1. Introduction
The spinal cord (SC) plays a crucial role in the system of the antigravity balance regulation. After the method of the electrical stimulation of the SC began to be actively used in the studies of the locomotion and postural regulation [
1], it became clear that much is still unknown about the involvement of the spinal networks in the postural control. In the experiments on the decerebrated cats, it was shown that the spinal networks can completely control the body weight support in the absence of supraspinal influences in the case of the stimulation of the L5 spinal segment [
2]. The results of a study in subjects who were unable to stand due to severe SC trauma show that with transcutaneous electrical stimulation (tES) of the SC at the level of the T11 or L1 vertebrae, all patients (n = 15) could independently control the vertical posture with the minimal external support of the knees or pelvis, and some of them (n = 7) -- without any support [
3]. Thus, in the absence of supraspinal influences, when the possibilities of the regulation by sensory systems and processes occurring in the brain are limited, activation of the spinal networks of the SC by stimulation of the lumbar thickening of the SC makes it possible to effectively control the vertical posture and support the body weight.
Normally, the maintenance of upright standing is a complex process that relies on the combined activation of different muscle groups. The use of tES of the SC in studies of the physiological mechanisms of postural maintenance has a significant advantage - this method allows non-invasive activation of SC networks, including those in normal subjects, i.e. to study SC neural networks in humans.
Human standing control solves two tasks simultaneously: one is to provide and distribute tonic muscle activity to stabilize body segments ("posture"), the other is to neutralize internal or external perturbations of body segments ("equilibrium") [
4]. The “posture” task is mainly performed by the extensor muscles, which are mainly composed of slow tonic fibers, and the “equilibrium” task, such as locomotion, is mainly performed by the flexor muscles, which are mainly composed of fast fibers. Within the lumbar thickening of the human spinal cord, the flexor and extensor nuclei are anatomically separated [
5], allowing tES to be used for targeted control of both flexors and extensors. Recent research has shown that tES at the L1-L2 vertebral level activates lower limb extensors during stepping [
6]. Thus, tES at the L1-L2 level may also affect the vertical postural stability via the changes in tonic extensor activity.
The stability of an individual's upright standing relies on the complex interaction of exteroceptive and interoceptive afferent streams and is significantly influenced by cognitive styles [
7,
8]. Two cognitive styles have been identified - field-dependent (FD) and field-independent (FI). Individuals of the FD style show "en bloc" oscillations of the head-shoulder–hip unit in the absence of vision or visual stimuli [
9]. Under the same conditions, in FI subjects, each body segment contributes independently to maintaining the upright posture. Thus, the activation of the flexor and extensor muscles of the legs in the FD and FI subjects is likely to have different effects on the vertical posture indicators.
We aimed to investigate the effect of tES-induced activation of the lower limb extensors on vertical postural stability in normal subjects. Because postural regulation is different in subjects with different cognitive styles, the effects of stimulation on FD and FI subjects will be analyzed separately.
Stimulation at the level of the L1-L2 vertebrae during stepping has been associated with increased coactivation of the leg muscles during the stance phase [
10]. Agonist-antagonist muscle coactivation is associated with the joint stiffness [
11]. If tES increases muscle coactivation (joint stiffness) as well as joint coordination while maintaining vertical posture differently in FD and FI subjects, then the effect of tES on vertical position will be different in FD and FI subjects.
2. Materials and Methods
The procedures and studies were performed in accordance with the tenets of the Declaration of Helsinki and approved by the Ethics Committee of the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences (Minutes # 1-02 dated 2023/02/01). All participants gave written informed consent.
2.1. Participants
The study included 16 volunteers (7 males, 9 females, 25 ± 5 years). The participants’ body mass index was 21.2 ± 2.5 kg/m
2. All participants rated themselves as healthy on the day of the study. All participants had right leg dominance as determined by the ball kick test [
12].
2.2. Procedure and Task
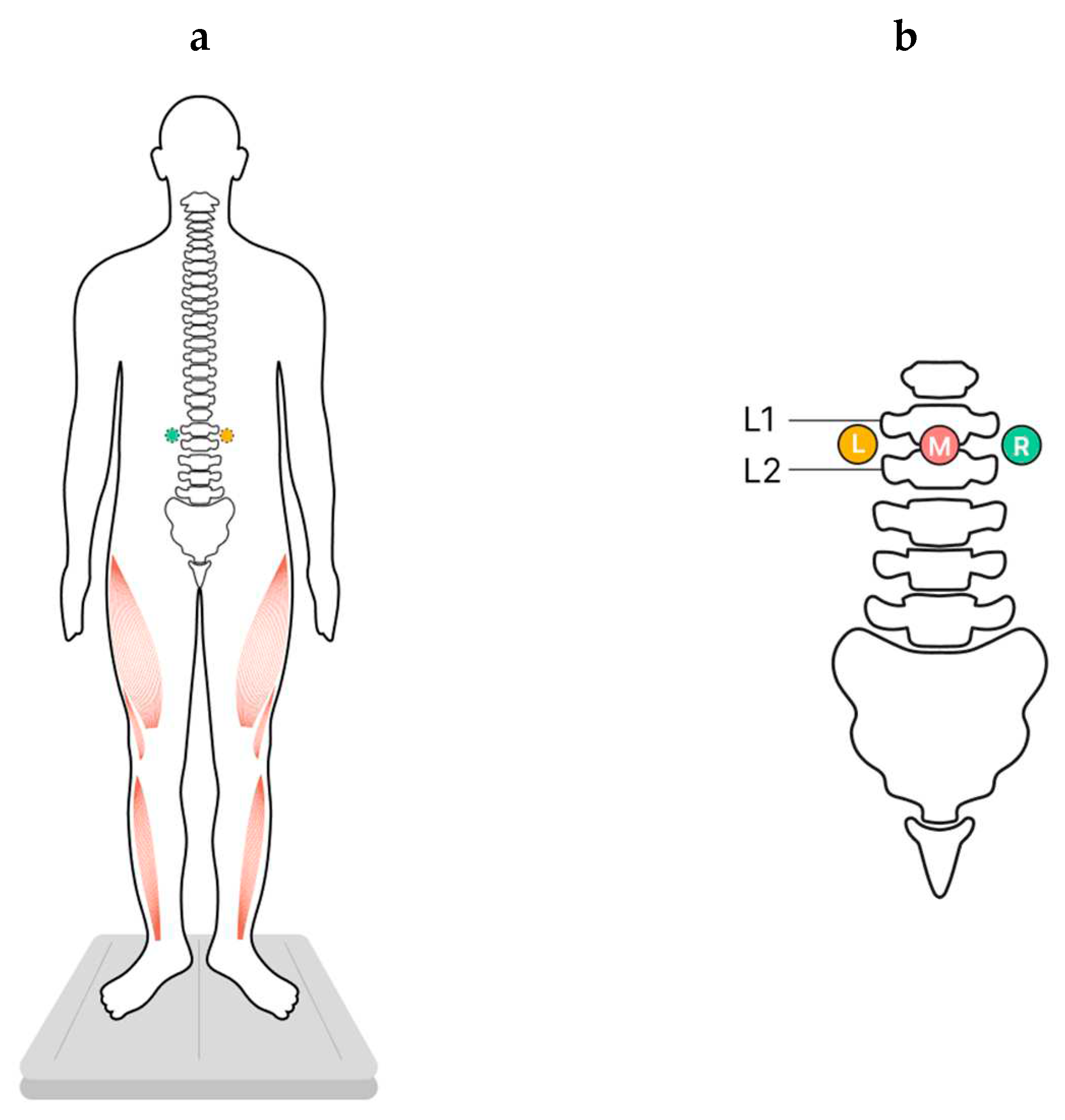
Changes in vertical posture were determined by stabilometry when participants stood on a force platform in the center of a soundproof anechoic chamber. The upright posture was standard (heels together, toes apart, hands down along the body) (
Figure 1a).
