Submitted:
04 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
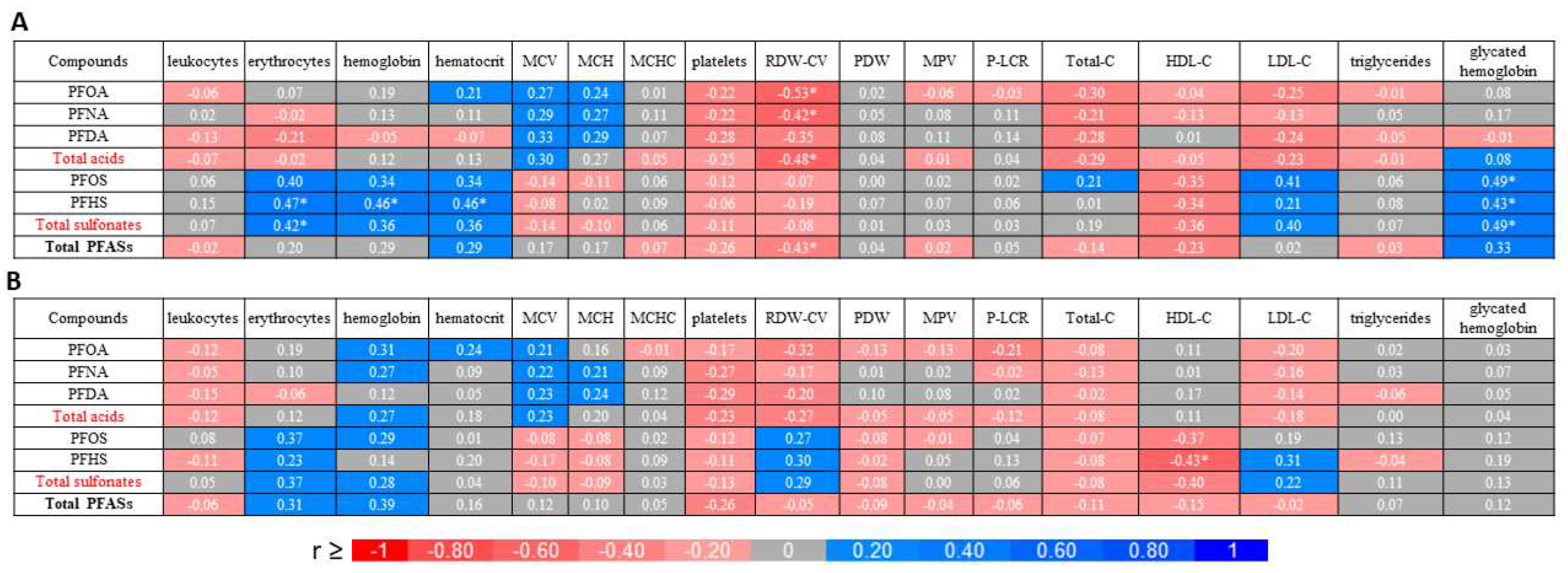
2.1. Perfluoroalkyl substances content
2.2. Blood parameters
3. Materials and Methods
3.1. The Chemicals, reagents, and study material
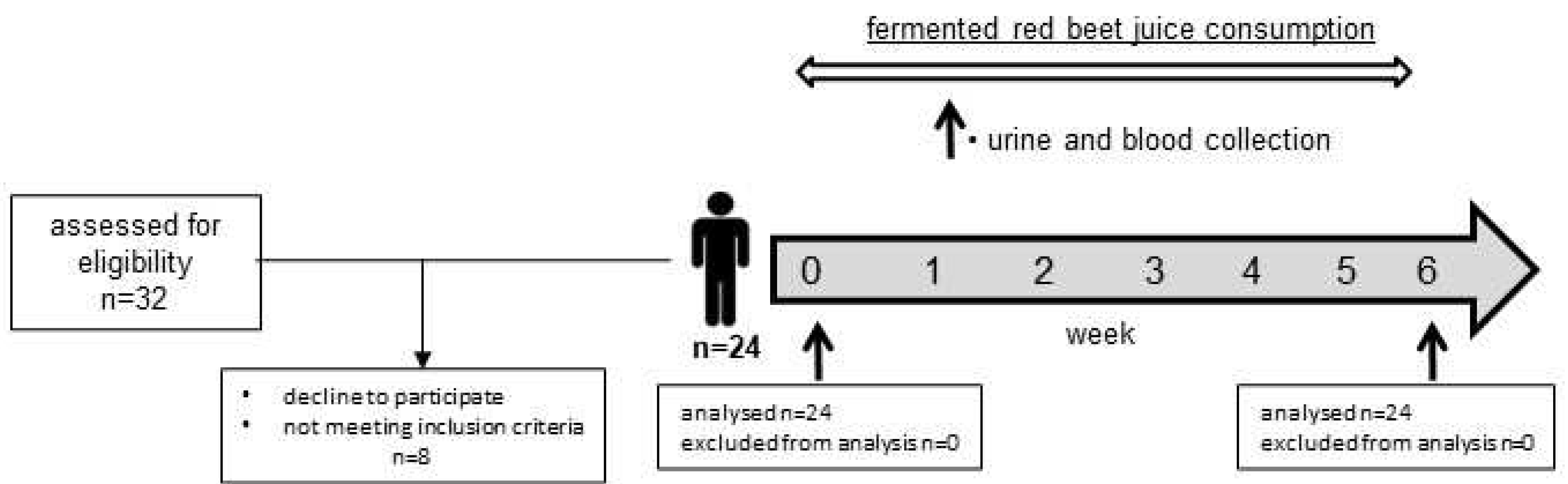
3.2. Characteristic of participants and study design
3.3. Ethical aspects
3.4. Plasma and urine samples preparation
3.5. Instrumental analysis
3.6. Analysis of blood parameters
3.7. Statistical analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellegrad, L.H.; Andersson, S.W.; Normen, A.L.; Andersson, H.A. Dietary plants sterols and cholesterol metabolism. Nutr. Rev. 2007, 65, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sznajder-Katarzyńska, K.; Surma, M.; Cieślik, E.; Wiczkowski, W. The perfluoroalkyl substances (PFASs) contamination of fruits and vegetables. Food Addit. Contam. Part A 2018, 35, 1776–1786. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Appleby, P.N.; Key, T.J. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2014, 100, 394S–398S. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Chen, W.Y.; Rosner, B.A.; Rulla, M.T.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int. J. Cancr 2018, 144, 1496–1510. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, Y.J.; Cai, L.B.; Xu, F.-R.; Xie. T.; He, Q.-Q. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit. Revi. Food Sci. Nutr. 2017, 57, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Płatosz, N.; Sawicki. T.; Wiczkowski, W. Profle of phenolic acids and flavonoids of red beet and its fermentation products. Does long-term consumption of fermented beetroot juice affect phenolics profle in human blood plasma and urine? Polish J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
- Crawford, N.M.; Fenton, S.E.; Strynar, M.; Hines, E.P.; Pritchard, D.A.; Steiner, A.Z. Effects of perfluorinated chemicals on thyroid function, markers ofovarian reserve, and natural fertility. Reprod. Toxicol. 2017, 69, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Poothong, S.; Thomsec, C.; Padilla-Sanchez, J.A.; Papadopoulou, E.; Haug, L.S. Distribution of novel and well-know poly-and perfluoroalkyl substances (PFASs) in human serum, plasma, and whole blood. Environ. Sci. Technol. 2017, 51, 13388–13396. [Google Scholar] [CrossRef]
- JOGSTEN I.E., PERELLO G., LLEBARIA X., BIGAS E., MARTI-CID R., KARRMAN A., DOMINGO J.L., Exposure to perfluorinated compounds in Catalonia, Spain, through consumption of various raw and cooked foodstuffs, including packaged food. Food Chem. Toxicol., 47, (7), 1577, 2009. 1577; 47.
- A. B. Lindstrom, M. J. Strynar, and E. L. Libelo, “Polyfluorinated compounds: past, present, and future,” Environmental Science & Technology, vol. 45, no. 19, pp. 7954–7961, 2011.
- M. P. Krafft and J. G. Riess, “Per- and polyfluorinated substances (PFASs): environmental challenges,” Current Opinion in Colloid & Interface Science, vol. 20, no. 3, pp. 192–212, 2015.
