Submitted:
07 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
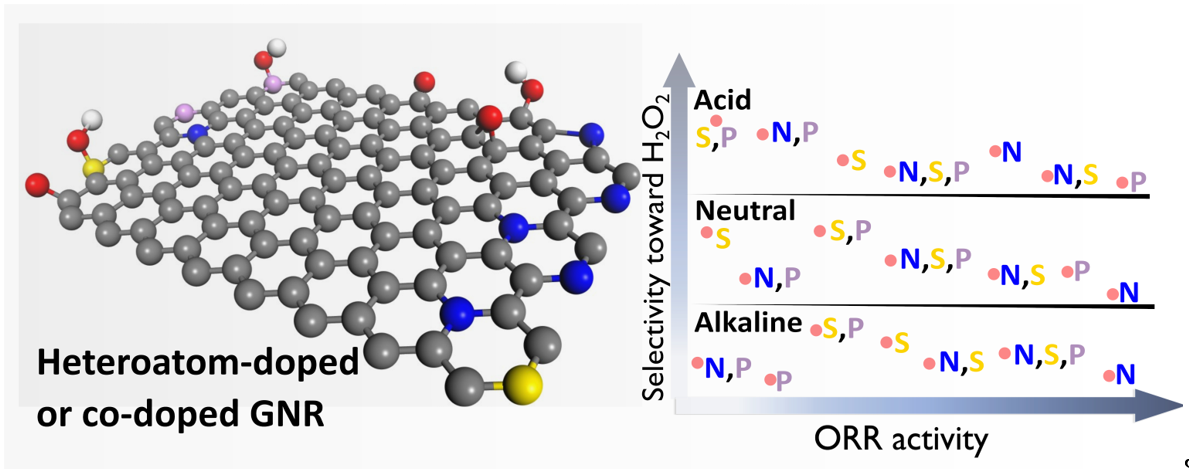
Keywords:
1. Introduction
2. Experimental Part
2.1. Reagents and Instruments
2.2. Electrode Preparation
2.3. Apparatuses, Measurements, and Material Characterization
2.4. GNR Synthesis and Syntheses of Different Doped GNR Samples
3. Results and Discussion
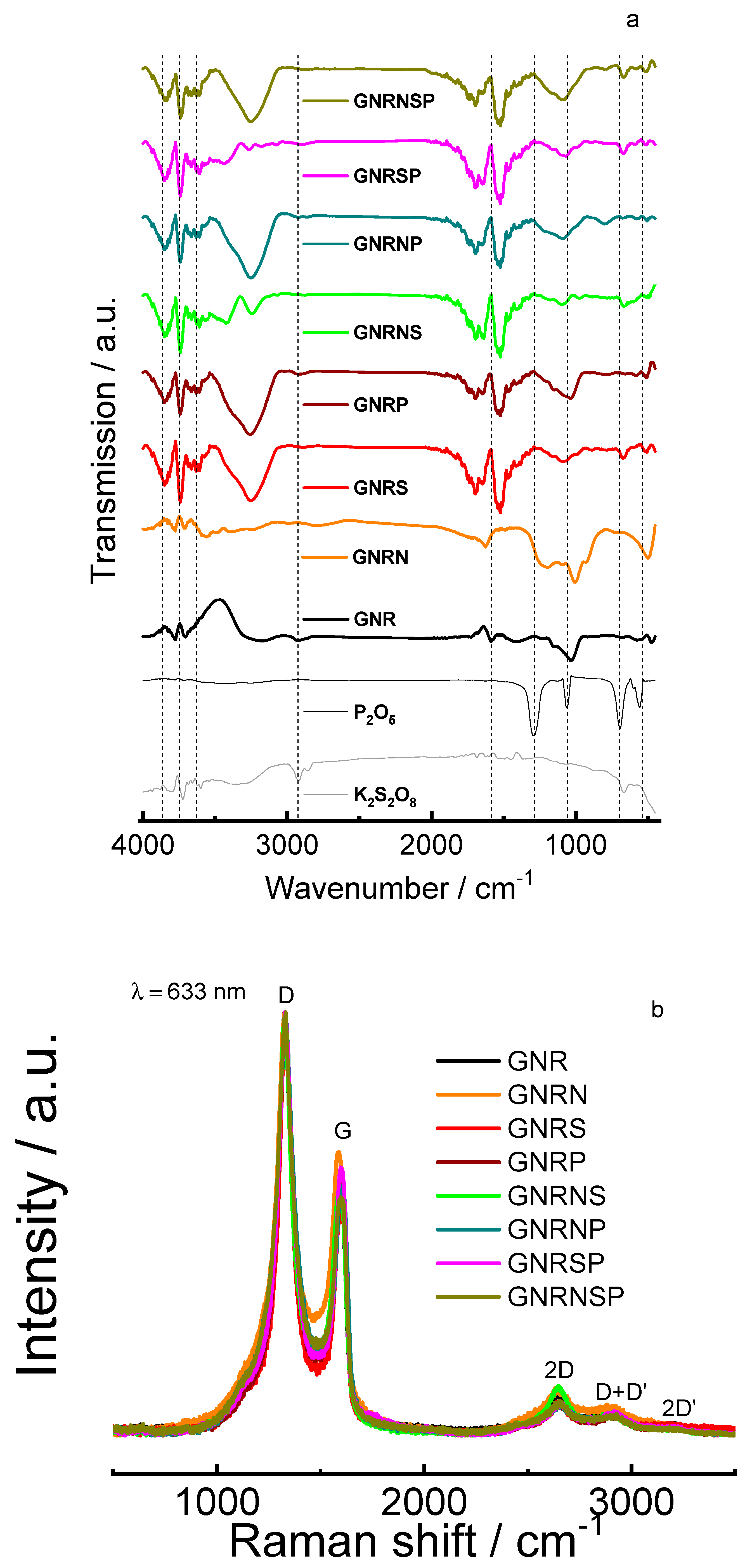
3.1. FT-IR and Raman Study
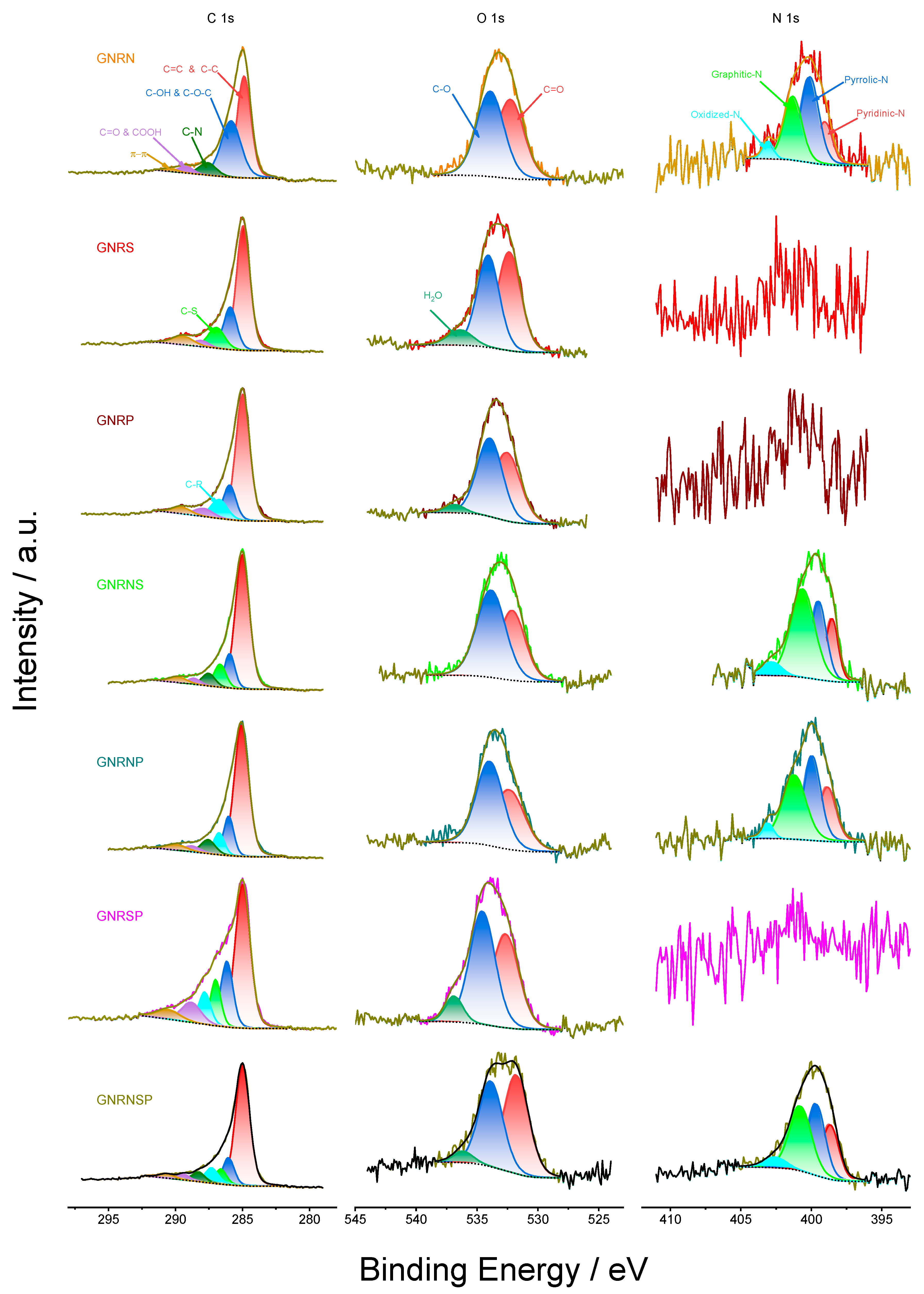
3.2. XPS, EA and TGA Study
3.3. XRD, TEM, and EDX Study
3.4. Electrochemical Study - ECSA and CV Profile
3.5. Analysis of ORR Activity and Selectivity
3.5.1. 0.5 M H2SO4 Solution
3.5.2. 0.1 M K2SO4 Solution
3.5.3. 0.1 M KOH Solution
3.6. Comparative Analyses and Assessment of Trends
4. Conclusions
Supplementary Materials
Acknowledgements
Conflict of Interest
References
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Narita, A.; Müllen, K. Graphene Nanoribbons: On-Surface Synthesis and Integration into Electronic Devices. Adv. Mater. 2020, 32, 2001893. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, C.; Shi, G. Graphene Based Catalysts. Energy Environ. Sci. 2012, 5, 8848. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Z.; Müllen, K. Nanographenes and Graphene Nanoribbons as Multitalents of Present and Future Materials Science. J. Am. Chem. Soc. 2022, 144, 11499–11524. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D. V; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal Unzipping of Carbon Nanotubes to Form Graphene Nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef]
- Mohanty, N.; Moore, D.; Xu, Z.; Sreeprasad, T.S.; Nagaraja, A.; Rodriguez, A.A.; Berry, V. Nanotomy-Based Production of Transferable and Dispersible Graphene Nanostructures of Controlled Shape and Size. Nat. Commun. 2012, 3, 844. [Google Scholar] [CrossRef]
- De Lima, F.; Maia, G. Oxidized/Reduced Graphene Nanoribbons Facilitate Charge Transfer to the Fe(CN)63-/Fe(CN)64- Redox Couple and towards Oxygen Reduction. Nanoscale 2015, 7, 6193–6207. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Ma, Y.; Qu, Y. Graphene and Their Hybrid Electrocatalysts for Water Splitting. ChemCatChem 2017, 9, 1554–1568. [Google Scholar] [CrossRef]
- Rogers, C.; Perkins, W.S.; Veber, G.; Williams, T.E.; Cloke, R.R.; Fischer, F.R. Synergistic Enhancement of Electrocatalytic CO2 Reduction with Gold Nanoparticles Embedded in Functional Graphene Nanoribbon Composite Electrodes. J. Am. Chem. Soc. 2017, 139, 4052–4061. [Google Scholar] [CrossRef]
- de Souza, M.K.R.; Cardoso, E. dos S.F.; Fortunato, G. V.; Lanza, M.R.V.; Nazário, C.E.; Zanoni, M.V.B.; Maia, G.; Cardoso, J.C. Combination of Cu-Pt-Pd Nanoparticles Supported on Graphene Nanoribbons Decorating the Surface of TiO2 Nanotube Applied for CO2 Photoelectrochemical Reduction. J. Environ. Chem. Eng. 2021, 9, 105803. [CrossRef]
- Cardoso, E.S.F.; Fortunato, G. V.; Maia, G. Use of Rotating Ring-Disk Electrodes to Investigate Graphene Nanoribbon Loadings for the Oxygen Reduction Reaction in Alkaline Medium. ChemElectroChem 2018, 5, 1691–1701. [Google Scholar] [CrossRef]