Participants were instructed to maintain the upright posture with their eyes closed in four experimental conditions. Three of these conditions involved tES of the spinal cord, applied at one of these loci: in the midline between the L1-L2 vertebrae and over the left or right dorsal roots of the spinal cord at the same level (
Figure 1b). The fourth condition consisted of standing without tES and served as a control. Position of the participant’s center of pressure (CoP) was recorded for each of the four experimental conditions.
Each recording lasted 70 sec. To determine the effect of tES on posture we analyzed the interval between 30 sec and 60 sec. Stimulation continued for 70 sec. We excluded the preceding 30 sec and the following 10 sec to avoid the influence of the on-stimulation effect and the effect of the waiting for the end of the recording. Breaks of 2-3 min were allowed between recordings to minimize fatigue. Participants were allowed to step off or rest on the force platform between recordings.
The order of the four recordings was randomized. After a short break (≤ 5 min), another random order of these four recordings followed. Each set of these four conditions was considered as an independent series, as a test and a retest. Thus, we obtained two measurements for each participant in all experimental conditions.
To prevent the possibility of voluntary or unconscious effort during the tES, participants were given a cognitive distraction task, which was to silently subtract a two-digit number from a three-digit-number [
7]. Mental arithmetic was also performed during the control recordings.
Cognitive style of the participants was determined using the Group Embedded Figures Test [
8]. This pencil-and-paper test is the most commonly used test of field dependence-independence [
13]. Those participants who scored < 2.5 on the test were classified as FD, and those who scored > 2.5 – as FI.
2.3. Stabilometry
The Stabilan-01-2 force plate system (Rhythm Ltd., Russia) with the StabMed 2.13 software was used for stabilometry [
14]. The system recorded the CoP positions with a sampling frequency of 50 Hz and a resolution of < 0.01 mm.
2.4. Transcutaneous Electrical Stimulation of the Spinal Cord and Dorsal Roots
Neostym-5 (Cosyma Ltd, Russia) was used for the tES. Stimulation was administered at a frequency of 20 Hz with monopolar modulated current pulses (1 ms, 5 kHz).
Adhesive cathodes (ø 2.5 cm, ValuTrode® Axelgaard Manufacturing Co.) were attached to the skin of the back: one was placed along the midline between the L1-L2 vertebrae (midline stimulation), and two were placed below and ~ 1.5 cm to the left and right of the midline electrode (along the dorsal roots of the spinal cord between the L1-L2 vertebrae) (
Figure 1b). Two adhesive anodes (5 * 10 cm
2, ValuTrode® Axelgaard Manufacturing Co.) were placed symmetrically above the iliac crests.
The intensity of the tES was adjusted to a maximum level that did not cause any pain or discomfort. The current intensity was selected for each tES site.
2.5. Analysis
CoP parameters, including the length of the CoP trajectory along the sagittal and frontal axes, the root mean square deviation (RMSD) of the CoP along the frontal and sagittal axes, and the area of the confidence ellipse (i.e., the ellipse area) were calculated (formulas for calculation in
Table S1). Increased values of these dependent parameters indicate decreased postural control, while decreased parameters indicate increased postural control.
Statistical analysis of CoP parameters was performed using Statistica v.10.A software package. The Shapiro-Wilk W test was used to determine whether the data followed a normal distribution. Where not all data were normally distributed, non-parametric statistics were used.
Values are presented as mean ± standard deviation or median [1st quartile (Q1), 3rd quartile (Q3)] depending on the data distribution. Changes in CoP parameters between control and experimental conditions are expressed as percentages: (experimental condition/control condition) * 100%.
The significance of differences between experimental conditions was determined using the Wilcoxon test. The significance of differences between parameters of the FI and FD participants was calculated using the Mann–Whitney U test.
3. Results
3.1. Cognitive Style. CoP parameters without tES
Of the 16 participants, eight were classified as FI and eight as FD subjects.
Three of the CoP parameters differed significantly between FI and FD participants in the control condition (
Table 1). Ellipse area, RMSD along the frontal and sagittal axes were ~2.5 (p = 0.01), 1.9 (p = 0.02) and 1.3 times (p = 0.03) higher in the FD participants than in the FI participants, respectively.
3.2. Current Intensities
Current intensities for the tES ranged from 10 to 57 mA. Intensities did not differ significantly for FD and FI participants (
Table 2).
