1. Introduction
Immune checkpoint blockade therapy has recently revolutionized the treatment and clinical outcome of several cancer types [
1]. The therapies targeting programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) have become the standard therapy for several human tumors. The therapeutic mAbs against PD-1 receptor (nivolumab and pembrolizumab), PD-L1 (atezolizumab, durvalumab, and avelumab), CTLA-4 (tremelimumab and ipilimumab) were approved by Food and Drug Administration (FDA) [
2]. These mAbs have been used in either monotherapy or combinatorial therapy with chemotherapy, radiation therapy, or other modalities [
1]. These antibodies improve the response rates, progression-free survival, and overall survival in patients with various types of cancers [
3,
4,
5].
PD-L1 (CD274) is a type I membrane protein expressed on non-lymphoid cells and a ligand of PD-1 [
6,
7]. PD-1 plays a critical role in the negative regulation of immune cells including cytotoxic T lymphocyte (CTL) and is important for antitumor immunity. CTLs are activated by recognizing presented antigens by T cell receptor (TCR)-CD3 complex [
8]. After the recognition, zeta-chain associated protein kinase (ZAP-70) is recruited to the TCR-CD3 complex and phosphorylated by lymphocyte protein tyrosine kinase, which transduces the downstream signaling [
9,
10]. In contrast, SH2-containing protein tyrosine phosphatase-2 (SHP-2) is recruited to PD-1 stimulated with PD-L1 [
11]. Since SHP-2 dephosphorylates phosphorylated ZAP-70, PD-1 can inhibit the activation signals in CTLs.
With the increase in life span in both humans and dogs, the cancer incidence has increased as well [
12]. Several naturally occurring tumors in dogs resemble human tumors in many respects. Therefore, the research on canine tumor therapy can generate knowledge that informs and prioritizes new tumor therapy in humans.
The development of dog PD-L1 (dPD-L1) mAbs has been reported [
13,
14,
15,
16,
17]. Using a canine chimeric mAb targeting PD-L1, tumor regression of undifferentiated sarcoma and oral melanoma was achieved [
14,
16,
17]. On the other hand, tumors that did not respond to the treatment also existed. Furthermore, the combination therapy of anti-dPD-L1 mAbs with hypofractionated radiotherapy is more effective to prolong overall survival [
18]. Therefore, the development of anti-dPD-L1 mAbs for diagnostic use is essential for the improvement of efficacy.
In this study, we established anti-dog PD-L1 (dPD-L1) mAbs (L1Mab-352 and L1Mab-354) by peptide immunization and showed the usefulness of the mAbs for immunohistochemical analysis in paraffin-embedded PD-L1-positive cells.
2. Materials and Methods
2.1. Preparation of cell lines
Chinese hamster ovary (CHO)-K1 and P3X63Ag8U.1 (P3U1) cells were obtained from the American Type Culture Collection (Manassas, VA).
The synthesized DNA (Eurofins Genomics KK, Tokyo, Japan) encoding signal sequence of N-terminus (
1-MRMFSVFTFMAYCHLLKA-
18) deleted dPD-L1 (dPD-L1, Accession No.: NM_001291972) was subsequently subcloned into a pCAGzeo_ssPA16 vector (IL2-signal sequence and PA16 tag added to N-terminus of construct). The amino acid sequence of the tag system was as follows: PA16 tag [
19,
20,
21,
22], sixteen amino acids (GLEGGVAMPGAEDDVV). The PA16 tag can be detected by an anti-human podoplanin mAb (clone NZ-1) [
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35]. The dPD-L1 plasmid was transfected into CHO-K1 cells, using a Neon transfection system (Thermo Fisher Scientific Inc., Waltham, MA). Stable transfectants were established through cell sorting using a cell sorter (SH800; Sony Corp., Tokyo, Japan), after which cultivation in a medium, containing 0.5 mg/mL of Zeocin (InvivoGen, San Diego, CA) was conducted.
CHO-K1, PA16-dPD-L1-overexpressed CHO-K1 (CHO/dPD-L1) and P3U1 cells were cultured in a Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc., Kyoto, Japan), with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). All cells were grown in a humidified incubator at 37oC, in an atmosphere of 5% CO2 and 95% air.
