Submitted:
08 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Statistical Analysis
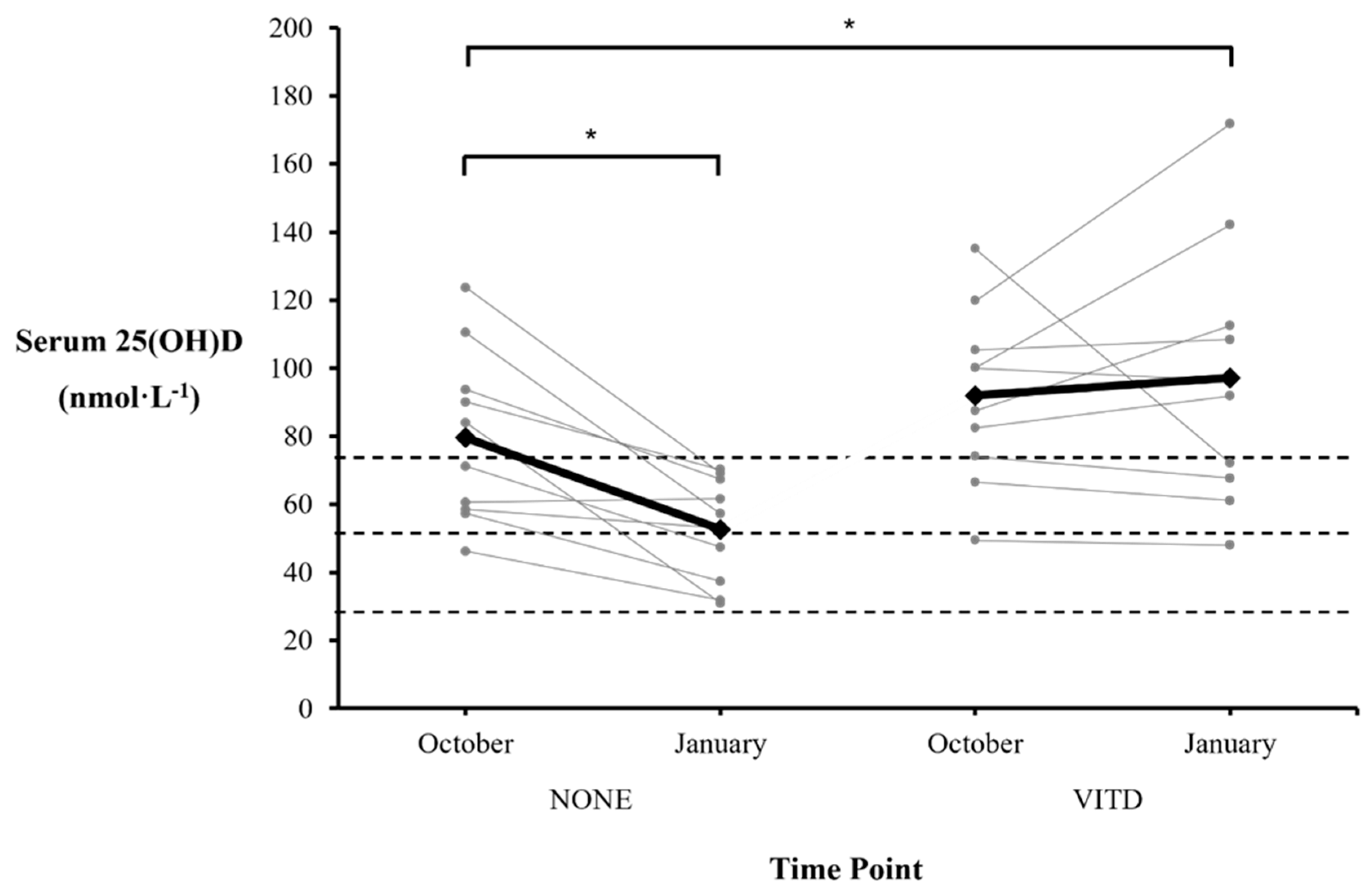
3. Results
3.1. Sub-group Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the athlete: current perspectives and new challenges. Sports Med 2018, 48, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Frommer, J.E.; McNeill, S.C.; Richtand, N.M.; Henley, J.W.; Potts Jr, J.T. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun 1977, 76, 107–114. [Google Scholar] [CrossRef]
- Carlberg, C.; Seuter, S.; de Mello, V.D.; Schwab, U.; Voutilainen, S.; Pulkki, K.; et al. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D₃ supplementation. PLoS One 2013, 8, e71042. [Google Scholar] [CrossRef] [PubMed]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med 2015, 45, 365–378. [Google Scholar] [CrossRef]
- Gillie, O. Sunlight robbery: a critique of public health policy on vitamin D in the UK. Mol Nutr Food Res 2010, 54, 1148–1163. [Google Scholar] [CrossRef]
- Todd, J.J.; Pourshahidi, L.K.; McSorley, E.M.; Madigan, S.M.; Magee, P.J. Vitamin D: recent advances and implications for athletes. Sports Med 2015, 45, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Newbury, J.W.; Foo, W.L.; Cole, M.; Kelly, A.L.; Chessor, R.J.; Sparks, S.A.; et al. Nutritional intakes of highly trained adolescent swimmers before, during, and after a national lockdown in the COVID-19 pandemic. PLoS One 2022, 17, e0266238. [Google Scholar] [CrossRef]
- Lewis, R.M.; Redzic, M.; Thomas, D.T. The effects of season-long vitamin D supplementation on collegiate swimmers and divers. Int J Sport Nutr Exerc Metab 2013, 23, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, M.S.; Frisard, M.I.; Rankin, J.W.; Zabinsky, J.S.; Mcmillan, R.P.; You, W.; et al. Effects of seasonal vitamin D3 supplementation on strength, power, and body composition in college swimmers. Int J Sport Nutr Exerc Metab 2020, 30, 165–173. [Google Scholar] [CrossRef]
- Galan, F.; Ribas, J.; Sánchez-Martinez, P.M.; Calero, T.; Sánchez, A.B.; Muñoz, A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin Nutr 2012, 31, 132–136. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Livne, N.; Raz, R.; Cohen, A.H.; Constantini, N.W. Vitamin D supplementation and physical performance in adolescent swimmers. Int J Sport Nutr Exerc Metab 2015, 25, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hollabaugh, W.L.; Meirick, P.J.; Matarazzo, C.P.; Burston, A.M.; Camery, M.E.; Ferrill-Moseley, K.A.; et al. Evaluation of a vitamin D screening and treatment protocol using a seasonal calculator in athletes. Curr Sports Med Rep 