3.3. CoP Parameters in Experimental Conditions
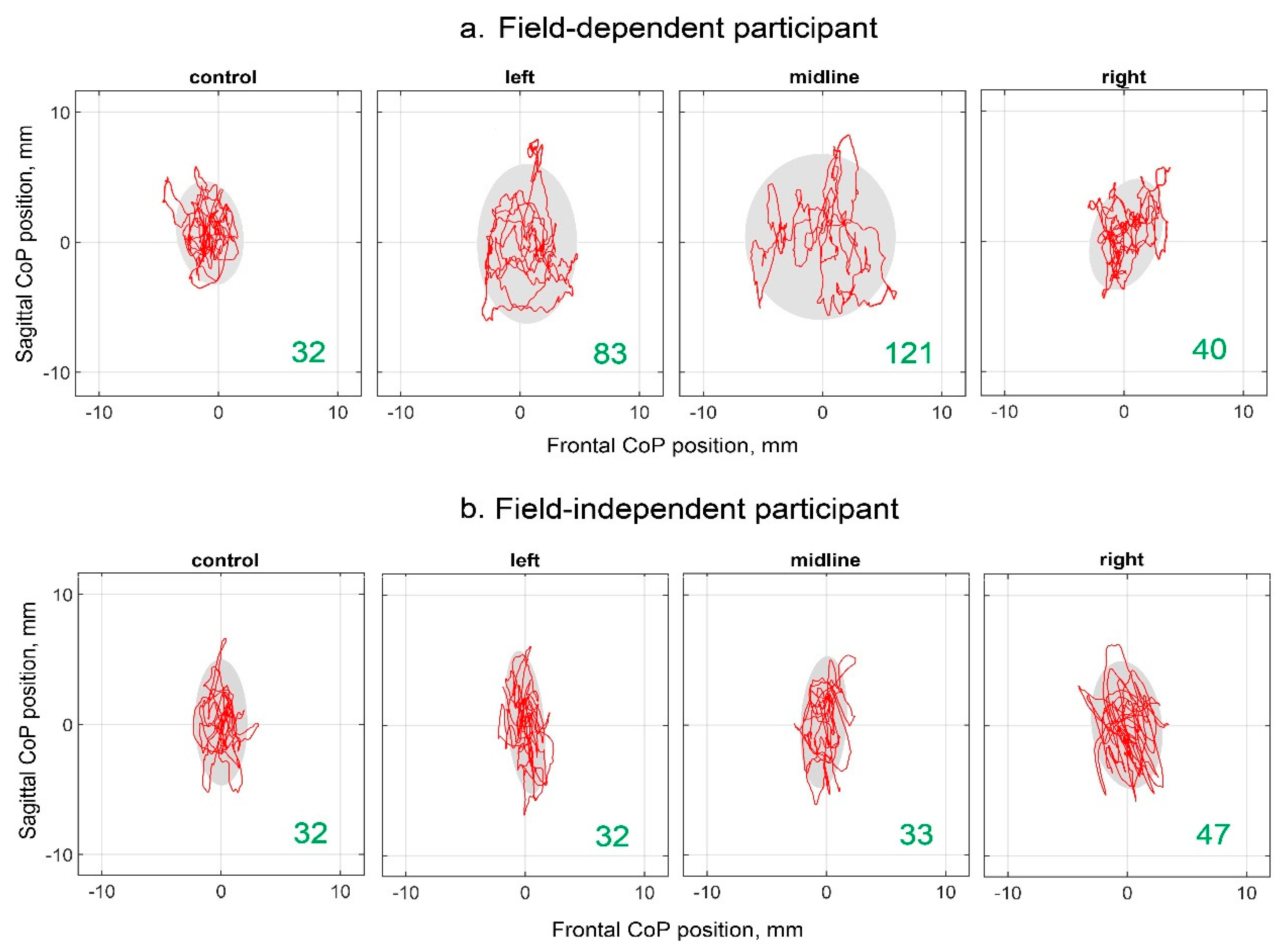
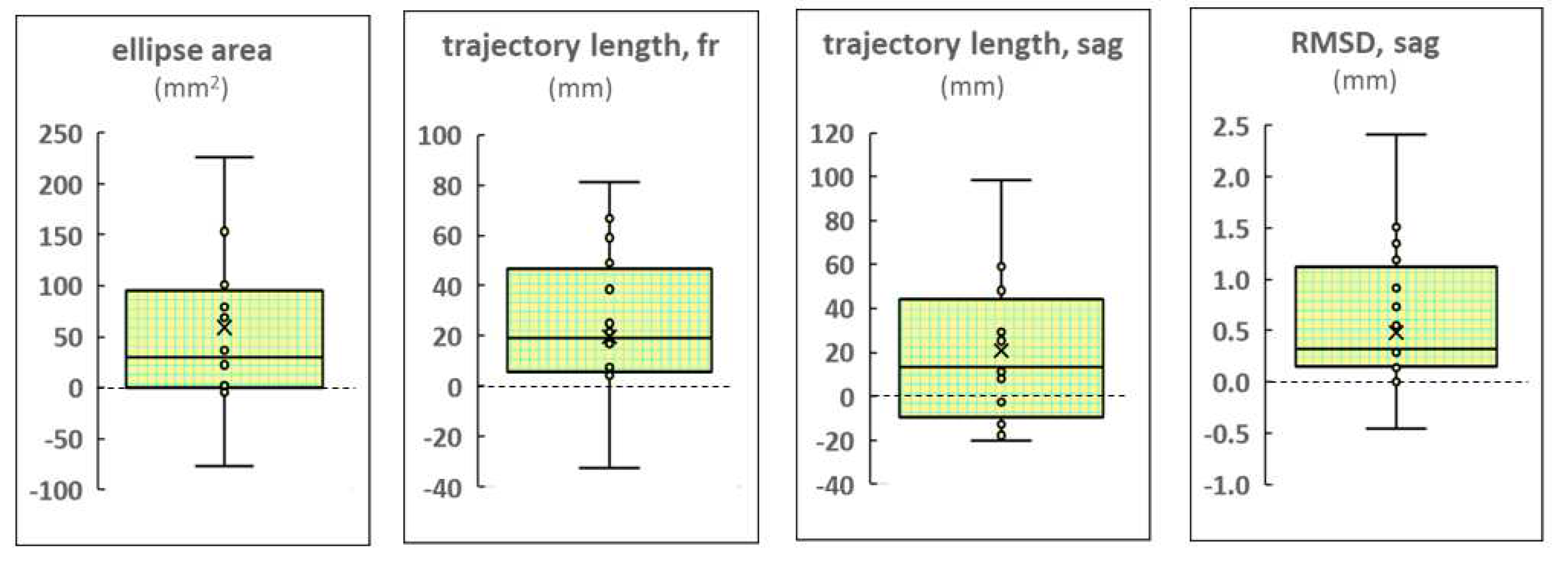
Examples of the CoP trajectories of the FD and FI participants in the different experimental conditions are shown in
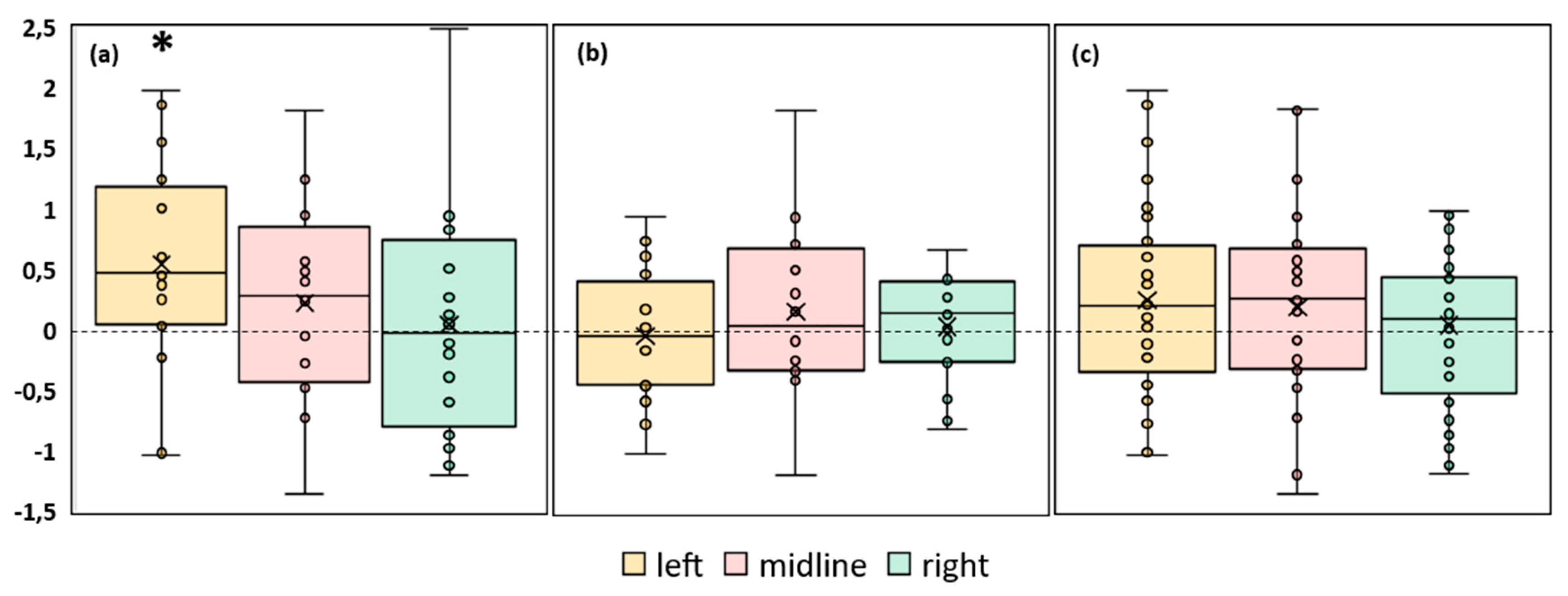
Figure 2. The increase in ellipse area with stimulation of the left dorsal root and midline at the level of the L1 vertebra relative to the control is clearly visible in the plots of the FD participant. This effect is not present in the plots of the FI participants.
3.3.1. Length of the CoP Trajectory
