Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. General characteristics
2.2. Risk analysis of SSI using logistic regression analysis
2.3. Comparison of outcomes of two groups (t-test, non-inferiority)
3. Discussion
4. Materials and Methods
4.1. Study population
4.2. Characteristics of patients
4.3. Outcomes
4.4. Ethics statement
4.5. Statistical analyses
Author Contributions
Funding
Conflicts of Interest
References
- Martone, W.J.; Nichols, R.L. Recognition, prevention, surveillance, and management of surgical site infections: introduction to the problem and symposium overview. Clinical Infectious Diseases 2001, 33, S67–S68. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, K.B.; Briggs, J.P.; Trivette, S.L.; Wilkinson, W.E.; Sexton, D.J. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection Control & Hospital Epidemiology 1999, 20, 725–730. [Google Scholar]
- Gorbach, S.L.; Condon, R.E.; Conte Jr, J.E.; Kaiser, A.B.; Ledger, W.J.; Nichols, R.L. Evaluation of new anti-infective drugs for surgical prophylaxis. Clinical infectious diseases 1992, 15, S313–S338. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.P.; Burton, R.C. Effects of prophylactic antibiotics on wound infection after elective colon and rectal surgery: 1960 to 1980. The American Journal of Surgery 1983, 145, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Peterson, K.D.; Mu, Y.; Banerjee, S.; Allen-Bridson, K.; Morrell, G.; Dudeck, M.A.; Pollock, D.A.; Horan, T.C. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009, 37, 783–805. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L. Prophylaxis for intraabdominal surgery. Reviews of infectious diseases 1984, 6, S276–S282. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surgical infections 2013, 14, 73–156. [Google Scholar] [CrossRef] [PubMed]
- Shatney, C.H. Antibiotic prophylaxis in elective gastro-intestinal tract surgery: a comparison of single-dose pre-operative cefotaxime and multiple-dose cefoxitin. Journal of Antimicrobial Chemotherapy 1984, 14, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lumley, J.; Siu, S.; Rllay, S.; Stitz, R.; Kemp, R.; Faoagali, J.; Nathanson, L.; White, S. Single dose ceftriaxone as prophylaxis for sepsis in colorectal surgery. Australian and New Zealand Journal of Surgery 1992, 62, 292–296. [Google Scholar] [CrossRef] [PubMed]
- AhChong, K.; Yip, A.; Lee, F.; Chiu, K. Comparison of prophylactic ampicillin/sulbactam with gentamicin and metronidazole in elective colorectal surgery: a randomized clinical study. Journal of Hospital Infection 1994, 27, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Control, C.f.D.; Prevention. Surgical site infection event SSI. National Healthcare Safety Network (NHSN) 2021. [Google Scholar]
