy with crico-hyoido-epiglottopexy for glottic carcinoma with anterior commissure involvement. Acta Otorhinolaryngol Ital. 2017, 37, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Alkan, U.; Nachalon, Y.; Shkedy, Y.; Yaniv, D.; Shvero, J.; Popovtzer, A. T1 squamous cell carcinoma of the glottis with anterior commissure involvement: Radiotherapy versus transoral laser microsurgery. Head Neck. 2017, 39, 1101–1105. [Google Scholar] [CrossRef]
- Baird, BJ.; Sung, CK.; Beadle, BM.; Divi, V. Treatment of early-stage laryngeal cancer: A comparison of treatment options. Oral Oncology. 2018, 87, 8–16. [Google Scholar] [CrossRef]
- Campo, F.; Zocchi, J.; Ralli, M.; De Seta, D.; Russo, FY.; Angeletti, D.; Minni, A.; Greco, A.; Pellini, R.; de Vincentiis, M. Laser Microsurgery Versus Radiotherapy Versus Open Partial Laryngectomy for T2 Laryngeal Carcinoma: A Systematic Review of Oncological Outcomes. Ear Nose Throat J. 2021, 100 1_suppl, 51S–58S. [Google Scholar] [CrossRef]
- Succo, G.; Crosetti, E.; Bertolin, A.; Piazza, C.; Molteni, G.; Cirillo, S.; Petracchini, M.; Tascone, M.; Sprio, AE.; Berta, GN.; Peretti, G.; Presutti, L.; Rizzotto, G. Treatment for T3 to T4a laryngeal cancer by open partial horizontal laryngectomies: Prognostic impact of different pathologic tumor subcategories. Head Neck. 2018, 40, 1897–1908. [Google Scholar] [CrossRef]
- de Vincentiis, M.; Greco, A.; Campo, F.; Candelori, F.; Ralli, M.; Di Traglia, M.; Colizza, A.; Cambria, F.; Zocchi, J.; Manciocco, V.; Spriano, G.; Pellini, R. Open partial horizontal laryngectomy for T2-T3-T4a laryngeal cancer: oncological outcomes and prognostic factors of two Italian hospitals. Eur Arch Otorhinolaryngol. 2022, 279, 2997–3004. [Google Scholar] [CrossRef]
- Mattioli, F.; Fermi, M.; Molinari, G.; Capriotti, V.; Melegari, G.; Bertolini, F.; D'Angelo, E.; Tirelli, G.; Presutti, L. pT3 N0 Laryngeal Squamous Cell Carcinoma: Oncologic Outcomes and Prognostic Factors of Surgically Treated Patients. Laryngoscope. 2021, 131, 2262–2268. [Google Scholar] [CrossRef]