- Sznajder-Katarzyńska, K.; Surma, M.; Cieślik, I. A review of perfluoroalkyl AIDS (PFAAs) in terms of sources, applications, human exposure, dietary intake, toxicity, legal regulation, and methods of determination. J. Chem. 2019, 2717528. [Google Scholar]
- Giesy, J.P.; Kannan, K. Peer Reviewed: Perfluorochemical Surfactants in the Environment. Environ. Sci. Technol. 2008, 36, 146A–152A. [Google Scholar] [CrossRef]
- Geueke, B. Dossier – Per- and polyfluoroalkyl substances (PFASs). Food Packag. Forum 2016, 1–13. [Google Scholar] [CrossRef]
- Ehresman, D.J.; Froehlich, J.W.; Olsen, G.W.; Chang, S.C.; Butenhoff, J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. [Google Scholar] [CrossRef] [PubMed]
- C. Lau, K. Anitole, C. Hodes, D. Lai, A. Pfahles-Hutchens, and J. Seed, “Perfluoroalkyl acids: a review of monitoring and toxicological findings,” Toxicological Sciences, vol. 99, no. 2, pp. 366–394, 2007.
- K. G´oralczyk, K. A. Pachocki, A. Hernik et al., “Perfluorinated chemicals in blood serum of inhabitants in central Poland in relation to gender and age,” Science of the Total Environment, vol. 532, pp. 548–555, 2015.
- A. K¨arrman, I. Ericson, B. van Bavel et al., “Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden,” Environmental Health Perspectives, vol. 115, no. 2, pp. 226–230, 2007.
- K. S. Guruge, S. Taniyasu, N. Yamashita et al., “Perfluorinated organic compounds in human blood serum and seminal plasma: a study of urban and rural tea worker populations in Sri Lanka,” Journal of Environmental Monitoring, vol. 7, no. 4, pp. 371–377, 2005.
- K. Inoue, F. Okada, R. Ito et al., “Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy,” Environmental Health Perspectives, vol. 112, no. 11, pp. 1204–1207, 2004.
- L. Domingo, I. E. Jogsten, U. Eriksson et al., “Human dietary exposure to perfluoroalkyl substances in Catalonia, Spain. Temporal trend,” Food Chemistry, vol. 135, no. 3, pp. 1575–1582, 2012.
- P. D. Jones, W. Hu, W. De Coen, J. L. Newsted, and J. P. Giesy, “Binding of perfluorinated fatty acids to serum proteins,” Environmental Toxicology and Chemistry, vol. 22, no. 11, pp. 2639–2649, 2003.
- F. P´erez, M. Nadal, A. Navarro-Ortega et al., “Accumulation of perfluoroalkyl substances in human tissues,” Environment International, vol. 59, pp. 354–362, 2013.
- Frisbee, S.J.; Shankar, A.; Knox, S.; Steenland, K.; Savitz, D.A.; Fletcher, T.; Ducatman, A.M. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents. Arch. Pediatr. Adolesc. Med. 2010, 164, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- G. Webster, Potential Human Health Effects of Perfluorinated Chemicals (PFCs), National Collaborating Centre for Environmental Health-NCCEH, Vancouver, Canada, 2010.
- Z. Wang, I. T. Cousins, M. Scheringer, and K. Hungerbuehler, “Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions,” Environment International, vol. 75, pp. 172–179, 2015.
- Organisation for Economic Co-operation and Development (OECD), “Results of the 2006 survey on production and use of PFOS, PFAS, PFOA, PFCA, their related substances and products/mixtures containing these substances,” in Proceedings of the ENVIRONMENT DIRECTORATE ?e Joint Meeting of the Chemicals Committee and Working Party on Chemicals, Pesticides and Biotechnology, ENV/JM/MONO(2006), p. 36, Paris, France, February 2006.
- M. P. Krafft and J. G. Riess, “Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution,” Biochimie, vol. 80, no. 5-6, pp. 489–514, 1998.
- S. Ganesan and N. Vasudevan, “Impacts of perfluorinated compounds on human health,” Bulletin of Environment, Pharmacology and Life Sciences, vol. 4, no. 7, pp. 183–191, 2015.
- L. Maestri, S. Negri, M. Ferrari et al., “Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry,” Rapid Communications in Mass Spectrometry, vol. 20, no. 18, pp. 2728–2734, 2006.
- K. Kannan, S. Corsolini, J. Falandysz et al., “Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries,” Environmental Science & Technology, vol. 38, no. 17, pp. 4489–4495, 2004.
- X. Wu, D. H. Bennett, A. M. Calafat et al., “Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California,” Environmental Research, vol. 136, pp. 264–273, 2015.
