Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Data analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: The right to sight: An analysis for the global burden of disease study. Lancet Glob Health 2021, 9, e144–e160. [CrossRef]
- Krupin, T.; Feitl, M.E.; Bishop, K.I. Postoperative intraocular pressure rise in open-angle glaucoma patients after cataract or combined cataract-filtration surgery. Ophthalmology 1989, 96, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Murchison, J.F., Jr.; Shields, M.B. Limbal-based vs. fornix-based conjunctival flaps in combined extracapsular cataract surgery and glaucoma filtering procedure. Am J Ophthalmol 1990, 109, 709–715. [Google Scholar] [CrossRef]
- Vaideanu, D.; Mandal, K.; Hildreth, A.; Fraser, S.G.; Phelan, P.S. Visual and refractive outcome of one-site phacotrabeculectomy compared with temporal approach phacoemulsification. Clin Ophthalmol 2008, 2, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Li, E.Y.; Tsoi, K.K.F.; Kwong, Y.Y.; Tham, C.C. Cost-effectiveness of phacoemulsification versus combined phacotrabeculectomy for treating primary angle closure glaucoma. J Glaucoma 2017, 26, 911–922. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, Y.M.; Elhusseiny, A.M.; Albalkini, A.S.; El Sheikh, R.H.; Osman, M.A. Mitomycin c-augmented phacotrabeculectomy versus phacoemulsification in primary angle-closure glaucoma: A randomized controlled study. J Glaucoma 2019, 28, 911–915. [Google Scholar] [CrossRef]
- Francis, B.A.; Wang, M.; Lei, H.; Du, L.; Minckler, D.; Green, R.; Roland, C. Changes in axial length following trabeculectomy and glaucoma drainage device surgery. Br J Ophthalmol 2005, 89, 17–20. [Google Scholar] [CrossRef]
- Claridge, K.; Galbraith, J.; Karmel, V.; Bates, A. The effect of trabeculectomy on refraction, keratometry and corneal topography. Eye 1995, 9, 292–298. [Google Scholar] [CrossRef]
- Husain, R.; Li, W.; Gazzard, G.; Foster, P.J.; Chew, P.T.; Oen, F.T.; Phillips, R.; Khaw, P.T.; Seah, S.K.; Aung, T. Longitudinal changes in anterior chamber depth and axial length in asian subjects after trabeculectomy surgery. Br J Ophthalmol 2013, 97, 852–856. [Google Scholar] [CrossRef]
- Kook, M.S.; Kim, H.B.; Lee, S.U. Short-term effect of mitomycin-c augmented trabeculectomy on axial length and corneal astigmatism. J Cataract Refract Surg 2001, 27, 518–523. [Google Scholar] [CrossRef]