- Cardoso, E.S.F.; Fortunato, G. V.; Maia, G. Modification of C, O, and N Groups for Oxygen Reduction Reaction on an Electrochemically Stabilized Graphene Nanoribbon Surface. J. Phys. Chem. C 2019, 123, 16308–16316. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Maia, G. Developing Efficient Catalysts for the OER and ORR Using a Combination of Co, Ni, and Pt Oxides along with Graphene Nanoribbons and NiCo2O4. J. Mater. Chem. A 2020, 8, 17691–17705. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Morelos-Gómez, A.; Vega-Díaz, S.; Tristán-López, F.; Elias, A.L.; Perea-López, N.; Muramatsu, H.; Hayashi, T.; Fujisawa, K.; Kim, Y.A.; et al. Formation of Nitrogen-Doped Graphene Nanoribbons via Chemical Unzipping. ACS Nano 2013, 7, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A Review of Oxygen Reduction Mechanisms for Metal-Free Carbon-Based Electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-Free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Noffke, B.W.; Li, Q.; Raghavachari, K.; Li, L. A Model for the PH-Dependent Selectivity of the Oxygen Reduction Reaction Electrocatalyzed by N-Doped Graphitic Carbon. J. Am. Chem. Soc. 2016, 138, 13923–13929. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Z.P.; Li, X.H.; Liu, M.Y.; Yang, G.; Piao, J.H.; Liang, Z.X. PH Effect on Electrochemistry of Nitrogen-Doped Carbon Catalyst for Oxygen Reduction Reaction. ACS Catal. 2015, 5, 4325–4332. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively Nitrogen-Doped Carbon Materials as Superior Metal-Free Catalysts for Oxygen Reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; et al. N-Doped Graphitized Carbon Nanohorns as a Forefront Electrocatalyst in Highly Selective O2 Reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Liu, X.; Chen, Y.; Zhao, Y.; Gao, S. Fabricating N, S Co-Doped Hierarchical Macro-Meso-Micro Carbon Materials as PH-Universal ORR Electrocatalysts**. ChemistrySelect 2022, 7, e202200044. [Google Scholar] [CrossRef]
- Kolle-Görgen, E.; Fortunato, G.; Ledendecker, M. Catalyst Stability in Aqueous Electrochemistry. Chem. Mater. 2022, 34, 10223–10236. [Google Scholar] [CrossRef]
- Xiang, T.; Wu, Z.; Sun, Z.; Cheng, C.; Wang, W.; Liu, Z.; Yang, J.; Li, B. The Synergistic Effect of Carbon Edges and Dopants towards Efficient Oxygen Reduction Reaction. J. Colloid Interface Sci. 2022, 610, 486–494. [Google Scholar] [CrossRef]
- Dong, F.; Cai, Y.; Liu, C.; Liu, J.; Qiao, J. Heteroatom (B, N and P) Doped Porous Graphene Foams for Efficient Oxygen Reduction Reaction Electrocatalysis. Int. J. Hydrogen Energy 2018, 43, 12661–12670. [Google Scholar] [CrossRef]
- Han, C.; Chen, Z. The Mechanism Study of Oxygen Reduction Reaction (ORR) on Non-Equivalent P, N Co-Doped Graphene. Appl. Surf. Sci. 2020, 511, 145382. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Xu, J.; Yan, X.; Zhao, T.S. Role of Phosphorus in Nitrogen, Phosphorus Dual-Doped Ordered Mesoporous Carbon Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media. Int. J. Hydrogen Energy 2018, 43, 1470–1478. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Z.; Kong, D.; Zhang, T.; Duan, X.; Zhou, S.; Niu, Y.; Shen, Y.; Sun, H.; Wang, S.; et al. New Insight to the Role of Edges and Heteroatoms in Nanocarbons for Oxygen Reduction Reaction. Nano Energy 2019, 66, 104096. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Han, L.; Lin, R.; Xiao, W.; Xuan, C.; Xin, H.L.; Wang, D. Nitrogen and Sulfur Co-Doping of Partially Exfoliated MWCNTs as 3-D Structured Electrocatalysts for the Oxygen Reduction Reaction. J. Mater. Chem. A 2016, 4, 5678–5684. [Google Scholar] [CrossRef]