- Del Bon, F.; Piazza, C.; Lancini, D.; Paderno, A.; Bosio, P.; Taboni, S.; Morello, R.; Montalto, N.; Missale, F.; Incandela, F.; Marchi, F.; Filauro, M.; Deganello, A.; Peretti, G.; Nicolai, P. Open Partial Horizontal Laryngectomies for T3⁻T4 Laryngeal Cancer: Prognostic Impact of Anterior vs. Posterior Laryngeal Compartmentalization. Cancers (Basel) 2019, 1, 289. [Google Scholar] [CrossRef]
- Campo, F.; Mazzola, F.; Bianchi, G.; Manciocco, V.; Ralli, M.; Greco, A.; Sperduti, I.; de Vincentiis, M.; Pellini, R. Partial laryngectomy for naïve pT3N0 laryngeal cancer: Systematic review on oncological outcomes. Head Neck. 2023, 45, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, L.; Tao, L.; Zhang, M.; Wu, H.; Chen, X.; Li, X.; Li, C.; Gong, H. Oncologic outcomes of surgical treatment for T3 glottic laryngeal squamous cell carcinoma. Head Neck. 2018, 40, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhu, YY.; Diao, WW.; Zhu, XL.; Shi, XH.; Li, WY.; Gao, ZQ.; Li, GJ.; Chen, XM. Matched-pair analysis of survival in the patients with T3 laryngeal squamous cell carcinoma treated with supracricoid partial laryngectomy or total laryngectomy. Onco Targets Ther. 2018, 11, 7947–7953. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Manciocco, V.; Simonelli, M.; Pagliuca, G.; D’Arcangelo, E.; & De Vincentiis, M.; & De Vincentiis, M. . Supracricoid partial laryngectomy in the treatment of laryngeal cancer: univariate and multivariate analysis of prognostic factors. Arch Otolaryngol Head Neck Surg. 2005, 131, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Crosetti, E.; Bertolin, A.; Molteni, G.; Bertotto, I.; Balmativola, D.; Carraro, M.; Sprio, AE.; Berta, GN.; Presutti, L.; Rizzotto, G.; Succo, G. Patterns of recurrence after open partial horizontal laryngectomy types II and III: univariate and logistic regression analysis of risk factors. Acta Otorhinolaryngol Ital. 2019, 39, 235–243. [Google Scholar] [CrossRef]
| Variables | Patients n.182 |
|---|---|
| Age | |
| Mean±SD (years) | 61.3±8.6SD |
| Follow-up | |
| mean±SD(months) | 98.6 ± 34.6SD |
| Sex | |
| M | 166 |
| F | 16 |
| Grading | |
| 1 | 39 |
| 2 | 120 |
| 3 | 23 |
| Subsite | |
| Supraglottic | 34 |
| Glottic | 148 |
| cT STAGE | |
| 1 | 105 |
| 2 | 56 |
| 3 | 21 |
| pT STAGE | |
| 1 | 102 |