2.2. Production of hybridomas
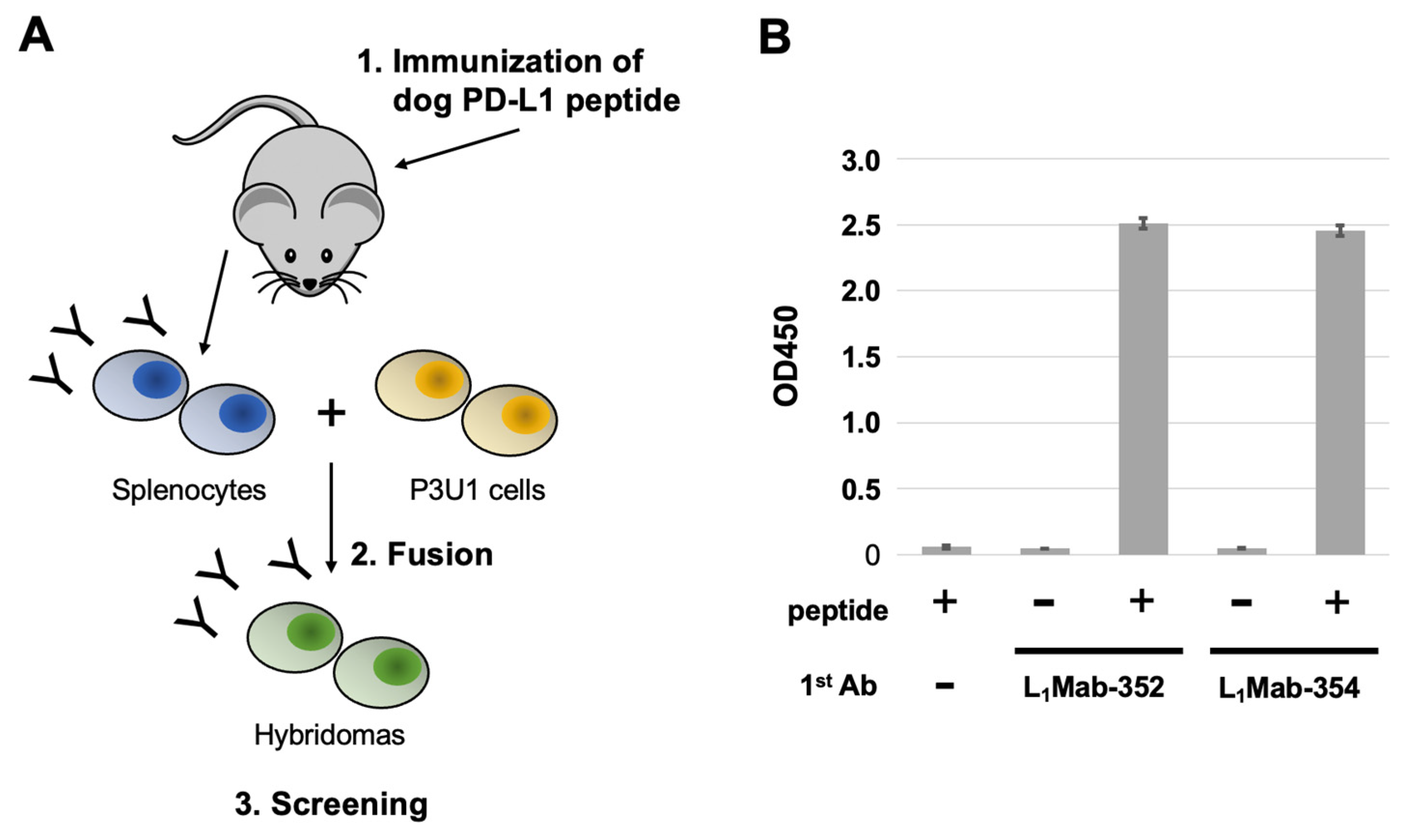
A five-week-old BALB/c mouse was purchased from CLEA Japan (Tokyo, Japan). The animal was housed under specific pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001). The dPD-L1 peptide (260-KKHGRMMDVEKC-271) and keyhole limpet hemocyanin-conjugated dPD-L1 peptide (dPD-L1 peptide-KLH) were purchased from Eurofins Japan.
To develop mAbs against dPD-L1, we intraperitoneally immunized one mouse with the dPD-L1 peptide-KLH (100 μg) plus Imject Alum (Thermo Fisher Scientific, Inc.). The procedure included three additional injections every week (100 μg), which were followed by a final booster intraperitoneal injection (100 μg), two days before harvesting splenocytes. The harvested splenocytes were subsequently fused with P3U1 cells, using PEG1500 (Roche Diagnostics, Indianapolis, IN). For the hybridoma selection, cells were cultured in the RPMI-1640 medium with 10% FBS, 100 units/mL of penicillin, 100 μg/mL of streptomycin, 0.25 μg/mL of amphotericin B, 5 μg/mL of Plasmocin, 5% Briclone (NICB, Dublin, Ireland), and hypoxanthine, aminopterin and thymidine (HAT; Thermo Fisher Scientific, Inc.). The supernatants were subsequently screened using enzyme-linked immunosorbent assay (ELISA) using the dPD-L1 peptide.