- Khan, Z.; Park, S.O.; Yang, J.; Park, S.; Shanker, R.; Song, H.-K.; Kim, Y.; Kwak, S.K.; Ko, H. Binary N,S-Doped Carbon Nanospheres from Bio-Inspired Artificial Melanosomes: A Route to Efficient Air Electrodes for Seawater Batteries. J. Mater. Chem. A 2018, 6, 24459–24467. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Huang, J.; Zhou, Y.; Xu, K.; Zhao, N.; Cheng, X. Soft Template-Assisted Method for Synthesis of Nitrogen and Sulfur Co-Doped Three-Dimensional Reduced Graphene Oxide as an Efficient Metal Free Catalyst for Oxygen Reduction Reaction. Carbon 2017, 122, 237–246. [Google Scholar] [CrossRef]
- Zhai, C.; Sun, M.; Zhu, M.; Song, S.; Jiang, S. A New Method to Synthesize Sulfur-Doped Graphene as Effective Metal-Free Electrocatalyst for Oxygen Reduction Reaction. Appl. Surf. Sci. 2017, 407, 503–508. [Google Scholar] [CrossRef]
- Zehtab Yazdi, A.; Roberts, E.P.L.; Sundararaj, U. Nitrogen/Sulfur Co-Doped Helical Graphene Nanoribbons for Efficient Oxygen Reduction in Alkaline and Acidic Electrolytes. Carbon 2016, 100, 99–108. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.-X.; Han, L.-L.; Liu, Y.-Y.; Guo, J.-P.; Xin, H.L.; Wang, D.-L. Rational Design of Three-Dimensional Nitrogen and Phosphorus Co-Doped Graphene Nanoribbons/CNTs Composite for the Oxygen Reduction. Chinese Chem. Lett. 2016, 27, 597–601. [Google Scholar] [CrossRef]
- Sibul, R.; Kibena-Põldsepp, E.; Mäeorg, U.; Merisalu, M.; Kikas, A.; Kisand, V.; Treshchalov, A.; Sammelselg, V.; Tammeveski, K. Sulphur and Nitrogen Co-Doped Graphene-Based Electrocatalysts for Oxygen Reduction Reaction in Alkaline Medium. Electrochem. commun. 2019, 109, 106603. [Google Scholar] [CrossRef]
- Palm, I.; Kibena-Põldsepp, E.; Mäeorg, U.; Kozlova, J.; Käärik, M.; Kikas, A.; Leis, J.; Kisand, V.; Tamm, A.; Tammeveski, K. Silicon Carbide-Derived Carbon Electrocatalysts Dual Doped with Nitrogen and Phosphorus for the Oxygen Reduction Reaction in an Alkaline Medium. Electrochem. commun. 2021, 125, 106976. [Google Scholar] [CrossRef]
- Palm, I.; Kibena-Põldsepp, E.; Mooste, M.; Kozlova, J.; Käärik, M.; Kikas, A.; Treshchalov, A.; Leis, J.; Kisand, V.; Tamm, A.; et al. Nitrogen and Phosphorus Dual-Doped Silicon Carbide-Derived Carbon/Carbon Nanotube Composite for the Anion-Exchange Membrane Fuel Cell Cathode. ACS Appl. Energy Mater. 2022, 5, 2949–2958. [Google Scholar] [CrossRef]
- Boruah, T.; Das, S.K.; Kumar, G.; Mondal, S.; Dey, R.S. Dual Active Sites in a Triazine-Based Covalent Organic Polymeric Framework Promoting Oxygen Reduction Reaction. Chem. Commun. 2022, 58, 5506–5509. [Google Scholar] [CrossRef]
- Mohmad, G.; Sarkar, S.; Biswas, A.; Roy, K.; Dey, R.S. Polymer-Assisted Electrophoretic Synthesis of N-Doped Graphene-Polypyrrole Demonstrating Oxygen Reduction with Excellent Methanol Crossover Impact and Durability. Chem. – A Eur. J. 2020, 26, 12664–12673. [Google Scholar] [CrossRef]
- Sarkar, S.; Biswas, A.; Kamboj, N.; Dey, R.S. Unveiling the Potential of an Fe Bis(Terpyridine) Complex for Precise Development of an Fe-N-C Electrocatalyst to Promote the Oxygen Reduction Reaction. Inorg. Chem. 2020, 59, 13453–13464. [Google Scholar] [CrossRef]
- Colthup, N. B.; Daly, L. H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy; 3rd edn.; Academic Press, Inc., 1990; ISBN 0-12-182554-X.