| 2 | 43 |
| 3 | 33 |
| 4 | 4 |
| cN STAGE | |
| N0 | 131 |
| N+ | 51 |
| pN STAGE | |
| N0 | 9 |
| N+ | 43 |
| Resection Margins | |
| Negative | 175 |
| Positive | 7 |
| Cartilage Invasion | |
| Negative | 174 |
| Positive | 8 |
| Perineural/Vascular Invasion | |
| Negative | 171 |
| Positive | 11 |
| OPHLs | |
| I | 27 |
| IIa | 84 |
| IIb | 19 |
| IIa+ARY | 23 |
| IIb+ARY | 29 |
| Variables | OS % | p | DFS% | p | DSS% | p | LFS% | p |
|---|---|---|---|---|---|---|---|---|
| Grading | 0.34 | 0.08 | 0.02 | 0.89 | ||||
| 1 | 92.31 | 84.62 | 94.87 | 92.31 | ||||
| 2 | 88.33 | 84.17 | 94.17 | 92.50 | ||||
| 3 | 78.26 | 65.22 | 78.26 | 95.65 | ||||
| Subsite | 0.12 | 0.82 | 0.04 | 0.10 | ||||
| Glottic | 89.19 | 81.76 | 93.92 | 91.22 | ||||
| Supraglottic | 82.35 | 82.35 | 85.29 | 100 | ||||
| cT Status | 0.29 | 0.003 | 0.037 | 0.10 | ||||
| 1 | 89.52 | 87.62 | 95.24 | 95.24 | ||||
| 2 | 85.71 | 76.79 | 89.29 | 91.07 | ||||
| 3 | 85.71 | 66.67 | 85.71 | 85.71 | ||||
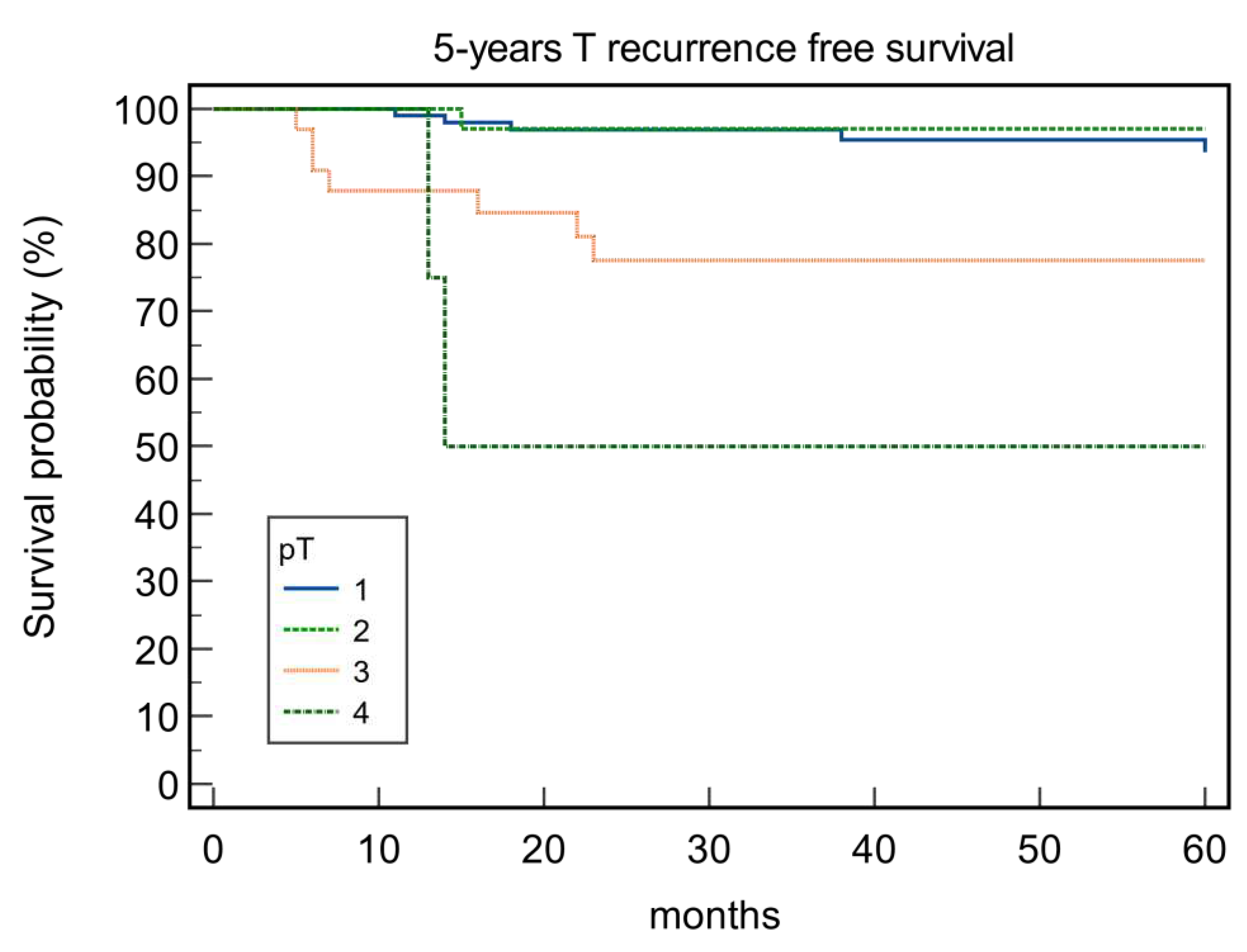
| pT Status | 0.53 | 0.001 | 0.17 | 0.0001 | ||||