- G.-W. Lien, T.-W. Wen, W.-S. Hsieh, K.-Y. Wu, C.-Y. Chen, and P.-C. Chen, “Analysis of perfluorinated chemicals in umbilical cord blood by ultra-high performance liquid chromatography/tandem mass spectrometry,” Journal of Chromatography B, vol. 879, no. 9-10, pp. 641–646, 2011.
- B. J. Apelberg, F. R. Witter, J. B. Herbstman et al., “Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth,” Environmental Health Perspectives, vol. 115, no. 11, pp. 1670–1676, 2007.
- R. Monroy, K. Morrison, K. Teo et al., “Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples,” Environmental Research, vol. 108, no. 1, pp. 56–62, 2008.
- L. Hanssen, A. A. Dudarev, S. Huber, J. Ř. Odland, E. Nieboer, and T. M. Sandanger, “Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan,” Science of the Total Environment, vol. 447, pp. 430–437, 2013.
- A. Karrman, J. L. Domingo, X. Llebaria et al., “Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples,” Environmental Science and Pollution Research, vol. 17, no. 3, pp. 750–758, 2010.
- J.-L. He, T. Peng, J. Xie et al., “Determination of 20 perfluorinated compounds in animal liver by HPLC-MS/MS,” Chinese Journal of Analytical Chemistry, vol. 43, no. 1, pp. 40–48, 2015.
- F. P´erez, M. Nadal, A. Navarro-Ortega et al., “Accumulation of perfluoroalkyl substances in human tissues,” Environment International, vol. 59, pp. 354–362, 2013.
- W. Volkel, O. Genzel-Borovicz´eny, H. Demmelmair et al., “Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study,” International Journal of Hygiene and Environmental Health, vol. 211, no. 3-4, pp. 440–446, 2008.
- S. Rainieri, N. Conlledo, T. Langerholc, E. Madorran, M. Sala, and A. Barranco, “Toxic effects of perfluorinated compounds at human cellular level and on a model vertebrate,” Food and Chemical Toxicology, vol. 104, pp. 14–25, 2017.
- Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) (2006) COT Statement on the Tolerable Daily Intake for Perfluorooctane Sulfonate. COT Statement 2006/09, Committee on Toxicity of Chemicals in Food, London, UK, 2006, https://cot.food.gov.uk/sites/default/files/cot/cotstatementpfos200609.pdf.
- G. L. Kennedy, J. L. Butenhoff, G. W. Olsen et al., “-etoxicology of perfluorooctanoate,” Critical Reviews in Toxicology, vol. 34, no. 4, pp. 351–384, 2004.
- A. M. Seacat, P. J. -omford, K. J. Hansen, G. W. Olsen, M. T. Case, and J. L. Butenhoff, “Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys,” Toxicological Sciences, vol. 68, no. 1, pp. 249–264, 2002.
- L. Zheng, G.-H. Dong, Y.-H. Jin, and Q.-C. He, “Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice,” Archives of Toxicology, vol. 83, no. 7, pp. 679 689, 2009.
- A. D. Benninghoff, G. A. Orner, C. H. Buchner, J. D. Hendricks, A. M. Duffy, and D. E. Williams, “Promotion of hepatocarcinogenesis by perfluoroalkyl acids in rainbow trout,” Toxicological Sciences, vol. 125, no. 1, pp. 69–78, 2012.
- J. W. Nelson, E. E. Hatch, and T. F. Webster, “Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population,” Environmental Health Perspectives, vol. 118, no. 2, pp. 197–202, 2010.
- J. C. DeWitt, M. M. Peden-Adams, J. M. Keller, and D. R. Germolec, “Immunotoxicity of perfluorinated compounds: recent developments,” Toxicologic Pathology, vol. 40, no. 2, pp. 300–311, 2012.
- M. R. Qazi, Z. Xia, J. Bogdanska et al., “-e atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short-term exposure to perfluorooctanesulfonate are high-dose phenomena mediated in part by peroxisome proliferator-activated receptoralpha (PPARα),” Toxicology, vol. 260, no. 1–3, pp. 68–76, 2009.
- P. Grandjean, E. W. Andersen, E. Budtz-Jørgensen et al., “Serum vaccine antibody concentrations in children exposed to perfluorinated compounds,” JAMA, vol. 307, no. 4, pp. 391–397, 2012.
- N. M. Crawford, S. E. Fenton, M. Strynar, E. P. Hines, D. A. Pritchard, and A. Z. Steiner, “Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility,” Reproductive Toxicology, vol. 69, pp. 53–59, 2017.