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; 7th edn.; John Wiley & Sons. Inc., 2005; ISBN 0-471-39362-2.
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.G.; Narita, A.; Hernandez, Y.; Balandina, T.; Mali, K.S.; De Feyter, S.; Feng, X.; Müllen, K. Structurally Defined Graphene Nanoribbons with High Lateral Extension. J. Am. Chem. Soc. 2012, 134, 18169–18172. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.; Zhong, Q.; Du, K.; Li, H.; Huang, J. Chemical Unzipping of Multiwalled Carbon Nanotubes for High-Capacity Lithium Storage. Electrochim. Acta 2014, 125, 170–175. [Google Scholar] [CrossRef]
- Wei, R.; Gu, Y.; Zou, L.; Xi, B.; Zhao, Y.; Ma, Y.; Qian, Y.; Xiong, S.; Xu, Q. Nanoribbon Superstructures of Graphene Nanocages for Efficient Electrocatalytic Hydrogen Evolution. Nano Lett. 2020, 20, 7342–7349. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, G.; Narita, A.; Orosco, M.; Feng, X.; Müllen, K.; Zhou, C. Deposition, Characterization, and Thin-Film-Based Chemical Sensing of Ultra-Long Chemically Synthesized Graphene Nanoribbons. J. Am. Chem. Soc. 2014, 136, 7555–7558. [Google Scholar] [CrossRef]
- Schwab, M.G.; Narita, A.; Osella, S.; Hu, Y.; Maghsoumi, A.; Mavrinsky, A.; Pisula, W.; Castiglioni, C.; Tommasini, M.; Beljonne, D.; et al. Bottom-Up Synthesis of Necklace-Like Graphene Nanoribbons. Chem. - An Asian J. 2015, 10, 2134–2138. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Zheng, W.; Osella, S.; Arisnabarreta, N.; Droste, J.; Serra, G.; Ivasenko, O.; Lucotti, A.; Beljonne, D.; et al. Cove-Edged Graphene Nanoribbons with Incorporation of Periodic Zigzag-Edge Segments. J. Am. Chem. Soc. 2022, 144, 228–235. [Google Scholar] [CrossRef]
- Narita, A.; Feng, X.; Hernandez, Y.; Jensen, S.A.; Bonn, M.; Yang, H.; Verzhbitskiy, I.A.; Casiraghi, C.; Hansen, M.R.; Koch, A.H.R.; et al. Synthesis of Structurally Well-Defined and Liquid-Phase-Processable Graphene Nanoribbons. Nat. Chem. 2014, 6, 126–132. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Fabrication of Nitrogen and Sulfur Co-Doped Graphene Nanoribbons with Porous Architecture for High-Performance Supercapacitors. Chem. Eng. J. 2017, 312, 180–190. [Google Scholar] [CrossRef]