2022, 21, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Leitch, B.A.; Wilson, P.B.; Ufholz, K.E.; Roemmich, J.N.; Orysiak, J.; Walch, T.J.; et al. Vitamin D awareness and intake in collegiate athletes. J Strength Cond Res 2021, 35, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Love, T.D.; Baker, D.F.; Healey, P.B.; Haszard, J.; Edwards, A.S.; et al. Knowledge and attitudes to vitamin D and sun exposure in elite New Zealand athletes: a cross-sectional study. J Int Soc Sports Nutr 2014, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Geiker, N.R.W.; Hansen, M.; Jakobsen, J.; Kristensen, M.; Larsen, R.; Jørgensen, N.R.; et al. Vitamin D status and muscle function among adolescent and young swimmers. Int J Sport Nutr Exerc Metab 2017, 27, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Newbury, J.W.; Sparks, S.A.; Cole, M.; Kelly, A.L.; Gough, L.A. Nutritional supplement use in a UK high-performance swimming club. Nutrients 2023, 15, 3306. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform 2022, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- World Aquatics. Swimming points. Available online: https://www.worldaquatics.com/swimming/points (accessed on 5 August 2023).
- Binks, M.J.; Bleakley, A.S.; Rathnayake, G.; Pizzutto, S.; Chang, A.B.; McWhinney, B.; et al. Can dried blood spots be used to accurately measure vitamin D metabolites? Clin Chim Acta 2021, 518, 70–77. [Google Scholar] [CrossRef]
- Heath, A.K.; Williamson, E.J.; Ebeling, P.R.; Kvaskoff, D.; Eyles, D.W.; English, D.R. Measurements of 25-hydroxyvitamin D concentrations in archived dried blood spots are reliable and accurately reflect those in plasma. J Clin Endocrinol Metab 2014, 99, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Man, P.W.; Heijboer, A.C.; van der Meer, I.M.; Lin, W.; Numans, M.E.; Lips, P.; Middelkoop, B.J.C.; et al. Agreement between measurement of 25-hydroxyvitamin D3 in dried blood spot samples and serum in a Chinese population in the Netherlands. J Steroid Biochem Mol Biol 2019, 195, 105472. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, R.; Allen, K.J.; Koplin, J.J.; Roche, P.; Greaves, R.F. Candidate reference method for determination of vitamin D from dried blood spot samples. Clin Chem Lab Med 2020, 58, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011, 96, 1911–1130. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and athletes. Curr Sports Med Rep 2010, 9, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, skeletal muscle function and athletic performance in athletes – a narrative review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988.