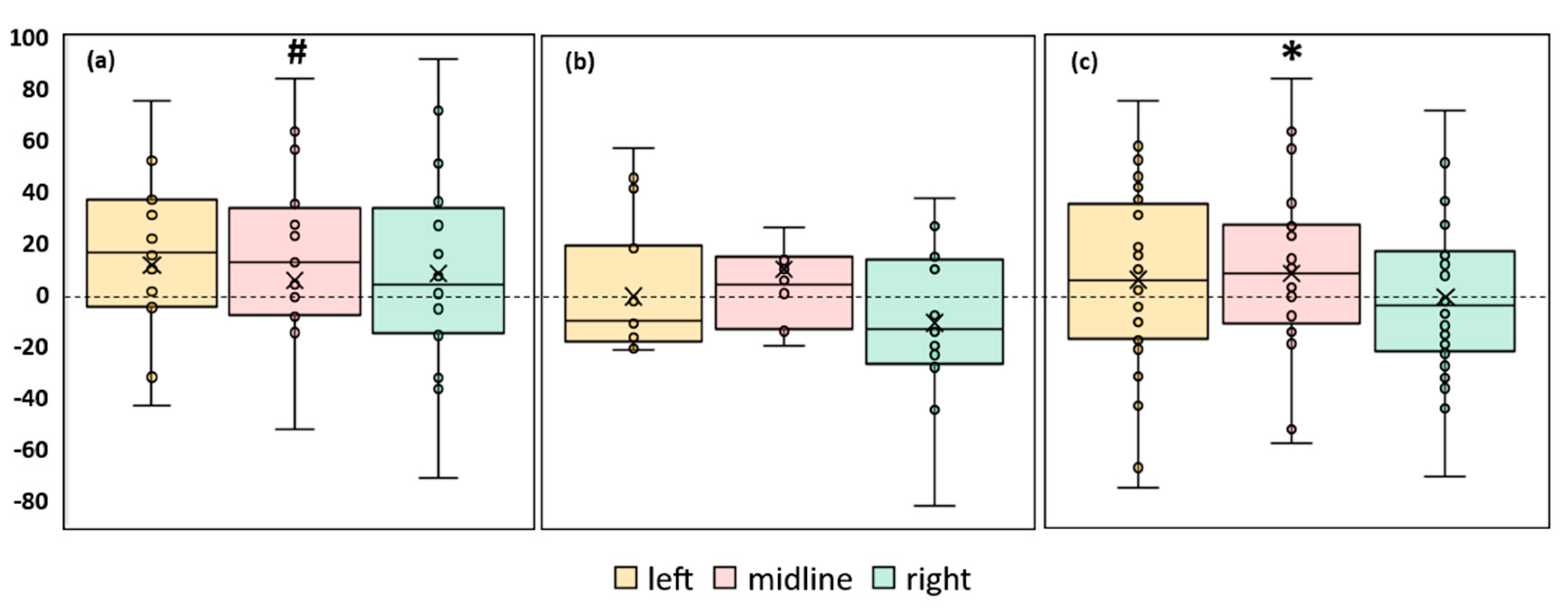
An analysis of the CoP trajectory length along the frontal axis of the FD participants showed a tendency to increase for 23% (p = 0.06) during the midline tES compared to the control condition (
Figure 3a,
Table S2). In the FI participants there were no significant changes between the values of this parameter in the control and stimulation conditions (
Figure 3b,
Table S2). The analysis of this CoP parameter in the combined group of participants revealed an increase of 8% (p = 0.04) during the midline tES compared to the control (
Figure 3c,
Table S2).