- Boone, C. V.; Maia, G. Pt–Pd and Pt–Pd–(Cu or Fe or Co)/Graphene Nanoribbon Nanocomposites as Efficient Catalysts toward the Oxygen Reduction Reaction. Electrochim. Acta 2017, 247, 19–29. [Google Scholar] [CrossRef]
- Boone, C. V.; Maia, G. Lowering Metal Loadings onto Pt–Pd–Cu/Graphene Nanoribbon Nanocomposites Affects Electrode Collection Efficiency and Oxygen Reduction Reaction Performance. Electrochim. Acta 2019, 303, 192–203. [Google Scholar] [CrossRef]
- Souza, A.S.; Bezerra, L.S.; Cardoso, E.S.F.; Fortunato, G. V.; Maia, G. Nickel Pyrophosphate Combined with Graphene Nanoribbon Used as Efficient Catalyst for OER. J. Mater. Chem. A 2021, 9, 11255–11267. [Google Scholar] [CrossRef]
- Martini, B.K.; Maia, G. Using a Combination of Co, Mo, and Pt Oxides along with Graphene Nanoribbon and MoSe2 as Efficient Catalysts for OER and HER. Electrochim. Acta 2021, 138907. [Google Scholar] [CrossRef]
- Cardoso, E.S.F.; Fortunato, G. V; Palm, I.; Kibena-Põldsepp, E.; Greco, A.S.; Júnior, J.L.R.; Kikas, A.; Merisalu, M.; Kisand, V.; Sammelselg, V.; et al. Effects of N and O Groups for Oxygen Reduction Reaction on One- and Two-Dimensional Carbonaceous Materials. Electrochim. Acta 2020, 344, 136052. [Google Scholar] [CrossRef]
- Fortunato, G. V.; Cardoso, E.S.F.; Martini, B.K.; Maia, G. Ti/Pt−Pd-Based Nanocomposite: Effects of Metal Oxides on the Oxygen Reduction Reaction. ChemElectroChem 2020, 7, 1610–1618. [Google Scholar] [CrossRef]
- Wilson, N.R.; Pandey, P.A.; Beanland, R.; Rourke, J.P.; Lupo, U.; Rowlands, G.; Römer, R.A. On the Structure and Topography of Free-Standing Chemically Modified Graphene. New J. Phys. 2010, 12, 125010. [Google Scholar] [CrossRef]
- Han, G.-F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.-P.; Kim, S.-W.; Kim, S.-J.; Bu, Y.; Fu, Z.; Lu, Y.; et al. Building and Identifying Highly Active Oxygenated Groups in Carbon Materials for Oxygen Reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2 : A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Majumdar, D. Recent Progress in Copper Sulfide Based Nanomaterials for High Energy Supercapacitor Applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Duraivel, M.; Nagappan, S.; Balamuralitharan, B.; Selvam, S.; Karthick, S.N.; Prabakar, K.; Ha, C.