- Kwok, S.; Lau, W.; Leung, K.; Ku, K.; Ho, W.; Li, A. Amoxycillin and clavulanic acid versus cefotaxime and metronidazole as antibiotic prophylaxis in elective colorectal resectional surgery. Chemotherapy 1993, 39, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Deierhoi, R.J.; Dawes, L.G.; Vick, C.; Itani, K.M.; Hawn, M.T. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. Journal of the American College of Surgeons 2013, 217, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Pochhammer, J.; Köhler, J.; Schäffer, M. Colorectal surgical site infections and their causative pathogens: differences between left-and right-side resections. Surgical infections 2019, 20, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, B.; Li, M.; Cao, J.; Liu, D.; Wang, Z.; Wang, Q.; Xiao, P.; Zhang, X.; Gao, Y. Multicenter surveillance study of surgical site infection and its risk factors in radical resection of colon or rectal carcinoma. BMC infectious diseases 2019, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.I.; Keskey, R.; Ackerman, M.T.; Zaborina, O.; Hyman, N.; Alverdy, J.C.; Shogan, B.D. Enterococcus faecalis Is Associated with Anastomotic Leak in Patients Undergoing Colorectal Surgery. Surgical Infections 2021. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Science translational medicine 2015, 7, 286ra268–286ra268. [Google Scholar] [CrossRef] [PubMed]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal infection—treatment and antibiotic resistance. Enterococci: from commensals to leading causes of drug resistant infection [Internet], 2014. [Google Scholar]
- Kalakouti, E.; Simillis, C.; Pellino, G.; Mughal, N.; Warren, O.; Mills, S.; Tan, E.; Kontovounisios, C.; Tekkis, P.P. Characteristics of Surgical Site Infection Following Colorectal Surgery in a Tertiary Center: Extended-spectrum β-Lactamase-producing Bacteria Culprits in Disease. Wounds: a compendium of clinical research and practice 2017, 30, 108–113. [Google Scholar] [PubMed]
- Bhattacharjee, A.; Sen, M.R.; Prakash, P.; Anupurba, S. Role of β-lactamase inhibitors in enterobacterial isolates producing extended-spectrum β-lactamases. Journal of antimicrobial chemotherapy 2008, 61, 309–314. [Google Scholar] [CrossRef] [PubMed]
| Variables | CFZ/MTZ N=556 |
AMP/SUL N=646 |
P-value |
|---|---|---|---|
| Age in years, mean ± standard deviation | 64.2 ± 12.0 | 64.4 ± 12.8 | 0.831 |
| Sex | |||
| Male | 318 (47.3) | 355 (52.7) | 0.449 |
| Female | 291 (45.0) | 291 (55.0) | |
| Surgical site | |||
| Colon | 267 (43.1) | 353 (56.9) | 0.021 |
| Rectum | 289 (49.8) | 291 (50.2) | |
| Operation time | |||
| <75% | 394 (46.3) | 457 (53.7) | 0.294 |
| ≥75% | 114 (50.4) | 112 (49.6) | |
| ASA score | |||
| I–II | 269 (50.9) | 260 (49.1) | 0.244 |
| III–IV | 253 (47.2) | 283 (52.8) | |
| Diabetes mellitus | |||
| No | 425 (49.5) | 433 (50.5) | 0.227 |
| Yes | 99 (44.8) | 122 (55.2) | |
| Current Smoking | |||