2.3. Purification of mAbs
The cultured supernatants of L1Mab-352 and L1Mab-354-producing hybridomas were filtrated with Steritop (0.22 μm, Merck KGaA, Darmstadt, Germany). The filtered supernatants were subsequently applied to 1 mL of Ab-Capcher ExTra (ProteNova, Inc., Kagawa, Japan). After washing with phosphate-buffer saline (PBS), bound antibodies were eluted with an IgG elution buffer (Thermo Fisher Scientific, Inc.), followed by immediate neutralization of eluates, using 1M Tris-HCl (pH 8.0). Finally, the eluates were concentrated, after which PBS was replaced with the elution buffer using Amicon Ultra (Merck KGaA).
2.4. ELISA
The dPD-L1 peptide was immobilized on Nunc Maxisorp 96-well immunoplates (Thermo Fisher Scientific, Inc.) at 1 μg/mL for 30 minutes at 37°C. After washing with PBS containing 0.05% Tween20 (PBST; NacalaiTesque, Inc.), wells were blocked with 1% bovine serum albumin (BSA) in PBST for 30 minutes at 37°C. Then, plates were incubated at 1 μg/mL of L1Mab-352 and L1Mab-354, followed by peroxidase-conjugated anti-mouse immunoglobulins (1:1000; Agilent Technologies, Inc., Santa Clara, CA). Finally, enzymatic reactions were conducted using the ELISA POD substrate TMB kit (Nacalai Tesque, Inc.) and stopped by the addition of 1M H2SO4. The absorbance at 450 nm was measured by using an iMark microplate reader (Bio-Rad Laboratories, Inc., Berkeley, CA).
To determine the dissociation constant (KD), L1Mab-352 and L1Mab-354 were serially diluted from 40 μg/mL to 2.4 ng/mL. The KD was calculated by fitting saturation binding curves to the built-in; one-site binding models in GraphPad PRISM 8 (GraphPad Software, Inc., La Jolla, CA).
2.5. Flow cytometric analysis
CHO-K1 and CHO/dPD-L1 cells were harvested after a brief exposure to 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA, Nacalai Tesque, Inc.). The cells were subsequently washed with 0.1% BSA in PBS and treated with 1 μg/mL NZ-1 for 30 min at 4°C. The cells were treated with 2 μg/mL Alexa Fluor 488-conjugated anti-rat IgG (Cell Signaling Technology, Inc., Danvers, MA). The fluorescence data were collected using the EC800 Cell Analyzer (Sony Corp.).
2.6. Immunohistochemical analysis of paraffin-embedded CHO/dPD-L1
Cell blocks were produced using iPGell (Genostaff Co., Ltd., Tokyo, Japan). To deparaffinize, rehydrate and retrieve antigen, the sections were autoclaved in EnVision FLEX Target Retrieval Solution, High pH (high pH; #DM828, Agilent Technologies, Inc.) at 121°C for 20 minutes. Then, sections were blocked using the Super Block T20 (PBS) Blocking Buffer (Thermo Fisher Scientific, Inc.), incubated with 50 μg/mL L1Mab-352 or L1Mab-354 for 1 h at room temperature, and treated with the Envision + Kit for mouse (Agilent Technologies, Inc.) for 30 min. Finally, color was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Agilent Technologies, Inc.) for 5 min, and counterstaining was performed using hematoxylin (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
To inhibit L1Mab-352 and L1Mab-354 binding, dPD-L1 peptide (final concentration: 20 μg/mL) was mixed with L1Mab-352 or L1Mab-354 (final concentration: 50 μg/mL). After incubation for 1.5 h at room temperature, immunohistochemical analysis was performed.
3. Results
3.1. Establishment of novel dPD-L1 mAs.
For this study, we immunized a mouse with a KLH-conjugated synthetic peptide corresponding to an intracellular region of dPD-L1. To produce hybridomas, the splenocytes from the mouse were fused with P3U1 cells by using polyethylene glycol. The wells which reacted with the synthetic peptide were selected by ELISA. After limiting dilution and additional screening, two clones L
1Mab-352 (mouse IgG
1, kappa) and L
1Mab-354 (IgG
1, kappa) were finally established (
Figure 1).