-S.; Kim, H.-J. Superior One-Pot Synthesis of a Doped Graphene Oxide Electrode for a High Power Density Supercapacitor. New J. Chem. 2018, 42, 11093–11101. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Zakar, S.; Haider, S.S. Role of Aqueous Electrolytes on the Performance of Electrochemical Energy Storage Device. J. Electroanal. Chem. 2020, 858, 113793. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G. V.; De Lima, F.; Maia, G. Oxygen-Reduction Reaction Strongly Electrocatalyzed by Pt Electrodeposited onto Graphene or Graphene Nanoribbons. J. Power Sources 2016, 302, 247–258. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Darvishzad, T.; Gryboś, J.; Stelmachowski, P.; Sojka, Z.; Kruczała, K. Switching the Locus of Oxygen Reduction and Evolution Reactions between Spinel Active Phase and Carbon Carrier upon Heteroatoms Doping. Catal. Today 2023, 418, 114043. [Google Scholar] [CrossRef]
- Chai, G.-L.; Hou, Z.; Ikeda, T.; Terakura, K. Two-Electron Oxygen Reduction on Carbon Materials Catalysts: Mechanisms and Active Sites. J. Phys. Chem. C 2017, 121, 14524–14533. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Zheng, Y.; Xia, B.; Zhou, X.; Xu, H.; Qiao, S. Tailoring Selectivity of Electrochemical Hydrogen Peroxide Generation by Tunable Pyrrolic-Nitrogen-Carbon. Adv. Energy Mater. 2020, 10, 2000789. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, X.; Xia, C.; Wu, Z.-Y.; Zhu, P.; Kim, J.Y.; Bai, X.; Gao, G.; Hu, Y.; Zhong, J.; et al. Highly Active and Selective Oxygen Reduction to H2O2 on Boron-Doped Carbon for High Production Rates. Nat. Commun. 2021, 12, 4225. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and Sulfur Doped Mesoporous Carbon as Metal-Free Electrocatalysts for the in Situ Production of Hydrogen Peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Qin, M.; Fan, S.; Wang, L.; Gan, G.; Wang, X.; Cheng, J.; Hao, Z.; Li, X. Oxygen and Nitrogen Co-Doped Ordered Mesoporous Carbon Materials Enhanced the Electrochemical Selectivity of O2 Reduction to H2O2. J. Colloid Interface Sci. 2020, 562, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A.; Niu, J. Doping Strategy, Properties and Application of Heteroatom-Doped Ordered Mesoporous Carbon. RSC Adv. 2021, 11, 5361–5383. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Waje, M.M.; Chen, Z.; Yan, Y.; Bozhilov, K.N.; Feng, P. Sulfonated Ordered Mesoporous Carbon as a Stable and Highly Active Protonic Acid Catalyst. Chem. Mater. 2007, 19, 2395–2397. [Google Scholar] [CrossRef]
- Xie, L.; Wang, P.; Li, Y.; Zhang, D.; Shang, D.; Zheng, W.; Xia, Y.; Zhan, S.; Hu, W. Pauling-Type Adsorption of O2 Induced Electrocatalytic Singlet Oxygen Production on N–CuO for Organic Pollutants Degradation. Nat. Commun. 2022, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, A.; Luerßen, B.; Mollenhauer, D.; Janek, J.; Schröder, D. Singlet Oxygen in Electrochemical Cells: A Critical Review of Literature and Theory. Chem. Rev. 2021, 121, 12445–12464. [Google Scholar] [CrossRef] [PubMed]
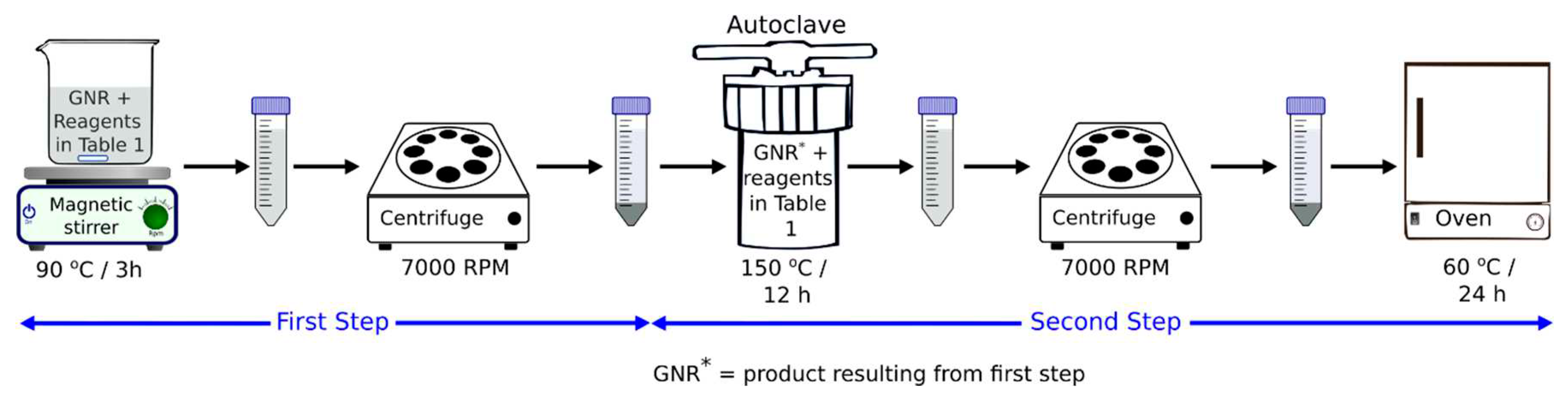
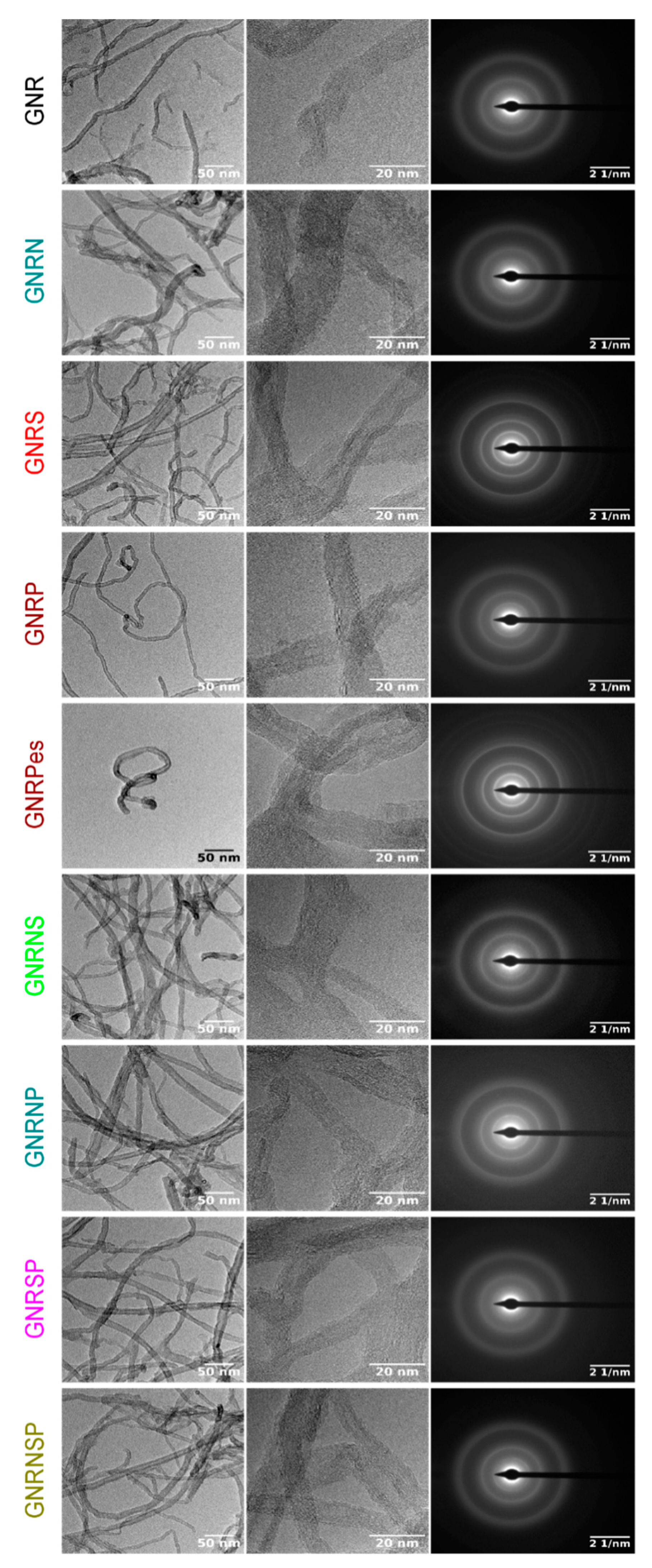
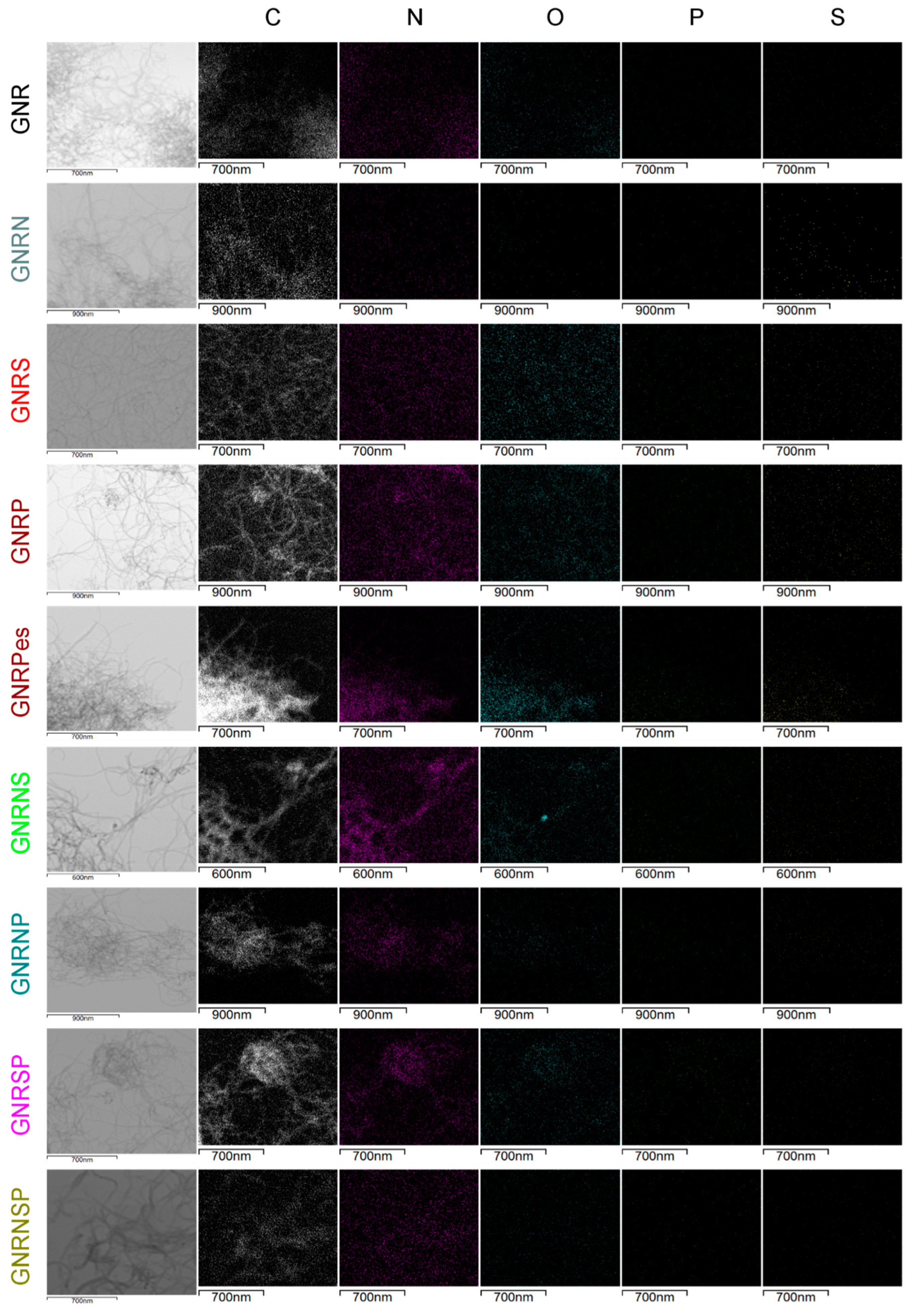
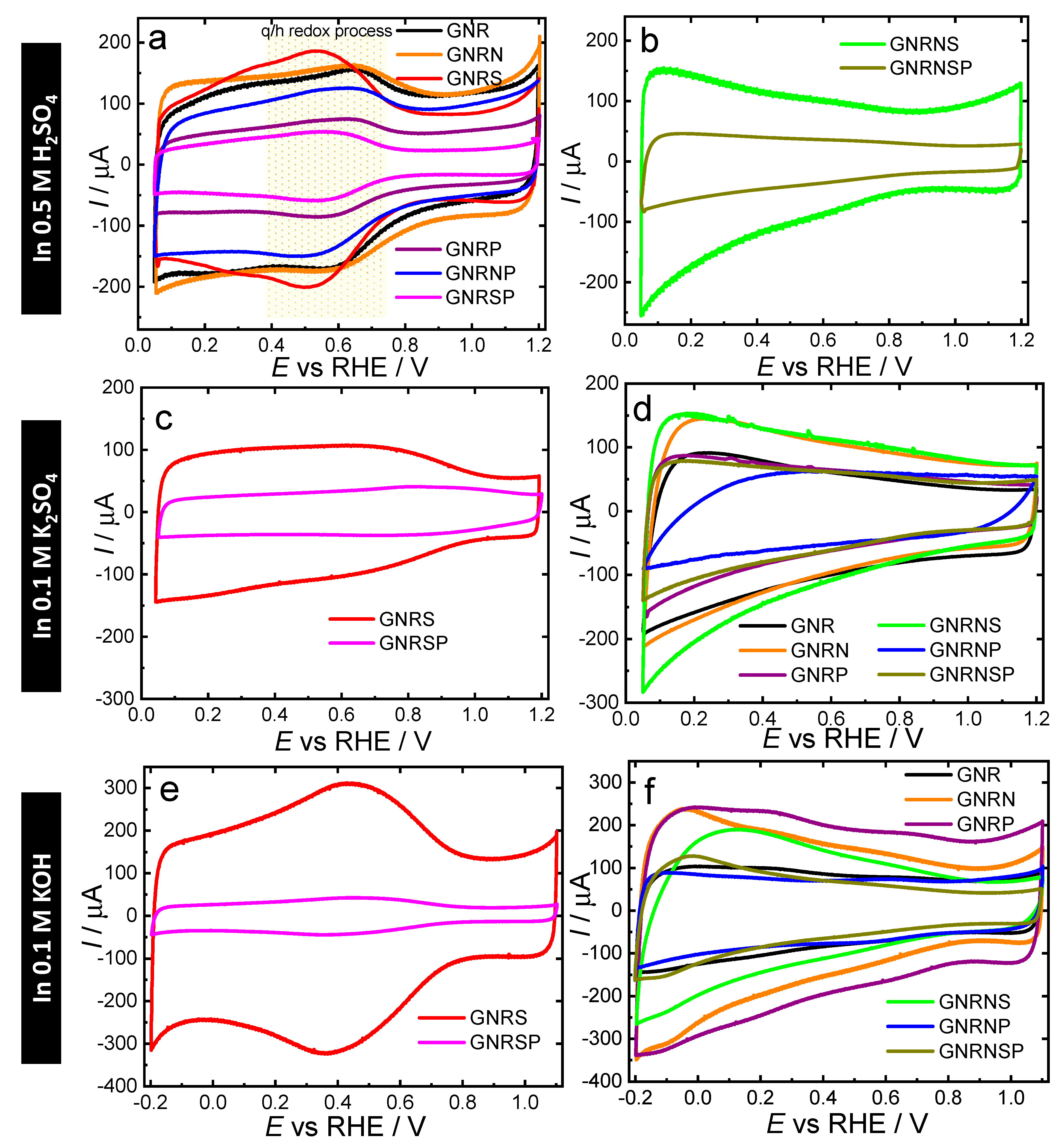
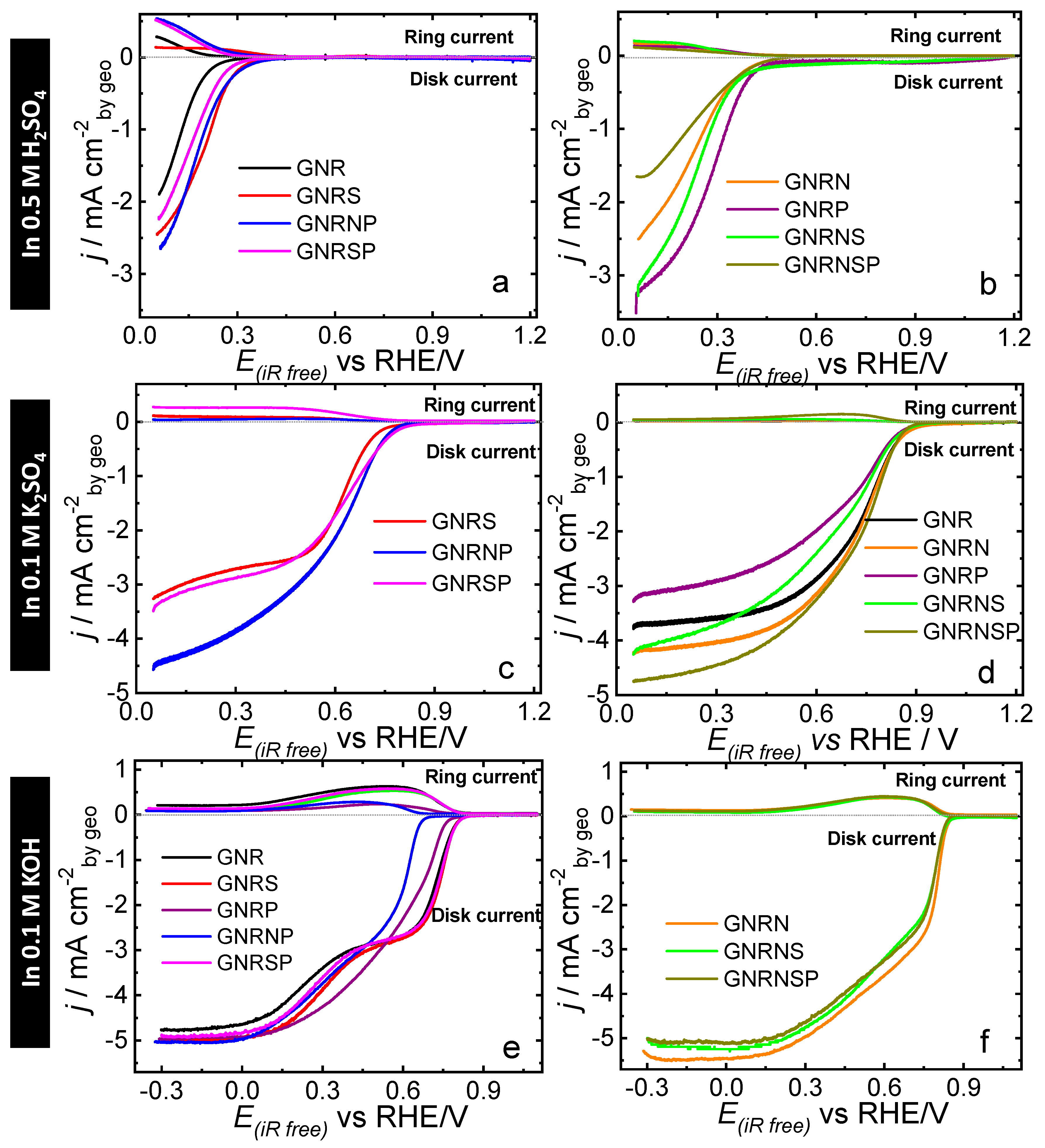
| Catalyst | First step Involved Heat Treatment at 95°C for 3h (Component Amounts as Shown Below), Followed by 10 Cycles of Washing/Centrifugation (7,000 rpm) with Water Replacement. | Second Step Was Executed in Autoclave System for 12 h at 150°C (Component Amounts as Shown Below), Followed by 10 Cycles of Washing/Centrifugation (7,000 rpm) with Water Replacement; Subsequently, the Material Was Dried at 60°C for 24 h | ||||||||
|
GNR (g) |
K2S2O8 (g) |
P2O5 (g) |
NH4OH (mL) | N2H6SO4 (g) | H2O (mL) | K2S2O8(g) | P2O5(g) | NH4OH (mL) | H2O (mL) | |
| GNRN | 0.02 | - | - | 1 | 1.06 | 30 | - | - | 30 | - |
| GNRS | 0.02 | 0.2 | - | - | - | 30 | 0.2 | - | - | 30 |
| GNRP | 0.02 | - | 0.2 | - | - | 30 | - | 0.2 | - | 30 |
| GNRNS | 0.02 | 0.2 | - | 1 | 1.06 | 30 | 0.2 | - | 30 | - |
| GNRNP | 0.02 | - | 0.2 | 1 | 1.06 | 30 | - | 0.2 | 30 | - |
| GNRSP | 0.02 | 0.2 | 0.2 | - | - | 30 | 0.2 | 0.2 | - | 30 |
| GNRNSP | 0.02 | 0.2 | 0.2 | 1 | 1.06 | 30 | 0.2 | 0.2 | 30 | - |