- M.-J. Lopez-Espinosa, D. Mondal, B. Armstrong, M. S. Bloom, and T. Fletcher, “-yroid function and 18 Journal of Chemistry perfluoroalkyl acids in children living near a chemical plant,” Environmental Health Perspectives, vol. 120, no. 7, pp. 1036–1041, 2012.
- N. Johansson, A. Fredriksson, and P. Eriksson, “Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice,” Neurotoxicology, vol. 29, no. 1, pp. 160–169, 2008.
- A. J. Filgo, E. M. Quist, M. J. Hoenerhoff, A. E. Brix, G. E. Kissling, and S. E. Fenton, “Perfluorooctanoic acid (PFOA)-induced liver lesions in two strains of mice following developmental exposures,” Toxicologic Pathology, vol. 43, no. 4, pp. 558–568, 2015.
- C. Fei, J. K. McLaughlin, R. E. Tarone, and J. Olsen, “Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort,” Environmental Health Perspectives, vol. 115, no. 11, pp. 1677–1682, 2007.
- T. I. Halldorsson, D. Rytter, L. S. Haug et al., “Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study,” Environmental Health Perspectives, vol. 120, no. 5, pp. 668–673, 2012.
- Fenton, S.E.; Ducatman, A.; Boobis, A. Per- and polyfluoroalhyl substances toxicity and human health review: current state of knowledge and strategies for informing future research. Environ. Toxicol Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Herzke D, Huber S, Bervoets L, D’Hollander W, Hajslova J, Pulkrabova J, Brambilla G, De Filippis SP, Klenow S, Heinemeyer G, et al. 2013. Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ Sci Pollut Res. 20:7930–7939.
- de Castro, A.P.R.B.; da Cunha, D.T.; Antunes, A.E.C.; Corona, L.P.; Bezerra, R.M.N. Effect of freeze-dried red beet (beta vulgaris l.) leaf supplementation on biochemical and anthropometrical parameters in overweight and obese individuals: a pilot study. Plant Foods Hum. Nutr. 2017, 74, 232–234. [Google Scholar] [CrossRef]
- Asgary, S.; Afshani, M.R.; Sahebkar, A.; Keshvari, M.; Taheri, M.; Jahanian, E.; Rafieian-Kopaei, M.; Malekian, F.; Sarrafzadegan, N. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: a randomized crossover pilot study. J. Hum. Hypertens. 2016, 30, 627–632. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Wiktor, A. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef]
- Nakamura, K.; seishima, R.; Matsui, S.; Shiggeta, K.; Okabayashi, K.; Kitagawa, Y. The prognostic impact of preoperative mean corpuscular volume in colorectal cancer. Jpn. J. Clin. Oncol. 2022, 52, 562–570. [Google Scholar] [CrossRef]
- Vinholt, P.J.; Hvas, A.M. , Frederiksen, H.; Bathum, L.; Jorgensen, M.K.; Nybo, M. Platelet count is associated with cardiovascular disease, cancer and mortality: A population-based cohort study. Thromb. Res. 2016, 148, 136–142. [Google Scholar] [CrossRef]
- Semple, J.M.; Italiano Jr., J. E.; Freedman, J. Platelets and the immune continuum. Nat. Rev, Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Rotander, A.; Toms, L.-M.L.; Aylward, L.; Kay, M.; Mueller, J.F. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environ. Int. 2015, 82, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Peraz, F.; Llorca, M.; Farré, M.; Barcelo, D. Automated analysis of perfluorinated compounds In human hair and urine samples by turbulent flow chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 2369–2378. [Google Scholar] [CrossRef]