The length of the CoP trajectory along the sagittal axis in both FI and FD participants did not show significant changes under the stimulation conditions compared to the control. A similar result was obtained in the combined group.
3.3.2. Ellipse Area
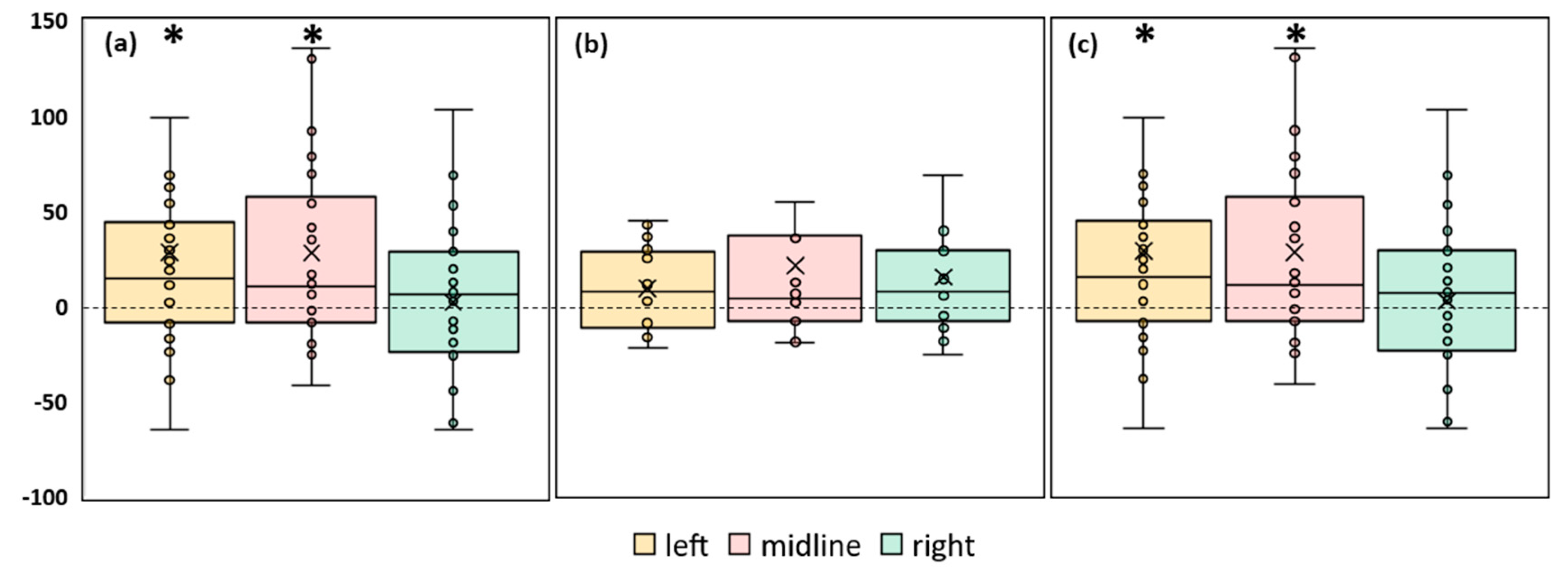
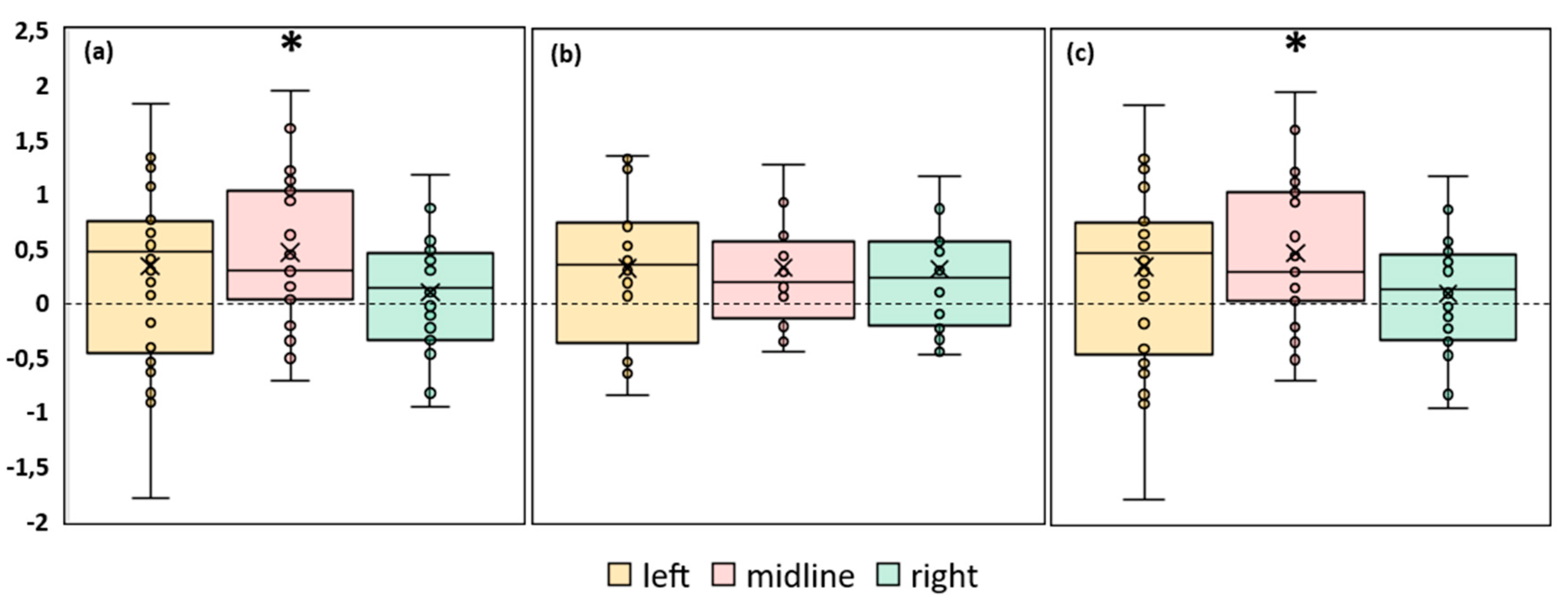
A significant increase in ellipse area during stimulation was observed in FD participants. The left roots and midline tES increased the ellipse area by ~30% (p = 0.02 and p = 0.04, respectively) (
Figure 4a,
Table S3). No significant changes in ellipse area were observed in FI participants (
Figure 4b,
Table S3). In the combined group this CoP parameter significantly increased by 23% during tES of the left root (p = 0.01) and by 27% during midline stimulation (p = 0.02) (
Figure 4c,
Table S3).