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Bernards, J.R.; Sato, K.; Haff, G.G.; Bazyler, C.D. Current research and statistical practices in sport science and a need for change. Sports 2017, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; McKenzie, J.E.; McDonald, S.; Baram, L.; Page, M.J.; Allman-Farinelli, M.; et al. Assessment of the methods used to develop vitamin D and calcium recommendations – a systematic review of bone health guidelines. Nutrients 2021, 13, 2423. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Interaction between vitamin D and calcium. Scand J Clin Lab Invest Suppl 2012, 243, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Turner, A.; Spina, C.; Decastro, S.; Chen, T.C.; Holick, M.F. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr 2004, 80, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.D.; Laing, E.M.; Hill Gallant, K.M.; Hall, D.B.; McCabe, G.P.; Hausman, D.B.; et al. A randomized trial of vitamin D₃ supplementation in children: dose-response effects on vitamin D metabolites and calcium absorption. J Clin Endocrinol Metab 2013, 98, 4816–4825. [Google Scholar] [CrossRef]
- Mazahery, H.; von Hurst, P.R. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Yousef, S.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Safety of high-dose vitamin D supplementation among children aged 0 to 6 years: a systematic review and meta-analysis. JAMA Netw Open 2022, 5, e227410. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am J Clin Nutr 2007, 85, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, M.; Hulver, M.; Eugene, E. Vitamin D practice patterns in National Collegiate Athletic Association Division I collegiate athletics programs. J Athl Train 2020, 55, 65–70. [Google Scholar] [CrossRef]
- Foo, W.L.; Faghy, M.A.; Sparks, A.; Newbury, J.W.; Gough, L.A. The effects of a nutrition education intervention on sports nutrition knowledge during a competitive season in highly trained adolescent swimmers. Nutrients 2021, 13, 2713. [Google Scholar] [CrossRef] [PubMed]
- Heaney, S.; O'Connor, H.; Michael, S.; Gifford, J.; Naughton, G. Nutrition knowledge in athletes: a systematic review. Int J Sport Nutr Exerc Metab 2011, 21, 248–261. [Google Scholar] [CrossRef]
- Philippou, E.; Middleton, N.; Pistos, C.; Andreou, E.; Petrou, M. The impact of nutrition education on nutrition knowledge and adherence to the Mediterranean Diet in adolescent competitive swimmers. J Sci Med Sport 2017, 20, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.M.; Dickson, T.; Hillier, S.; Stoner, L.; Scarrott, C.; Draper, N. A comparison of capillary, venous, and salivary cortisol sampling after intense exercise. Int J Sports Physiol Perform 2014, 9, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.K.; Gebretsadik, T.; Koestner, N.; Newman, M.S.; Liu, Z.; Carroll, K.N.; et al. Agreement of blood spot card measurements of vitamin D levels with serum, whole blood specimen types and a dietary recall instrument. PLoS One 2011, 6, e16602. [Google Scholar] [CrossRef]
| Swimmers | Age (years) | Height (m) | Body Mass (kg) | WA Points* |
|---|---|---|---|---|
| Male (n = 8) | 18 ± 2 | 1.80 ± 0.04 | 72.6 ± 8.3 | 705 ± 83 |
| Female (n = 12) | 16 ± 2 | 1.70 ± 0.09 | 62.1 ± 6.9 | 690 ± 55 |
| Combined (n = 20) | 17 ± 2 | 1.74 ± 0.09 | 66.3 ± 9.0 | 696 ± 66 |
| NONE | VITD | ||||
|---|---|---|---|---|---|
| Swimmer | Vitamin D3 dose (IU·day-1) | Δ from October (nmol·L-1) | Swimmer | Vitamin D3 dose (IU·day-1) | Δ from October (nmol·L-1) |
| 1 | 0 | -26.3 | 11 | 400 | -5.4 |
| 2 | 0 | -53.2 | 12 | 2500 | +25.0 |
| 3 | 0 | -53.0 | 13 | 4000 | +41.9 |
| 4 | 0 | -54.6 | 14 | 2000 | +9.5 |
| 5 | 0 | -23.8 | 15 | 2500 | -3.7 |
| 6 | 0 | -5.5 | 16 | 1000 | -62.9 |
| 7 | 0 | -19.9 | 17 | 2500 | +3.1 |
| 8 | 0 | +1.0 | 18 | 2500 | +52.0 |
| 9 | 0 | -14.3 | 19 | 2500 | -6.3 |
| 10 | 0 | -19.9 | 20 | 400 | -1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