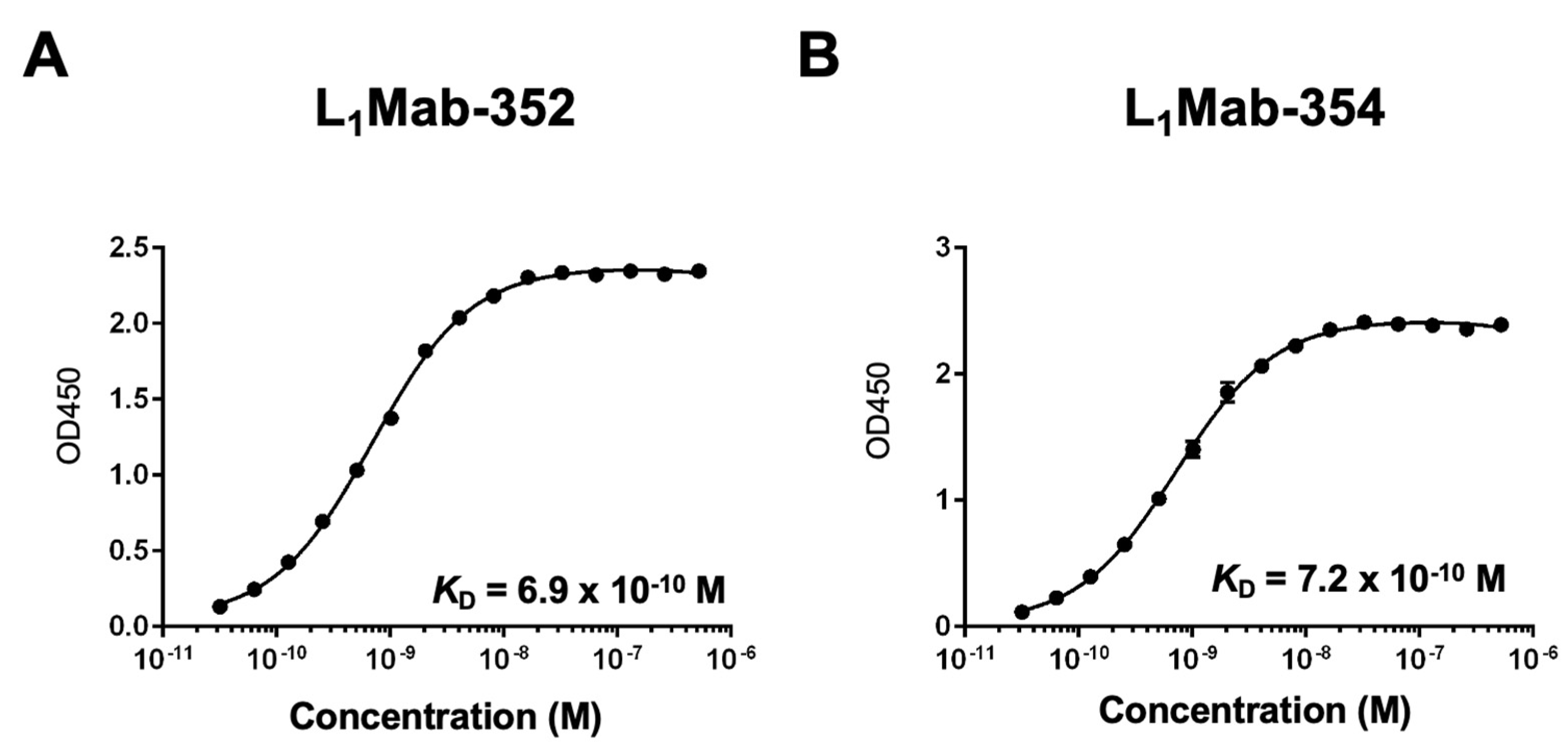
3.2. Kinetic analyses of L1Mab-352 and L1Mab-354 against the dPD-L1 peptide.
To determine the
KD of L
1Mab-352 and L
1Mab-354 with the dPD-L1 peptide, we conducted the kinetic analysis using ELISA. After immobilization of the dPD-L1 peptide on immunoplates, serially diluted L
1Mab-352 and L
1Mab-354 were added. The mean of the absorbance at 450 nm was plotted versus the concentrations of L
1Mab-352 and L
1Mab-354. The
KD values of L
1Mab-352 and L
1Mab-354 for the dPD-L1 peptide were calculated as 6.9 ×10
-10 M and 7.2 ×10
-10 M, respectively (
Figure 2).