3.3.3. RMSD of the CoP
The significant 18% increase in RMSD along the frontal axis (p = 0.02) was observed in FD participants during tES of the left roots (
Figure 5a,
Table S4). No significant changes in this parameter were observed during stimulation in the FI participants and in the combined group (
Figure 5b,c,
Table S4).
The RMSD along the sagittal axis increased by 15% (p = 0.01) during the midline tES in FD participants (
Figure 6a,
Table S5). There were no significant changes in this RMSD in FI participants (
Figure 6b,
Table S5). In the combined group the parameter increased by 12% (p = 0.008) during the midline tES (
Figure 6c,
Table S5).
3.3.4. Difference in CoP Paramaters between Left and Right tES
In the FD participants, most of the CoP parameters obtained during tES of the left roots were significantly greater than those obtained during the stimulation of the right root (
Figure 7). The ellipse area was greater by 37% (p < 0.02), the RMSD along the sagittal axis - by 13% (p < 0.03), the trajectory length along the frontal axis - by 13% (p < 0.03) (Tables S3, S5, S2, respectively), and the trajectory length along the sagittal axis by 10% (p < 0.04). This effect was absent in FI participants and in the combined group.
4. Discussion
4.1. Differences in Postural Control Responses to Spinal tES in Patients with Motor Disorders and Healthy Subjects
Previously, electrical stimulation of the cervical and lumbar segments of the spinal cord combined with multi-session activity-based training has been shown to rehabilitate voluntary movements and independent body weight support in patients with severe spinal cord injury [
3,
15,
16]. Acute spinal tES improved postural stability in 11 of 12 patients (ages 2-50 years) with cerebral palsy [
17]. In subjects with multiple sclerosis, postural stability was improved during tES of the spinal cord when standing with eyes closed, presumably by modulating proprioceptive function [
18]. Research in laboratory animals has shown that spinal cord stimulation activates antigravity tonic muscles and restores spinal networks that control body balance [
2,
19].
The effect of the spinal tES on balance control was studied in healthy adults [
20,
21]. Tests included forward, backward, left and right trunk perturbations without and with midline stimulation between the L2-L3 vertebrae. The disturbances were caused by the forces applied to the pelvis. Continuous monophasic tES consisted of pulses of 5-10 mA, at a frequency of 30 Hz. Balance control was characterized based on participants’ responses to force perturbations. It was found that the acute effect of tES was to increase muscle activity during the forward perturbation, but this was accompanied by reduced balance performance in that direction.
We investigated the effect of the tES on postural control when healthy adult subjects performed steady-state upright postures. Midline tES reduced the postural stability as evidenced by the increase in CoP trajectory length along the frontal axis, confidence ellipse area and RMSD along the sagittal axis at midline stimulation (Figures 3c, 4c and 6c, respectively).
Thus, the spinal tES at the L1-L2 vertebral level, which activates the lower limb extensors [
6], initiated and restored the independent upright posture in patients with motor disorders and decreased postural stability in healthy subjects. The reasons for these different effects can be speculated. The tES of the balance networks of the spinal cord replaces the missing or malfunctioning supraspinal control of balance in the patients, so that the independent upright posture is rehabilitated during stimulation. In healthy subjects, this tES may disrupt the normally functioning supraspinal regulation of vertical posture; therefore, antigravitational stability decreases under this effect. This assumption is supported by the different results of the tES on upright posture in FD and FI participants.
4.2. Upright Posture of FD Participants Responds to Spinal tES and One of FI Participants Does Not
In 1948 the Rod and Frame test, in which the rod and outer frame are rotated independently at variable angles, was introduced to assess subjective vertical position in space [
22]. Subjects were divided into two groups, each referred to as a particular cognitive style, based on their response to the test. Subjects who oriented the rod to tilt in the direction of the tilted frame were identified as field-dependent (FD), because they relied on the visual field defined by the frame to estimate verticality. Later, it was shown that not only the visual system, but multiple sensory systems are involved in generating an internal representation of the body in space, perceiving one's own movement, and maintaining upright posture [
23].
Isableu et al. showed that cognitive style has a significant impact on postural control strategy [
24]. Different efficiencies in postural performance were exhibited by FD and FI subjects – FD subjects were less stable than FI subjects. Similarly, in our study (
Table 1), increased values of ellipse area, RMSD
front, and RMSD
sag in FD participants compared to FI participants indicate decreased postural control in FD participants in control conditions.