| 1 | 89.22 | 88.24 | 95.10 | 95.10 | ||||
| 2 | 83.72 | 76.74 | 86.05 | 97.67 | ||||
| 3 | 87.88 | 75.76 | 90.91 | 84.85 | ||||
| 4 | 100 | 25.00 | 100 | 50.00 | ||||
| cN Status | 0.19 | 0.041 | 0.008 | 0.50 | ||||
| N0 | 88.50 | 83.97 | 94.66 | 93.13 | ||||
| N+ | 86.27 | 76.47 | 86.26 | 92.16 | ||||
| pN Status | 0.08 | 0.008 | 0.06 | 0.008 | ||||
| N0 | 89.21 | 84.89 | 95.53 | 84.89 | ||||
| N+ | 83.72 | 72.09 | 88.37 | 72.09 | ||||
| Resection Margins | 0.35 | 0.86 | 0.47 | 0.41 | ||||
| Negative | 87.36 | 81.61 | 91.95 | 93.10 | ||||
| Positive | 100 | 85.71 | 100 | 85.71 | ||||
| Cartilage invasion | 0.76 | 0.41 | 0.42 | 0.45 | ||||
| Negative | 87.93 | 82.18 | 92.53 | 93.10 | ||||
| Positive | 87.50 | 75.00 | 87.50 | 87.50 | ||||
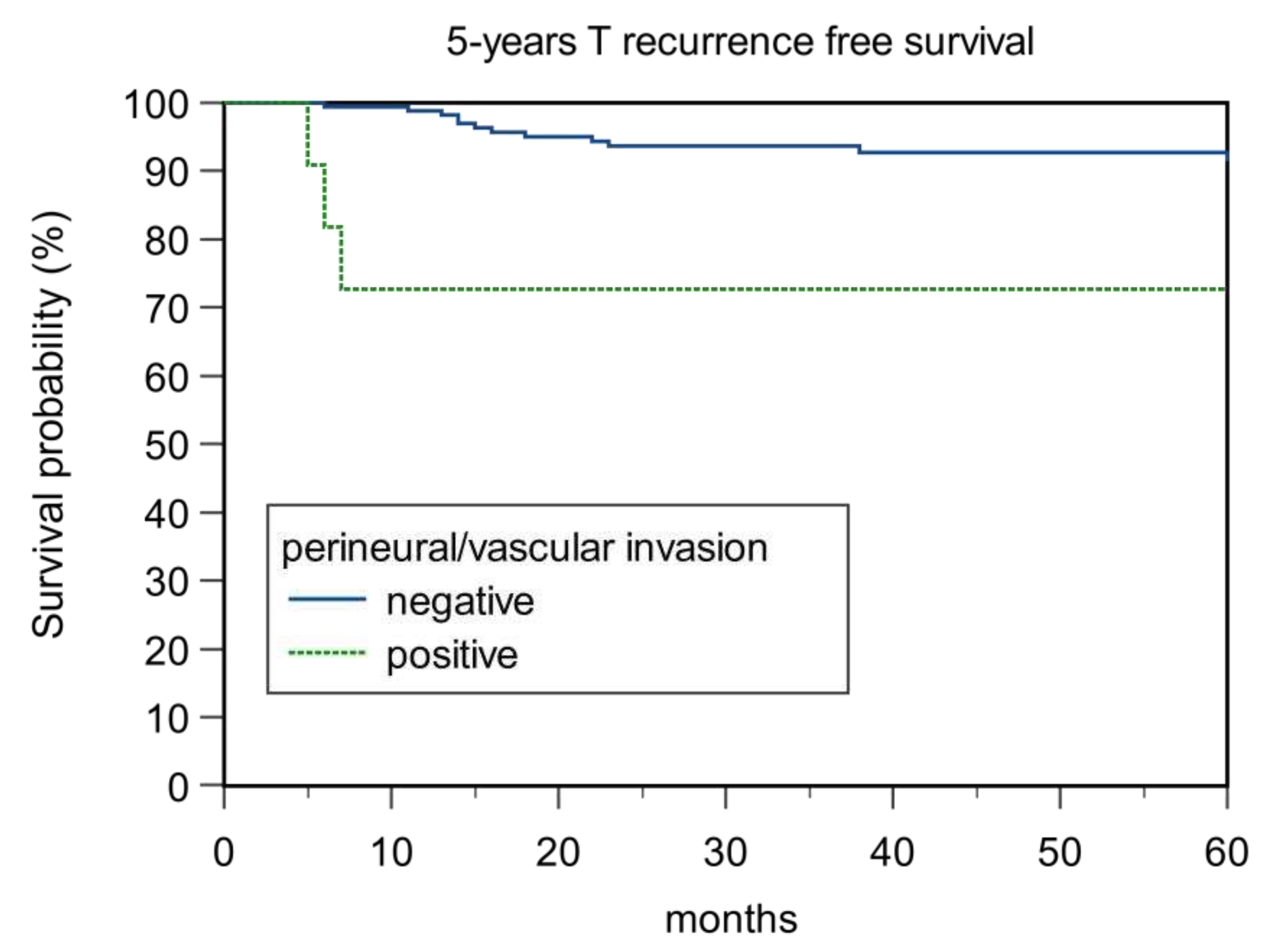
| Perineural/Vascular Invasion | 0.001 | 0.0006 | <0.0001 | 0.35 | ||||
| Negative | 88.89 | 87.04 | 93.57 | 92.98 | ||||
| Positive | 72.73 | 63.64 | 72.73 | 90.91 | ||||
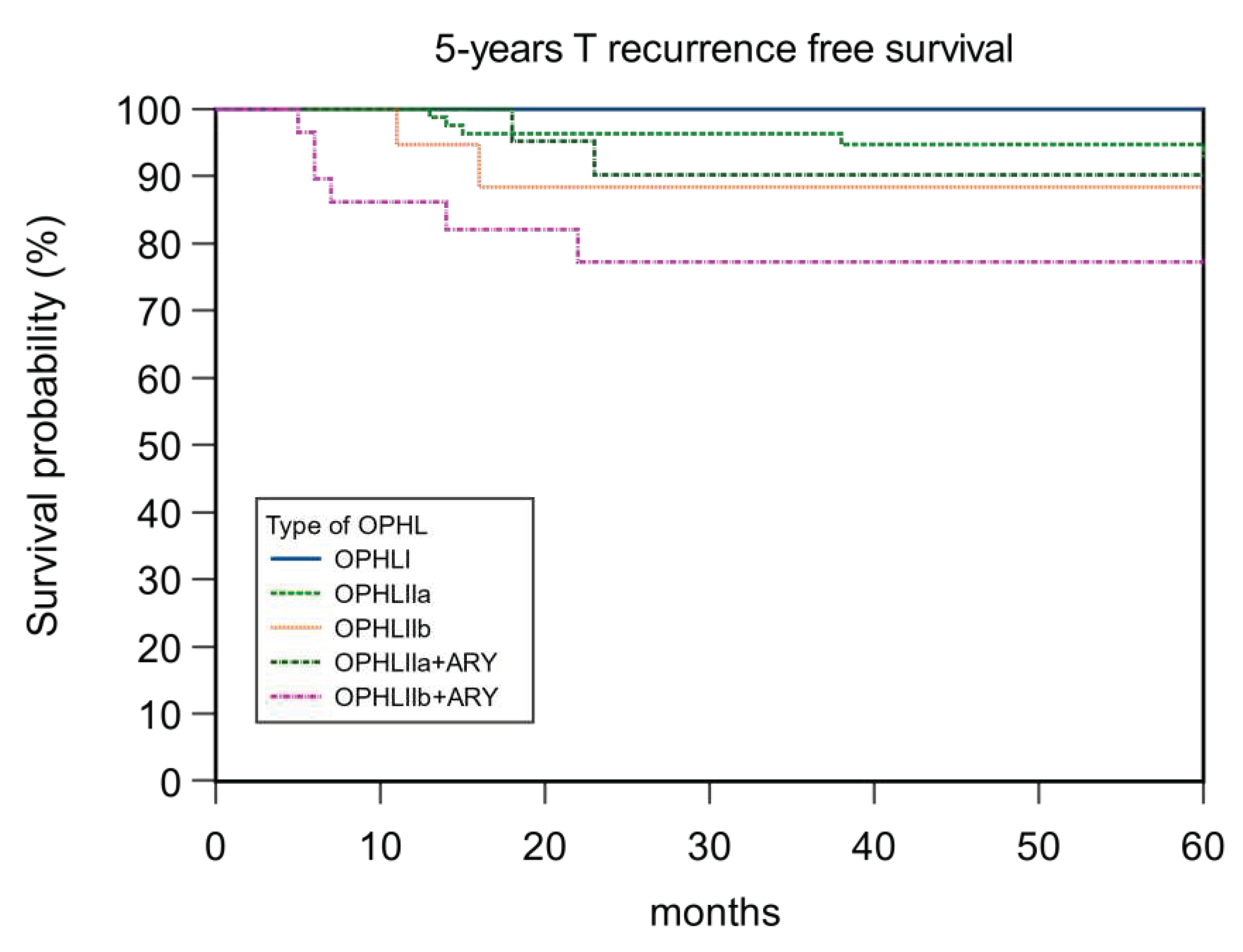
| OPHL | 0.58 | 0.041 | 0.80 | 0.16 | ||||
| I | 81.48 | 81.48 | 85.19 | 100 | ||||