| Catalyst | Dopant Element (at%)* | Cdl (µF) | ECSA** (cm²) | Electrolyte | Eonset (VRHE) *** | E1/2 (VRHE) | X HO2‒ (%)**** |
nav**** |
|---|---|---|---|---|---|---|---|---|
| GNR | - | 2248.4 | 132.3 | 0.5 M H2SO4 | 0.26 | 0.13 | 76.7 | 2.5 |
| GNR | - | 1265.8 | 74.5 | 0.1 M K2SO4 | 0.89 | 0.73 | 5.2 | 3.9 |
| GNR | - | 1287.6 | 58.5 | 0.1 M KOH | 0.83 | 0.66 | 55.7 | 2.9 |
| GNRN | N (2.5 at%) | 2709.5 | 159.4 | 0.5 M H2SO4 | 0.41 | 0.24 | 57.7 | 2.8 |
| GNRN | N (2.5 at%) | 1228.4 | 72.3 | 0.1 M K2SO4 | 0.90 | 0.72 | 5.3 | 3.9 |
| GNRN | N (2.5 at%) | 1454.4 | 66.1 | 0.1 M KOH | 0.86 | 0.74 | 29.9 | 3.4 |
| GNRS | S (nq) | 2262.4 | 133.1 | 0.5 M H2SO4 | 0.33 | 0.20 | 52.3 | 3.0 |
| GNRS | S (nq) | 1394.9 | 82.1 | 0.1 M K2SO4 | 0.76 | 0.62 | 23.8 | 3.5 |
| GNRS | S (nq) | 1921.8 | 87.4 | 0.1 M KOH | 0.83 | 0.66 | 41.4 | 3.2 |
| GNRP | P (nq) | 1409.4 | 82.9 | 0.5 M H2SO4 | 0.45 | 0.28 | 35.3 | 3.3 |
| GNRP | P (nq) | 754.5 | 44.4 | 0.1 M K2SO4 | 0.88 | 0.68 | 13.7 | 3.7 |
| GNRP | P (nq) | 2316.8 | 105.3 | 0.1 M KOH | 0.79 | 0.58 | 26.7 | 3.5 |
| GNRNS | N (3.6 at%) S (nq) |
1190.0 | 70.0 | 0.5 M H2SO4 | 0.42 | 0.24 | 41.5 | 3.2 |
| GNRNS | N (3.6 at%) S (nq) |
1112.1 | 65.4 | 0.1 M K2SO4 | 0.88 | 0.66 | 12.5 | 3.7 |
| GNRNS | N (3.6 at%) S (nq) |
892.3 | 40.6 | 0.1 M KOH | 0.85 | 0.71 | 30.5 | 3.4 |
| GNRNP | N (4.3 at%) P (nq) |
2389.6 | 140.6 | 0.5 M H2SO4 | 0.33 | 0.18 | 91.5 | 2.2 |
| GNRNP | N (4.3 at%) P (nq) |
667.5 | 39.3 | 0.1 M K2SO4 | 0.79 | 0.60 | 11.9 | 3.8 |
| GNRNP | N (4.3 at%) P (nq) |
906.9 | 41.2 | 0.1 M KOH | 0.69 | 0.53 | 30.1 | 3.4 |
| GNRSP | P (nq) S (nq) |
722.5 | 42.5 | 0.5 M H2SO4 | 0.30 | 0.17 | 94.5 | 2.1 |
| GNRSP | P (nq) S (nq) |
635.9 | 37.4 | 0.1 M K2SO4 | 0.80 | 0.62 | 53.1 | 2.9 |
| GNRSP | P (nq) S (nq) |
175.1 | 8.0 | 0.1 M KOH | 0.83 | 0.65 | 48.7 | 3.0 |
| GNRNSP | N (4.6 at%) P (nq) S (nq) |
881.4 | 51.8 | 0.5 M H2SO4 | 0.41 | 0.25 | 48.6 | 3.0 |
| GNRNSP | N (4.6 at%) P (nq) S (nq) |
735.5 | 43.3 | 0.1 M K2SO4 | 0.87 | 0.71 | 18.9 | 3.6 |
| GNRNSP | N (4.6 at%) P (nq) S (nq) |
624.7 | 28.4 | 0.1 M KOH | 0.85 | 0.72 | 32.4 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