Strategies of the segmental coordination of the head, shoulders, and hips during upright standing were studied in FD and FI subjects [
9]. Segmental stabilization strategies differed among these individuals. FD subjects showed increased hip stabilization, and this stabilization was associated with "en bloc" operation of the head/shoulder and/or shoulder/hip units, which induced a corresponding stability of the entire trunk and head. In FI subjects, the head, shoulders and hips moved independently from each other during postural control.
The relationship between joint stabilization and upright postural instability has been demonstrated [
25]. The young adults stood on a force plate during 60 sec without and with immobilized joints (only knees constrained, knees and hips, and knees, hips and trunk). It was shown that increased body stability was achieved when more joints were free to move.
As we hypothesized, tES decreased upright postural stability in the FD participants, but not in the FI participants (
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). The intensity of the stimulation current was the same in the subgroups (
Table 2), thus, the different effect of the tES on the upright posture of the FD and FI participants is related to the different strategies of the body control in these subjects. It has been shown that tES at the level of the L1 vertebra during the stance phase of the stepping increases the coactivation of the antagonist muscles [
10]. Coactivation index values increased by ~10% for thigh muscles and ~5% for ankle muscles. Increased agonist-antagonist muscle coactivation results in increased apparent joint stiffness [
11].
In FI subjects, body segments move independently from each other during steady state standing [
9]. The increased stiffness of the ankle and knee joints induced by tES could be compensated by the increased flexion of the other segments during postural control, that is why the recorded postural parameters were not altered significantly in these participants.
In FD subjects, body segments are “en bloc” during postural control [
9]. The increased stiffness of the leg joints during tES increased the overall stabilization of the joints, and the instability of the upright posture was similar to that previously demonstrated when the joints were mechanically stabilized [
25].
4.3. Left and Right Spinal Root tES Have Different Effects on Postural Balance
Computational modeling of spinal tES showed that midline spinal stimulation increased current density around the spinal segments, but was clearly concentrated along the dorsal roots on both sides, and the lumbar lateral cathode stimulated the vast majority of the lumbar nerve roots on the side of stimulation [
26].
The effects of tES of the right and left spinal roots differed in all participants as well as in the FD subgroup. Stimulation of the left spinal root significantly decreased postural control, whereas stimulation of the right spinal root did not (
Figure 2,
Figure 4 and
Figure 5). In FD participants, the values of two CoP parameters during tES of the left root, which did not increase significantly compared to these parameters without stimulation, were significantly higher than the values of these parameters during stimulation of the right root. These parameters were the RMSD along the sagittal axis and the trajectory length along the frontal axis (
Figure 7). The results show that postural control decreased during tES of the left root compared to the control without stimulation and with stimulation of the right root.
All participants in our study had right leg dominance, so their left leg was the supporting leg [
12]. Thus, the tES of the dorsal roots at the L1 vertebra on the side of the supporting leg reduces postural control, and the one on the side of the dominant leg does not.
4.4. “Posture” and “Equilibrium” Tasks in Standing and Spinal tES
As mentioned above, the control of balance during standing solves two processes simultaneously: one is the stabilization of the body segments to resist gravity ("posture"), the other is the compensation of perturbations of the body segments ("equilibrium") [
4]. The “posture” task is mainly performed by the extensor muscles, and the “equilibrium” task is mainly performed by the flexor muscles. Spinal stimulation at the L1-L2 mainly activates the extensors and at T11-T12 mainly the flexors [
6].
We have shown that tES of the spinal cord at the L1-L2 vertebrae reduces postural control during quiet standing when the "equilibrium" task is virtually absent. Omofuma and colleagues [
20,
21] stimulated the similar level of the spinal cord and tested balance control during the trunk perturbations when the "equilibrium" task was solved simultaneously with the “posture” task. They found that the balance suffered. In both studies the tES interfered with the control of the antigravity tonic muscles. As a next step, we plan to study the effects of stimulation at the T11-T12 vertebrae to activate the flexor muscles during vertical postural perturbations.
5. Conclusions
Spinal tES at L1-L2 vertebrae, used for neurorehabilitation of standing in patients with motor disorders, reduced balance control in healthy participants.
Cognitive style is important in postural control. Separate analysis of results in FD and FI subgroups showed that tES did not affect postural control in FI subjects.
We attribute the effect to an increase in lower limb joint stiffness, which is critical for postural control in FD subjects.
The tES of the dorsal roots at the L1 vertebra on the side of the supporting leg reduces postural control, and the one on the side of the dominant leg does not.
This non-invasive spinal cord stimulation can be used to study the regulatory mechanisms of upright standing in healthy subjects.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Analyzed center of pressure (CoP) parameters; Table S2. Length of the CoP trajectory along the frontal axis based on participant’s cognitive type and tES condition (mm); Table S3. Ellipse area based on participant’s cognitive type and tES condition (mm2); Table S4. RMSD along the frontal axis based on participant’s cognitive type and tES condition (mm); Table S4. RMSD along the frontal axis based on participant’s cognitive type and tES condition (mm).
Author Contributions
Conceptualization, T.M. and I.A.; methodology, T.M. and N.S.; validation, O.T. and A.G.; formal analysis, O.T.; investigation, N.S.; data curation, O.T. and A.G.; writing—original draft preparation, N.S.; writing—review and editing, T.M.; visualization, N.S. and A.G.; supervision, I.A.; project administration, I.A.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 23-25-00226.
Institutional Review Board Statement
The procedures and studies were performed in accordance with the tenets of the Declaration of Helsinki and approved by the Ethics Committee of the Sechenov Insti-tute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences (Minutes # 1-02 dated 2023/02/01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Nikita Shamantsev for the design of Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gerasimenko, Y.P.; McKinney, Z.; Sayenko, D.G.; Gad, P.; Gorodnichev, R.M.; Grundfest, W.; Edgerton, V.R.; Kozlovskaya, I.B. Spinal and Sensory Neuromodulation of Spinal Neuronal Networks in Humans. Hum. Physiol. 2017, 43, 492–500. [Google Scholar] [CrossRef]
- Musienko, P.; Courtine, G.; Tibbs, J.E.; Kilimnik, V.; Savochin, A.; Garfinkel, A.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Somatosensory Control of Balance during Locomotion in Decerebrated Cat. J. Neurophysiol. 2012, 107, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.; Havton, L.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury | Journal of Neurotrauma Available online:. Available online: https://www.liebertpub.com/doi/abs/10.1089/neu.2018.5956 (accessed on 26 June 2023).