| IIa | 89.29 | 88.10 | 96.43 | 94.05 | ||||
| IIb | 89.47 | 73.68 | 84.21 | 89.46 | ||||
| IIa+ARY | 86.96 | 78.26 | 93.30 | 91.30 | ||||
| IIb+ARY | 89.66 | 72.41 | 93.10 | 86.21 |
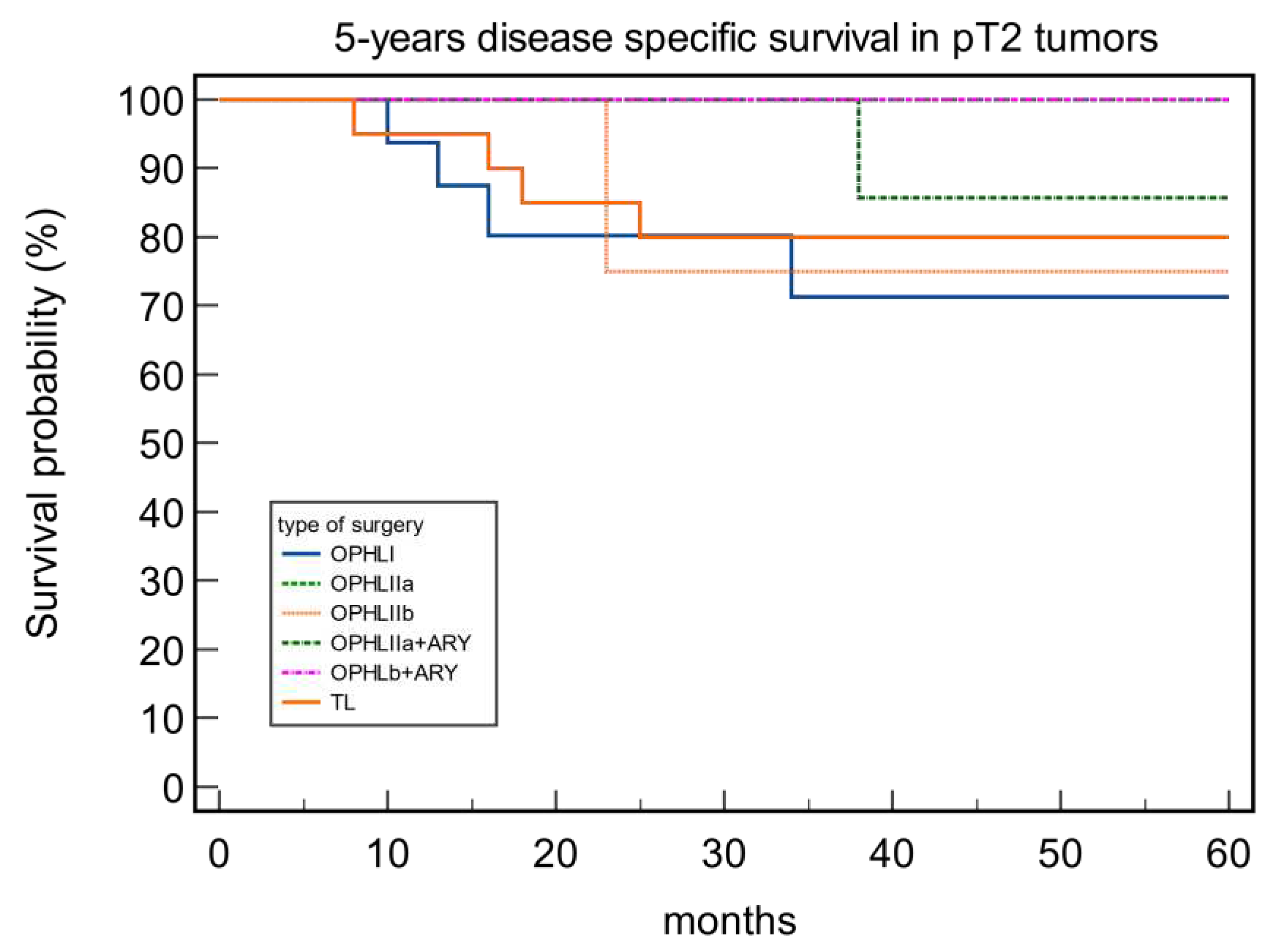
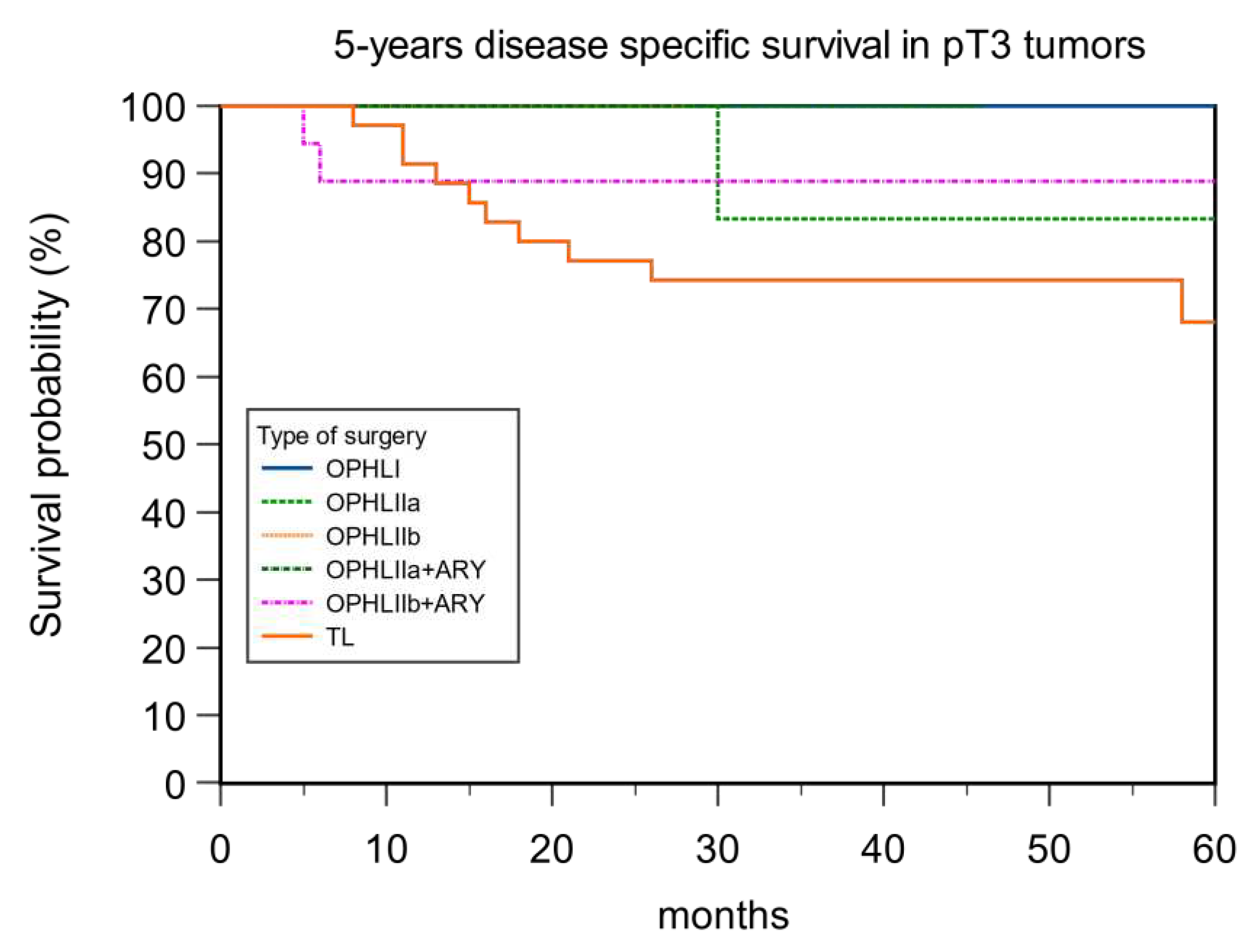
| Type of Surgery | DSS pT2 | DSS pT3 |
|---|---|---|
| OPHL I | 75% | 100% |
| OPHL IIa | 100% | 85.71% |
| OPHL IIb | 75% | 100% |
| OPHLIIa+ARY | 88% | 100% |
| OPHLIIb+ARY | 100% | 88.89% |
| Total Laryngectomy | 80% | 68.57% |
| p value | 0.54 | 0.63 |
| OPHL I | OPHL IIa | OPHL IIb | OPHL IIa + ARY | OPHL IIb + ARY | Total | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | ||
| T recurrence | 0 | 0 | 5 | 5.9 | 2 | 10.5 | 2 | 8.6 | 6 | 20.5 | 15 | 8.2 | 0.004 |
| N recurrence | 3 | 11.1 | 4 | 4.7 | 1 | 5.2 | 0 | 0 | 1 | 3.4 | 9 | 4.94 | 0.17 |
| Second/Distant metastasis | 4 | 14.8 | 3 | 3.5 | 0 | 0 | 4 | 17.3 | 2 | 1.8 | 13 | 7.14 | 0.058 |
| Variables | No Recurrence N.167 |
Recurrence N.15 |
P value |
|---|---|---|---|
|
Grading 1 2 3 |
36 109 22 |
3 11 1 |
0.73 |
|
Subsite Glottic Supraglottic |
133 34 |
15 0 |
0.053 |