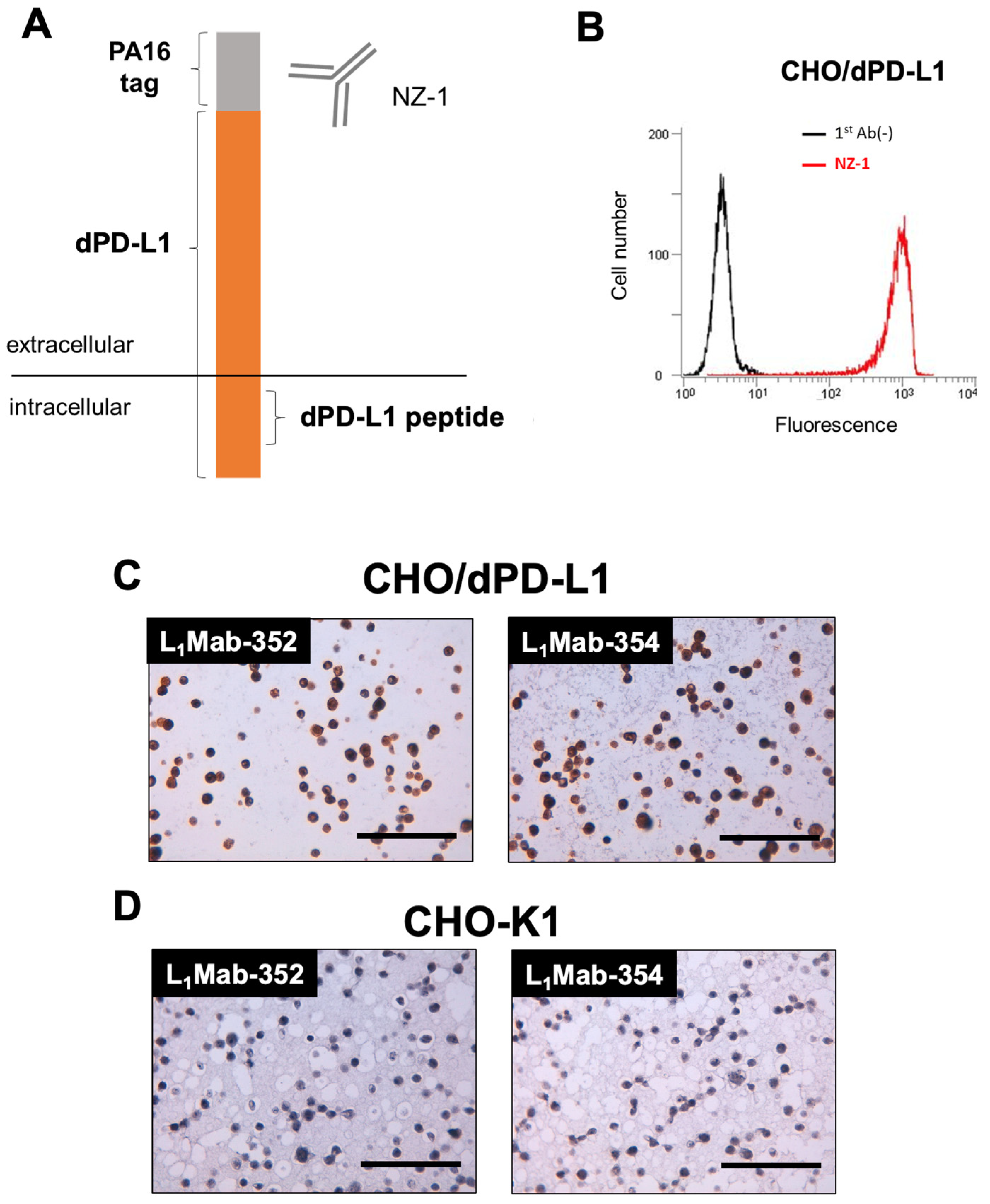
3.3. Immunohistochemical analysis of L1Mab-352 and L1Mab-354 using paraffin-embedded CHO/dPD-L1.
We next performed immunohistochemical analysis against paraffin-embedded CHO/dPD-L1 cells (
Figure 3A) using L
1Mab-352 and L
1Mab-354. We first confirmed the cell surface expression of dPD-L1 using flow cytometry (
Figure 3B). Then, we prepared the paraffin-embedded CHO-K1 and CHO/dPD-L1 cells, and stained the section using L
1Mab-352 and L
1Mab-354. As shown in
Figure 3C,D, L
1Mab-352 and L
1Mab-354 stained to the section of CHO/dPD-L1, but not that of CHO-K1. These results indicated that L
1Mab-352 and L
1Mab-354 can stain dPD-L1 in paraffin-embedded samples.