| No | 425 (48.5) | 451 (51.5) | 1.000 |
| Yes | 99 (48.8) | 104 (51.2) | |
| Obesity | |||
| No | 320 (44.0) | 407 (56.0) | <0.001 |
| Yes | 204 (58.0) | 148 (42.0) | |
| Wound class | |||
| Clean or clean/contaminated | 515 (49.0) | 535 (51.0) | 1.000 |
| Contaminated/dirty | 8 (50.0) | 8 (50.0) | |
| Surgical approach | |||
| Minimally invasive | 416 (47.3) | 463 (52.7) | 0.240 |
| Open | 140 (43.3) | 183 (56.7) | |
| Improper timing of administration | |||
| No | 534 (46.4) | 617 (53.6) | 1.000 |
| Yes | 22 (46.8) | 25 (53.2) | |
| Oral kanamycin | |||
| No | 218 (44.0) | 278 (56.0) | 0.196 |
| Yes | 338 (47.9) | 368 (52.1) |
| Variables | Surgical site infection | Univariate | Multivariate | |
|---|---|---|---|---|
| Antibiotics group | 0.940 (0.843–1.047) | - | ||
| CFZ/MTZ (n=556) | 21 (55.3) | |||
| AMP/SUL (n=646) | 17 (44.7) | |||
| Age, years | 1.256 (0.617–2.558) | - | ||
| 18–59 (n=405) | 11 (28.9) | |||
| 60–99 (n=797) | 27 (71.1) | |||
| Sex | 0.444* (0.214–0.922) | - | ||
| Male (n=673) | 28 (73.7) | |||
| Female (n=529) | 10 (26.3) | |||
| Site | 1.868 (0.957–3.648) | - | ||
| Colon (n=620) | 14 (36.8) | |||
| Rectum (n=580) | 24 (63.2) | |||
| Operation time | 2.103* (1.053–4.199) | - | ||
| <75% (n=851) | 24 (64.9) | |||
| ≥75% (n=226) | 13 (35.1) | |||
| ASA score | 0.663 (0.340–1.293) | - | ||
| I–II (n=529) | 22 (59.5) | |||
| III–IV (n=536) | 15 (40.5) | |||
| Diabetes mellitus | 1.258 (0.585–2.707) | - | ||
| No (n=858) | 28 (75.7) | |||
| Yes (n=221) | 9 (24.3) | |||
| Current smoking | 3.484*** (1.784–6.803) | 3.489*** (1.784-6.822) | ||
| No (n=876) | 21 (56.8) | |||
| Yes (n=203) | 16 (43.2) | |||
| Obesity | 0.560 (0.253–1.237) | - | ||
| No (n=727) | 29 (78.4) | |||
| Yes (n=352) | 8 (21.6) | |||
| Wound class | 1.878 (0.241–14.606) | - | ||
| Clean or clean/contaminated (n=1,050) | 36 (97.3) | |||
| Contaminated/dirty (n=16) | 1 (2.7) | |||
| Surgical approach | 1.433 (0.724–2.835) | - | ||
| Minimally invasive (n=879) | 25 (65.8) | |||
| Open (n=323) | 13 (34.2) | |||
| Improper timing of administration | 0.654 (0.326-1.314) | - | ||
| No (n=47) | 3 (7.9) | |||
| Yes (n=1,151) | 35 (92.1) | |||
| Oral kanamycin | 1.212 (0.620-2.366) | - | ||
| No (n=496) | 14 (36.8) | |||
| Yes (n=706) | 24 (63.2) | |||
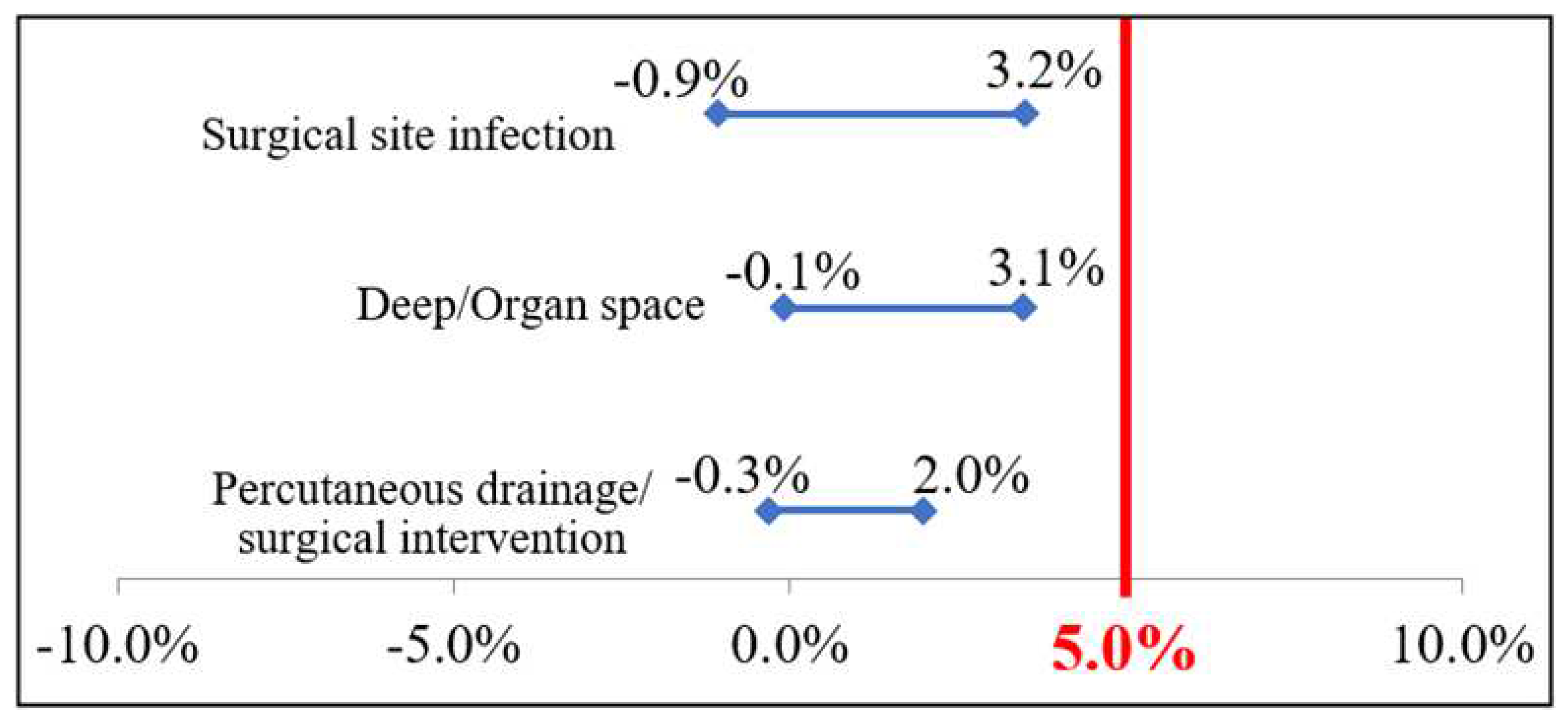
| CFZ/MTZ | AMP/SUL | RD | 95% confidence interval of the difference | P-value | |||
|---|---|---|---|---|---|---|---|
| M ± SD | M ± SD | ||||||
| Primary endpoint |
Surgical site infection | 0.04 ± 0.191 | 0.03 ± 0.160 | 0.05 | -0.009 | 0.032 | 0.264 |
| Secondary endpoint |
Deep/Organ space | 0.03 ± 0.162 | 0.01 ± 0.111 | 0.05 | -0.001 | 0.031 | 0.073 |
| Percutaneous drainage/surgical intervention | 0.01 ± 0.119 | 0.01 ± 0.079 | 0.05 | -0.003 | 0.020 | 0.167 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