- Surma, M.; Wiczkowski, W.; Zieliński, H.; Cieślik, E. Determination of selected perfluorinated acids (PFCAs) and perfluorinated sulfonates (PFASs) in food contact materials using LC-MS/MS. Packag. Technol. Sci. 2015, 28, 789–799. [Google Scholar] [CrossRef]
- Niedzwiedzka, E.; Wadolowska, L.; Kowalkowska, J. Reproducibility of a non-quantitative food frequency questionnaire (62-Item FFQ-6) and PCA-driven dietary pattern identification in 13-21-year-old females. Nutrients 2019, 1, 2183. [Google Scholar] [CrossRef]
| No | Compounds | Rt (min) |
[M]- (m/z) |
MS/MS (m/z) |
Sample |
|---|---|---|---|---|---|
| Perfluorocarboxilic acids | |||||
| 1 | PFOA | 2.02 | 413 | 369 | J, B, U |
| 2 | PFNA | 2.43 | 463 | 419 | B |
| 3 | PFDA | 2.86 | 513 | 469 | B |
| Perfluoroalkane sulfonates | |||||
| 4 | PFOS | 2.97 | 499 | 80 | B |
| 5 | PFHS | 2.11 | 399 | 80 | B |
| No | Compounds | Blood plasma samples |
p T0 vs T1 |
|
|---|---|---|---|---|
| T0 | T1 | |||
| Acids | ||||
| 1 | PFOA | 0.94 (0.76-1.56) | 0.93 (0.77-1.52) | 0.627 |
| 2 | PFNA | 0.39 (0.26-0.58) | 0.34 (0.23-0.58) | < 0.001** |
| 3 | PFDA | 0.30 (0.20-0.46) | 0.23 (0.17-0.38) | < 0.001** |
| Total acids | 1.65 (1.29-2.94) | 1.49 (1.19-2.64) | 0.009** | |
| Sulfonates | ||||
| 4 | PFOS | 0.59 (0.44-0.80) | 0.71 (0.53-0.96) | < 0.001** |
| 5 | PFHS | 0.11 (0.09-0.17) | 0.25 (0.15-0.37) | < 0.001** |
| Total sulfonates | 0.67 (0.53-0.92) | 0.96 (0.68-1.37) | < 0.001** | |
| Total of PFASs | 2.66 (1.90-4.47) | 2.62 (2.08-4.17) | 0.031* | |
| Blood parameters | Samples |
p T0 vs T1 |
|
|---|---|---|---|
| T0 | T1 | ||
| Hematology | |||
| Leukocytes [k/μL] | 5.2 (4.9-6.0) | 5.1 (4.6-5.8) | 0.961 |
| Erythrocytes [mln/μL] | 4.6 (4.3-5.1) | 4.7 (4.3-4.9) | 0.246 |
| Hemoglobin [g/dL] | 13.2 (12.9-14.7) | 13.6 (12.7-14.1) | 0.721 |
| Hematocrit [%] | 41.0 (39.0-45.0) | 40.0 (37.0-42.0) | 0.013* |
| MCV [fl] | 88.0 (85.0-92.0) | 87.0 (84.0-90.0) | 0.001** |
| MCH [pg] | 29.0 (28.0-30.0) | 29.0 (28.0-31.0) | 0.686 |
| MCHC [g/dL] | 32.9 (32.3-33.5) | 33.4 (33.1-34.2) | < 0.001** |
| RDW-CV [%] | 13.0 (12.0-14.0) | 13.0 (12.0-13.0) | 0.208 |
| Platelets [k/μL] | 273.0 (215.0-318.0) | 262.0 (202.0-299.0) | 0.002** |
| PDW [fl] | 14.0 (13.0-15.0) | 14.0 (12.0-15.0) | 0.083 |
| MPV [fl] | 11.2 (10.7-11.9) | 10.9 (10.4-11.7) | < 0.001** |
| P-LCR [%] | 34.0 (29.0-38.0) | 32.0 (28.0-38.0) | < 0.001** |
| Blood biochemistry | |||
| Total-C [mg/dL] | 184.0 (165.0-197.0) | 168.0 (155.0-194.0) | 0.074 |
| HDL-C [mg/dL] | 69.0 (64.0-86.0) | 73.0 (57.0-88.0) | 0.897 |
| LDL-C [mg/dL] | 80.6 (64.8-109.8) | 76.4 (66.4-98.4) | 0.128 |
| Triglycerides [mg/dL] | 80.0 (48.0-102.0) | 71.0 (58.0-81.0) | 0.412 |
| Glycated hemoglobin [%] | 5.2 (5.0-5.3) | 5.2 (5.1-5.3) | 0.877 |
| Characteristics | Total |
|---|---|
| Sample size | 24 |
| Sample percentage | 100.0 |
| Age (years), mean (SD) | 29.5 (3.6) |
| Gender, n (%) | |
| women | 19 (79.2) |
| men | 5 (20.8) |
| BMI (kg/m2), mean (SD) | 24.9 (1.8) |
| Residence, n (%) | |
| Urban | 17 (70.8) |
| Rural | 7 (29.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