- Law, S.K.; Mansury, A.M.; Vasudev, D.; Caprioli, J. Effects of combined cataract surgery and trabeculectomy with mitomycin c on ocular dimensions. Br J Ophthalmol 2005, 89, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.W.; Lee, Y.H.; Kim, D.W.; Lee, T.; Hong, S.; Seong, G.J.; Kim, C.Y. Effect of trabeculectomy on the accuracy of intraocular lens calculations in patients with open-angle glaucoma. Clin Exp Ophthalmol 2016, 44, 465–471. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, C.E.; Park, J.H.; Seo, S.; Lee, K.W. Refractive error induced by combined phacotrabeculectomy. jkos 2018, 59, 1173–1180. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Su, C.-C.; Wang, T.-H.; Huang, J.-Y. Refractive outcomes of cataract surgery in patients receiving trabeculectomy—a comparative study of combined and sequential approaches. J Formos Med Assoc 2021, 120, 415–421. [Google Scholar] [CrossRef]
- Ong, C.; Nongpiur, M.; Peter, L.; Perera, S.A. Combined approach to phacoemulsification and trabeculectomy results in less ideal refractive outcomes compared with the sequential approach. J Glaucoma 2016, 25, e873–e878. [Google Scholar] [CrossRef]
- Tzu, J.H.; Shah, C.T.; Galor, A.; Junk, A.K.; Sastry, A.; Wellik, S.R. Refractive outcomes of combined cataract and glaucoma surgery. J Glaucoma 2015, 24, 161–164. [Google Scholar] [CrossRef]
- Chan, J.C.; Lai, J.S.; Tham, C.C. Comparison of postoperative refractive outcome in phacotrabeculectomy and phacoemulsification with posterior chamber intraocular lens implantation. J Glaucoma 2006, 15, 26–29. [Google Scholar] [CrossRef]
- Olsen, T. Calculation of intraocular lens power: A review. Acta Ophthalmol Scand 2007, 85, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.X.; Van Heerden, A.; Atik, A.; Petsoglou, C. Intraocular lens power formula accuracy: Comparison of 7 formulas. J Cataract Refract Surg 2016, 42, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Cashwell, L.F.; Martin, C.A. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology 1999, 106, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.X.; Chang, D.F. Intraocular lens power formulas, biometry, and intraoperative aberrometry: A review. Ophthalmology 2021, 128, e94–e114. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Eom, Y.; Yoon, E.G.; Choi, Y.; Song, J.S.; Jeong, J.W.; Park, S.K.; Kim, H.M. Algorithmic intraocular lens power calculation formula selection by keratometry, anterior chamber depth and axial length. Acta Ophthalmol 2022, 100, e701–e709. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Kamiya, K.; Iida, Y.; Kasahara, M.; Shoji, N. Predictability of combined cataract surgery and trabeculectomy using barrett universal ii formula. PLoS One 2022, 17, e0270363. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of intraocular lens calculation formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, M.; Huang, Y.; Chen, B.; Lam, D.S.; Congdon, N. Corneal hysteresis is correlated with reduction in axial length after trabeculectomy. Curr Eye Res 2012, 37, 381–387. [Google Scholar] [CrossRef]
- Haddad, J.S.; Rocha, K.M.; Yeh, K.; Waring, G.O.t. Lens anatomy parameters with intraoperative spectral-domain optical coherence tomography in cataractous eyes. Clin Ophthalmol 2019, 13, 253–260. [Google Scholar] [CrossRef]
- Khan, A.M.; Waldner, D.M.; Luong, M.; Sanders, E.; Crichton, A.; Ford, B.A. Stabilization of refractive error and associated factors following small incision phacoemulsification cataract surgery. BMC Ophthalmol 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Ostri, C.; Holfort, S.K.; Fich, M.S.; Riise, P. Automated refraction is stable 1 week after uncomplicated cataract surgery. Acta Ophthalmol (Copenh) 2018, 96, 149–153. [Google Scholar] [CrossRef]
- Kang, Y.S.; Sung, M.S.; Heo, H.; Ji, Y.S.; Park, S.W. Long-term outcomes of prediction error after combined phacoemulsification and trabeculectomy in glaucoma patients. BMC Ophthalmol 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Chung, J.K.; Wi, J.M.; Lee, K.B.; Ahn, B.H.; Hwang, Y.H.; Kim, M.; Jung, J.J.; Yoo, Y.C. Long-term comparison of postoperative refractive outcomes between phacotrabeculectomy and phacoemulsification. J Cataract Refract Surg 2018, 44, 964–970. [Google Scholar] [CrossRef]
| Phacotrabeculectomy (N = 48) |
Phacoemulsification (N = 48) |
P value | |
|---|---|---|---|
| Age, years | 66.1 ± 11.1 | 68.9 ± 10.0 | 0.198 a |
| Sex (Male/Female) | 19/29 | 20/28 | 0.835 b |
| AL, mm | 23.5 ± 1.5 | 23.6 ± 1.6 | 0.814 a |
| ACD, µm | 2.77 ± 0.54 | 2.99 ± 0.54 | 0.047 a |
| Average K, Diopter | 44.3 ± 1.6 | 44.4 ± 1.4 | 0.722 a |
| Preoperative IOP, mmHg | 27.6 ± 11.5 | 13.0 ± 3.9 | <0.001 a |