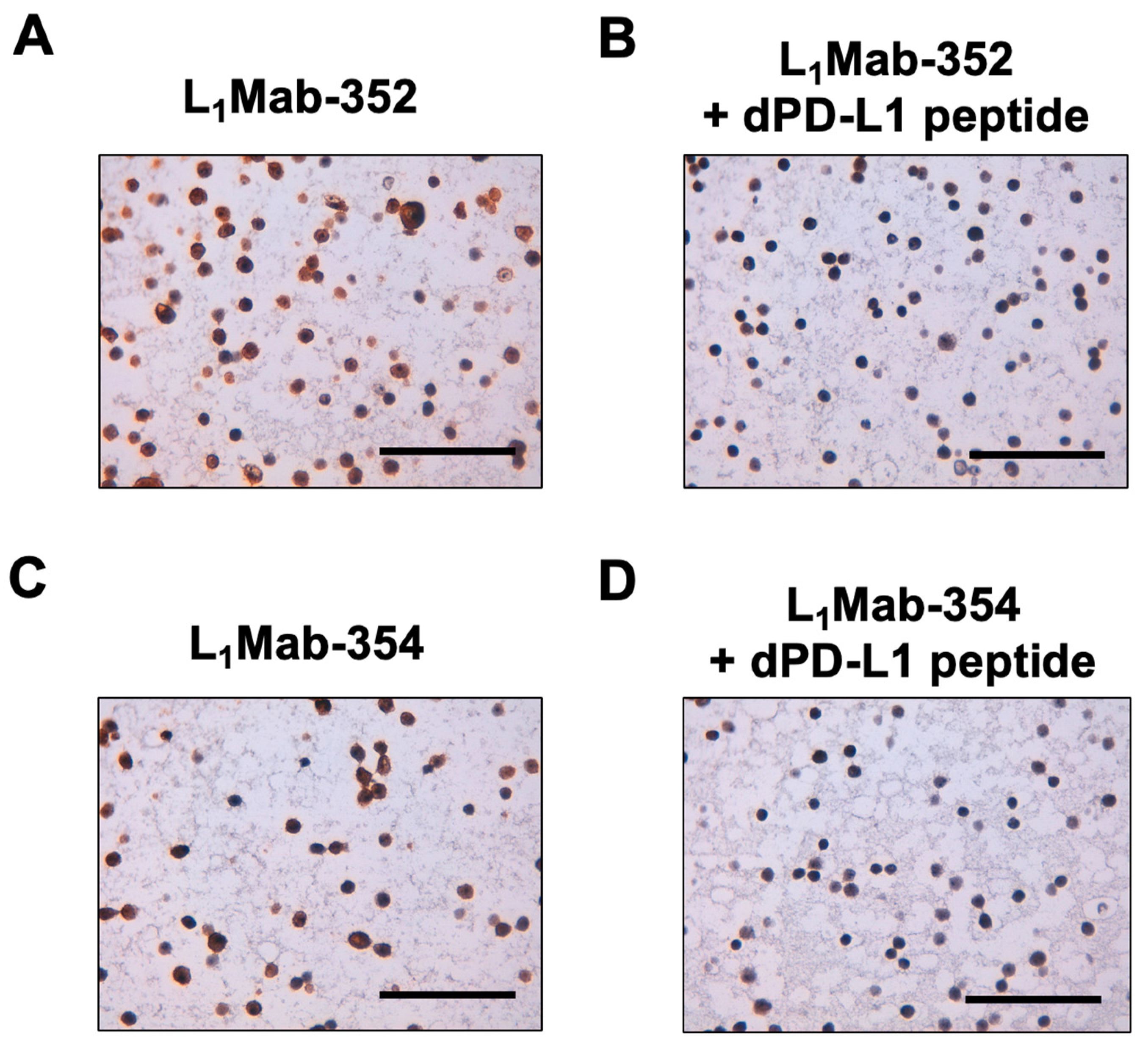
3.4. Peptide blocking of L1Mab-352 and L1Mab-354 in Immunohistochemical anayisis.
To confirm the specificity, we performed peptide blocking in the immunohistochemical analysis. As shown in
Figure 4, the reactivity of L
1Mab-352 and L
1Mab-354 was blocked in the presence of dPD-L1 peptide. This result indicated that the signals of staining with L
1Mab-352 and L
1Mab-354 are caused by the specific binding to dPD-L1.
4. Discussion
Immune checkpoint blockade therapies have improved the patients’ outcomes with various types of tumors [
36]. However, only 30% of patients receive the benefit from the therapy [
37]. Immunohistochemistry analyses to determine the PD-L1-positive tumor cells are widely validated and used as predictive biomarkers to select the patients for immune checkpoint blockade therapy [
38]. However, different diagnostic anti-PD-L1 mAb clones were approved for specific therapeutic ones by FDA. For instance, clone 22C3 has been utilized as a predictive biomarker for pembrolizumab in several cancers. A clone 28-8 has been approved as a complementary assay for nivolumab. Each clone has a different cut-off point and cancer-specific scoring algorithm [
2]. Both 22C3 and 28-8 recognize the extracellular domain of PD-L1, and exhibit similar subcellular patterns of PD-L1 expression in tumor and immune cells [
2]. However, different staining patterns by 22C3 and 28-8 are also reported [
39].
In this study, we developed novel dPD-L1 mAbs (L
1Mab-352 and L
1Mab-354) using peptide immunization of the intracellular domain, and showed the usefulness for immunohistochemical analysis in paraffin-embedded dPD-L1-positive cells (
Figure 3 and
Figure 4). Further studies are essential to show whether L
1Mab-352 and L
1Mab-354 apply to formalin-fixed paraffin-embedded canine tumors. An anti-dPD-L1 mAb (6C11-3A11) which recognizes the extracellular domain of dPD-L1 was reported to apply to immunohistochemistry [
17]. The comparison of the staining pattern of mAbs targeting dPD-L1 extracellular and intracellular domains could provide supportive information in human cancer diagnosis.