|
cT 1 2 3 |
100 51 16 |
5 5 5 |
0.014 |
|
pT 1 2 3 4 |
97 42 26 2 |
5 1 7 2 |
0.0002 |
|
cN N0 N+ |
122 45 |
9 6 |
0.28 |
|
pN N0 N+ |
129 38 |
10 5 |
0.35 |
|
Resection Margin negative positive |
161 6 |
14 1 |
0.55 |
|
Perineural Invasion Negative positive |
159 8 |
12 3 |
0.018 |
|
Cartilage Invasion Negative positive |
160 7 |
14 1 |
0.65 |
|
Type of OPHL I IIa IIb IIa+ARY IIb+ARY |
27 79 17 21 23 |
0 5 2 2 6 |
0.0049 |
| Variables | Laryngectomy Free Yes Not N.% N.% |
p |
|---|---|---|
|
Grading 1 2 3 |
36 3 111 9 22 1 |
0.89 |
|
Subsite Glottic Supraglottic |
135 13 34 0 |
0.13 |
|
cT status 1 2 3 |
100 5 51 5 18 3 |
0.09 |
|
pT status 1 2 3 4 |
97 5 42 1 28 5 2 2 |
0.0009 |
|
cN status N0 N+ |
122 9 47 4 |
0.81 |
|
pN status N0 N+ |
129 10 40 3 |
0.96 |
|
Resection margins Negative Positive |
163 12 6 1 |
0.45 |
|
Cartilage invasion Negative Positive |
162 12 7 1 |
0.45 |
|
Periveural/Vascular Invasion Negative Positive |
159 12 10 1 |
0.79 |
|
OPHL I IIa IIb IIa+ARY IIb+ARY |
27 0 79 5 17 2 21 2 25 4 |
0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