| ΔIOP, mmHg | 16.5 ± 12.1 | 2.1 ± 2.6 | <0.001 a |
| Prediction error, Diopter | −0.23 ± 0.59 | −0.31 ± 0.31 | 0.436 |
| Absolute prediction error, Diopter | 0.51 ± 0.37 | 0.38 ± 0.22 | 0.033 |
| Diagnosis | OAG (39.6%) ACG (60.4%) |
| Univariable analysis | Multivariable analysis (Model 1) a | Multivariable analysis (Model 2) a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Age, years | −0.001 | −0.007, 0.005 | 0.765 | ||||||
| Female (vs. male sex) | −0.062 | −0.190, 0.066 | 0.341 | ||||||
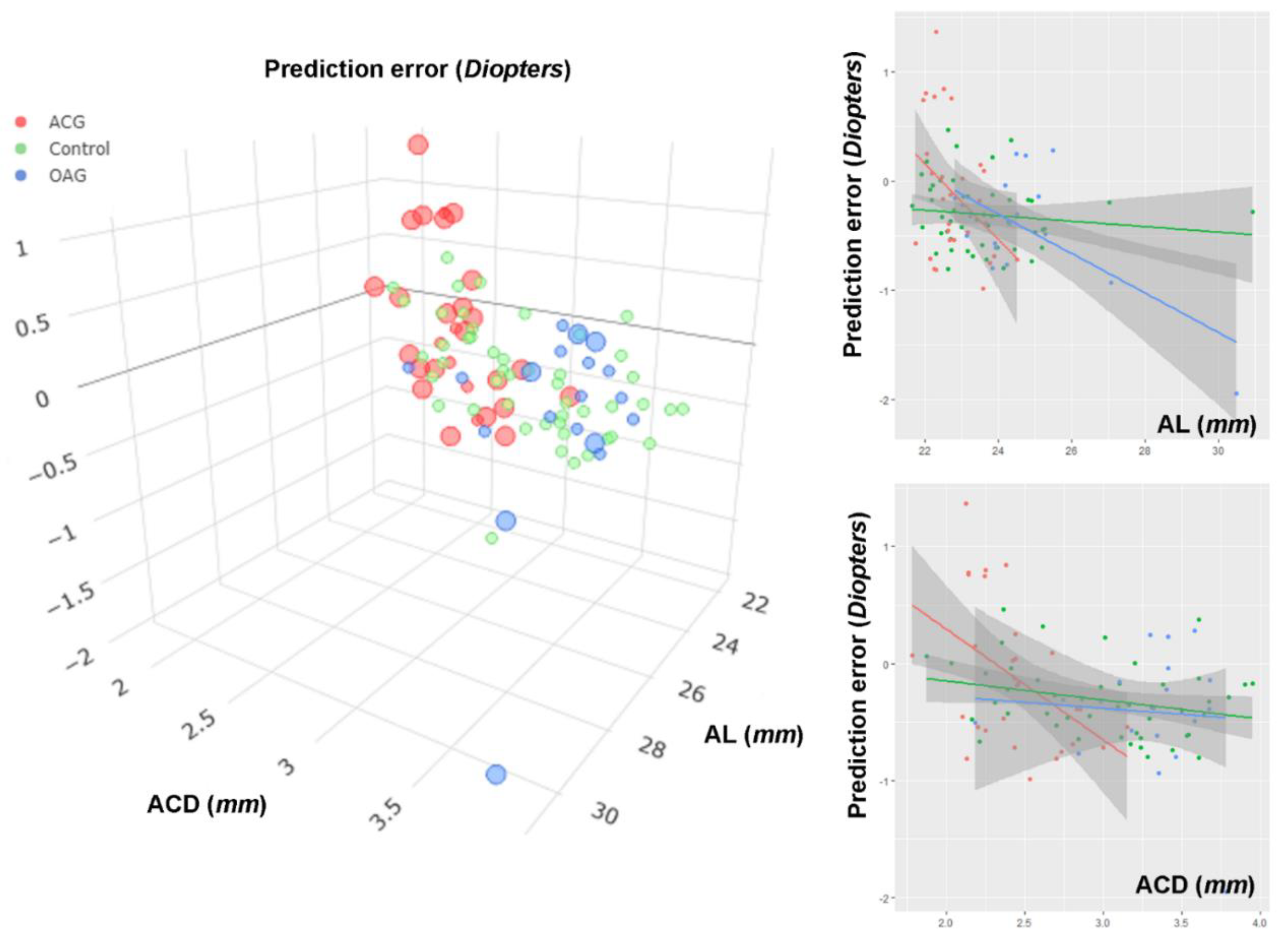
| AL, mm | 0.046 | 0.006, 0.087 | 0.026 | 0.053 | 0.013, 0.092 | 0.010 | 0.047 | 0.008, 0.087 | 0.020 |
| ACD, µm | 0.031 | −0.086, 0.148 | 0.598 | ||||||
| Average K, Diopter | −0.009 | −0.051, 0.034 | 0.694 | ||||||
| ΔIOP, mmHg | 0.006 | 0.001, 0.012 | 0.030 | 0.007 | 0.002, 0.012 | 0.012 | |||
| Phacotrabeculectomy (vs. control group) | 0.135 | 0.011, 0.258 | 0.033 | 0.138 | 0.018, 0.259 | 0.025 | |||
| Univariable analysis | Multivariable analysis (Model 1) a | Multivariable analysis (Model 2) a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age, years | 1.030 | 0.982, 1.081 | 0.220 | ||||||
| Female (vs. male sex) | 1.784 | 0.655, 4.856 | 0.257 | ||||||
| AL, mm | 0.570 | 0.341, 0.953 | 0.032 | 0.637 | 0.380, 1.067 | 0.087 | |||
| ACD, µm | 0.180 | 0.062, 0.517 | 0.001 | 0.277 | 0.091, 0.842 | 0.024 | |||
| Average K, Diopter | 1.186 | 0.862, 1.631 | 0.296 | ||||||
| ΔIOP, mmHg | 1.072 | 1.028, 1.118 | 0.001 | 1.066 | 1.021, 1.113 | 0.004 | 1.051 | 1.005, 1.099 | 0.031 |
| Univariable analysis | Multivariable analysis (Model 1) a |
Multivariable analysis (Model 2) a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Age, years | 0.007 | −0.008, 0.023 | 0.359 | ||||||
| Female (vs. male sex) | 0.402 | 0.068, 0.736 | 0.019 | 0.146 | −0.206, 0.498 | 0.407 | 0.296 | −0.063, 0.654 | 0.103 |
| AL, mm | −0.195 | −0.297, −0.094 | <0.001 | −0.213 | −0.346, −0.080 | 0.002 | |||
| ACD, µm | −0.418 | −0.720, −0.115 | 0.008 | −0.472 | −0.957, 0.013 | 0.056 | |||
| Average K, Diopter | 0.064 | −0.043, 0.171 | 0.236 | ||||||
| ΔIOP, mmHg | 0.012 | −0.002, 0.026 | 0.084 | 0.011 | −0.004, 0026 | 0.133 | 0.005 | −0.011, 0.020 | 0.556 |
| ACG (vs. OAG) | 0.294 | −0.049, 0.638 | 0.091 | −0.277 | −0.709, 0.155 | 0.203 | −0.273 | −0.814, 0.268 | 0.315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