Currently, several anti-dPD-L1 mAbs have been developed for canine tumor therapy [
16,
17,
40]. Like human tumors, standardization of PD-L1 immunohistochemistry will be required in the future.
Author Contributions
T.O. and T.T. performed the experiments. M.K.K. and Y.K. designed the experiments. T.O., H.S., and Y.K. analyzed the data. T.O. and H.S. wrote the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work is appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Japan Agency for Medical Research and Development (AMED) under grant nos. JP22ama121008 (to Y.K.), JP22am0401013 (to Y.K.), 23bm1123027h0001 (to Y.K.), JP22ck0106730 (to Y.K.), and JP21am0101078 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI), grant nos. 21K20789 (to T.T.), 22K06995 (to H.S.), 21K07168 (to M.K.K.), and 22K07224 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Alturki, N.A. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Vranic, S.; Gatalica, Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol Biomed 2023, 23, 15–25. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol 2005, 6, 223. [Google Scholar] [CrossRef]
- Kreileder, M.; Barrett, I.; Bendtsen, C.; Brennan, D.; Kolch, W. Signaling Dynamics Regulating Crosstalks between T-Cell Activation and Immune Checkpoints. Trends Cell Biol 2021, 31, 224–235. [Google Scholar] [CrossRef]
- Chan, A.C.; Irving, B.A.; Fraser, J.D.; Weiss, A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A 1991, 88, 9166–9170. [Google Scholar] [CrossRef]
- Weiss, A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell 1993, 73, 209–212. [Google Scholar] [CrossRef]
- Sheppard, K.A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 2004, 574, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Baioni, E.; Scanziani, E.; Vincenti, M.C.; Leschiera, M.; Bozzetta, E.; Pezzolato, M.; Desiato, R.; Bertolini, S.; Maurella, C.; Ru, G. Estimating canine cancer incidence: findings from a population-based tumour registry in northwestern Italy. BMC Vet Res 2017, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS One 2016, 11, e0157176. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci Rep 2017, 7, 8951. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Withers, S.S.; Chang, H.; Spanier, J.A.; De La Trinidad, V.L.; Panesar, H.; Fife, B.T.; Sciammas, R.; Sparger, E.E.; Moore, P.F.; et al. Development of canine PD-1/PD-L1 specific monoclonal antibodies and amplification of canine T cell function. PLoS One 2020, 15, e0235518. [Google Scholar] [CrossRef]
- Igase, M.; Nemoto, Y.; Itamoto, K.; Tani, K.; Nakaichi, M.; Sakurai, M.; Sakai, Y.; Noguchi, S.; Kato, M.; Tsukui, T.; et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci Rep 2020, 10, 18311. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis Oncol 2021, 5, 10. [Google Scholar] [CrossRef]
- Deguchi, T.; Maekawa, N.; Konnai, S.; Owaki, R.; Hosoya, K.; Morishita, K.; Nakamura, M.; Okagawa, T.; Takeuchi, H.; Kim, S.; et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Kaneko, M.K.; Kato, Y. Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C(44)Mab-5. Biochem Biophys Rep 2018, 14, 64–68. [Google Scholar] [CrossRef]
- Tamura, R.; Oi, R.; Akashi, S.; Kaneko, M.K.; Kato, Y.; Nogi, T. Application of the NZ-1 Fab as a crystallization chaperone for PA tag-inserted target proteins. Protein Sci 2019, 28, 823–836. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsunaga, Y.; Arimori, T.; Kitago, Y.; Ogasawara, S.; Kaneko, M.K.; Kato, Y.; Takagi, J. Tailored placement of a turn-forming PA tag into the structured domain of a protein to probe its conformational state. J Cell Sci 2016, 129, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; Nogi, T.; Kato, Y.; Takagi, J. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014, 95, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kaneko, M.K.; Kuno, A.; Uchiyama, N.; Amano, K.; Chiba, Y.; Hasegawa, Y.; Hirabayashi, J.; Narimatsu, H.; Mishima, K.; et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem Biophys Res Commun 2006, 349, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Chalise, L.; Kato, A.; Ohno, M.; Maeda, S.; Yamamichi, A.; Kuramitsu, S.; Shiina, S.; Takahashi, H.; Ozone, S.; Yamaguchi, J.; et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Delta combination therapy against glioblastoma. Mol Ther Oncolytics 2022, 26, 265–274. [Google Scholar] [CrossRef]
- Ishikawa, A.; Waseda, M.; Ishii, T.; Kaneko, M.K.; Kato, Y.; Kaneko, S. Improved anti-solid tumor response by humanized anti-podoplanin chimeric antigen receptor transduced human cytotoxic T cells in an animal model. Genes Cells 2022, 27, 549–558. [Google Scholar] [CrossRef]
- Tamura-Sakaguchi, R.; Aruga, R.; Hirose, M.; Ekimoto, T.; Miyake, T.; Hizukuri, Y.; Oi, R.; Kaneko, M.K.; Kato, Y.; Akiyama, Y.; et al. Moving toward generalizable NZ-1 labeling for 3D structure determination with optimized epitope-tag insertion. Acta Crystallogr D Struct Biol 2021, 77, 645–662. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Ohishi, T.; Nakamura, T.; Inoue, H.; Takei, J.; Sano, M.; Asano, T.; Sayama, Y.; Hosono, H.; Suzuki, H.; et al. Development of Core-Fucose-Deficient Humanized and Chimeric Anti-Human Podoplanin Antibodies. Monoclon Antib Immunodiagn Immunother 2020, 39, 167–174. [Google Scholar] [CrossRef]
- Abe, S.; Kaneko, M.K.; Tsuchihashi, Y.; Izumi, T.; Ogasawara, S.; Okada, N.; Sato, C.; Tobiume, M.; Otsuka, K.; Miyamoto, L.; et al. Antitumor effect of novel anti-podoplanin antibody NZ-12 against malignant pleural mesothelioma in an orthotopic xenograft model. Cancer Sci 2016, 107, 1198–1205. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Abe, S.; Ogasawara, S.; Fujii, Y.; Yamada, S.; Murata, T.; Uchida, H.; Tahara, H.; Nishioka, Y.; Kato, Y. Chimeric Anti-Human Podoplanin Antibody NZ-12 of Lambda Light Chain Exerts Higher Antibody-Dependent Cellular Cytotoxicity and Complement-Dependent Cytotoxicity Compared with NZ-8 of Kappa Light Chain. Monoclon Antib Immunodiagn Immunother 2017, 36, 25–29. [Google Scholar] [CrossRef]
- Ito, A.; Ohta, M.; Kato, Y.; Inada, S.; Kato, T.; Nakata, S.; Yatabe, Y.; Goto, M.; Kaneda, N.; Kurita, K.; et al. A Real-Time Near-Infrared Fluorescence Imaging Method for the Detection of Oral Cancers in Mice Using an Indocyanine Green-Labeled Podoplanin Antibody. Technol Cancer Res Treat 2018, 17, 1533033818767936. [Google Scholar] [CrossRef]
- Shiina, S.; Ohno, M.; Ohka, F.; Kuramitsu, S.; Yamamichi, A.; Kato, A.; Motomura, K.; Tanahashi, K.; Yamamoto, T.; Watanabe, R.; et al. CAR T Cells Targeting Podoplanin Reduce Orthotopic Glioblastomas in Mouse Brains. Cancer Immunol Res 2016, 4, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, T.; Yoneda, K.; Mori, M.; Kanayama, M.; Kuroda, K.; Kaneko, M.K.; Kato, Y.; Tanaka, F. Detection of Circulating Tumor Cells (CTCs) in Malignant Pleural Mesothelioma (MPM) with the "Universal" CTC-Chip and An Anti-Podoplanin Antibody NZ-1. 2. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kaneko, M.K.; Kunita, A.; Ito, H.; Kameyama, A.; Ogasawara, S.; Matsuura, N.; Hasegawa, Y.; Suzuki-Inoue, K.; Inoue, O.; et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci 2008, 99, 54–61. [Google Scholar] [CrossRef]
- Kato, Y.; Vaidyanathan, G.; Kaneko, M.K.; Mishima, K.; Srivastava, N.; Chandramohan, V.; Pegram, C.; Keir, S.T.; Kuan, C.T.; Bigner, D.D.; et al. Evaluation of anti-podoplanin rat monoclonal antibody NZ-1 for targeting malignant gliomas. Nucl Med Biol 2010, 37, 785–794. [Google Scholar] [CrossRef]
- Kubli, S.P.; Berger, T.; Araujo, D.V.; Siu, L.L.; Mak, T.W. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov 2021, 20, 899–919. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Akhtar, M.; Rashid, S.; Al-Bozom, I.A. PD-L1 immunostaining: what pathologists need to know. Diagn Pathol 2021, 16, 94. [Google Scholar] [CrossRef]
- Rimm, D.L.; Han, G.; Taube, J.M.; Yi, E.S.; Bridge, J.A.; Flieder, D.B.; Homer, R.; West, W.W.; Wu, H.; Roden, A.C.; et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017, 3, 1051–1058. [Google Scholar] [CrossRef]
- Oh, W.; Kim, A.M.J.; Dhawan, D.; Kirkham, P.M.; Ostafe, R.; Franco, J.; Aryal, U.K.; Carnahan, R.H.; Patsekin, V.; Robinson, J.P.; et al. Development of an Anti-canine PD-L1 Antibody and Caninized PD-L1 Mouse Model as Translational Research Tools for the Study of Immunotherapy in Humans. Cancer Res Commun 2023, 3, 860–873. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).