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.; Islam, R.; Latypov, T.; Basa, P.; Joseph, O.J.; Knudsen, B.; Siddiqui, A.M.; Summer, P.; Staehnke, L.J.; Grahn, P.J.; et al. Segment-Specific Orientation of the Dorsal and Ventral Roots for Precise Therapeutic Targeting of Human Spinal Cord. Mayo Clin. Proc. 2021, 96, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Moshonkina, T.; Grishin, A.; Bogacheva, I.; Gorodnichev, R.; Ovechkin, A.; Siu, R.; Edgerton, V.R.; Gerasimenko, Y. Novel Non-Invasive Strategy for Spinal Neuromodulation to Control Human Locomotion Available online:. Available online: https://www.frontiersin.org/articles/10.3389/fnhum.2020.622533/full (accessed on 28 July 2023).
- Woollacott, M.; Shumway-Cook, A. Attention and the Control of Posture and Gait: A Review of an Emerging Area of Research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wapner, S.; Demick, J. Field Dependence-Independence: Bio-Psycho-Social. Factors Across the Life Span; Psychology Press, 2014; ISBN 978-1-317-78287-2.
- Isableu, B.; Ohlmann, T.; Crémieux, J.; Amblard, B. Differential Approach to Strategies of Segmental Stabilisation in Postural Control. Exp. Brain Res. 2003, 150, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Moshonkina, T.R.; Shandybina, N.; Moiseev, S.; Grishin, A.; Gerasimenko, Y.P. Muscle Coactivation Phenomenon in the Modulation of Walking by Electrical Stimulation of the Spinal Cord | SpringerLink Available online:. Available online: https://link.springer.com/article/10.1134/S0362119721020092 (accessed on 27 June 2023).
- Latash, M.L. Muscle Coactivation: Definitions, Mechanisms, and Functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T.; Noé, F. Does Monopedal Postural Balance Differ between the Dominant Leg and the Non-Dominant Leg? A Review. Human. Mov. Sci. 2020, 74, 102686. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Allinson, C.W. Cognitive Style and Its Relevance for Management Practice Available online:. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1467-8551.1994.tb00068.x (accessed on 7 August 2023).
- Sliva, S.S. Domestic Computer Stabilography: Engineering Standards, Functional Capabilities, and Fields of Application. Biomed. Eng. 2005, 39, 31–34. [Google Scholar] [CrossRef]
- Megía García, A.; Serrano-Muñoz, D.; Taylor, J.; Avendaño-Coy, J.; Gómez-Soriano, J. Transcutaneous Spinal Cord Stimulation and Motor Rehabilitation in Spinal Cord Injury: A Systematic Review. Neurorehabil Neural Repair. 2020, 34, 3–12. [Google Scholar] [CrossRef]
- Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.-P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J. Clin. Med. 2022, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Hastings, H.; Zhong, H.; Seth, G.; Kandhari, S.; Edgerton, V.R. Transcutaneous Spinal Neuromodulation Reorganizes Neural Networks in Patients with Cerebral Palsy | SpringerLink Available online:. Available online: https://link.springer.com/article/10.1007/s13311-021-01087-6 (accessed on 10 June 2023).
- Roberts, B.W.R.; Atkinson, D.A.; Manson, G.A.; Markley, R.; Kaldis, T.; Britz, G.W.; Horner, P.J.; Vette, A.H.; Sayenko, D.G. Transcutaneous Spinal Cord Stimulation Improves Postural Stability in Individuals with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 52, 103009. [Google Scholar] [CrossRef] [PubMed]
- Musienko, P.E.; Zelenin, P.V.; Orlovsky, G.N.; Deliagina, T.G. Facilitation of Postural Limb Reflexes With Epidural Stimulation in Spinal Rabbits. J. Neurophysiol. 2010, 103, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Carrera, R.M.; Omofuma, I.; Yasin, B.; Agrawal, S.K. The Effect of Transcutaneous Spinal Cord Stimulation on Standing Postural Control in Healthy Adults. IEEE Robot. Autom. Lett. 2022, 7, 8268–8275. [Google Scholar] [CrossRef]
- Omofuma, I. Effect of Transcutaneous Spinal Cord Stimulation on Balance and Neurophysiological Characteristics in Young Healthy Adults 2023.
- Witkin, H.A.; Asch, S.E. Studies in Space Orientation. IV. Further Experiments on Perception of the Upright with Displaced Visual Fields. J. Exp. Psychol. 1948, 38, 762–782. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.-P.; Lacquaniti, F. Perceptual-Motor Styles. Exp. Brain Res. 2021, 239, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Isableu, B.; Ohlmann, T.; Crémieux, J.; Amblard, B. Selection of Spatial Frame of Reference and Postural Control Variability. Exp. Brain Res. 1997, 114, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.B. de; Freitas, S.M.S.F.; Duarte, M.; Latash, M.L.; Zatsiorsky, V.M. Effects of Joint Immobilization on Standing Balance. Human. Mov. Sci. 2009, 28, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.; Brown, E.H.; Mesbah, S.; Gonnelli, F.; Pisolkar, T.; Edgerton, V.R.; Ovechkin, A.V.; Gerasimenko, Y.P. Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia. J. Clin. Med. 2022, 11, 3670. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Study design. Participants stood on a force plate in a standard position while the transcutaneous electrical stimulation (tES) of the spinal cord was applied to modulate extensor activity in one of three experimental conditions, or stood in the control condition without tES. (a) – The position of the participants, ventral view, activated extensors marked in red. (b) – The positions of the cathodes for tES relative to the spine, dorsal view, L- left, M – midline, R - right.
Figure 1.
Study design. Participants stood on a force plate in a standard position while the transcutaneous electrical stimulation (tES) of the spinal cord was applied to modulate extensor activity in one of three experimental conditions, or stood in the control condition without tES. (a) – The position of the participants, ventral view, activated extensors marked in red. (b) – The positions of the cathodes for tES relative to the spine, dorsal view, L- left, M – midline, R - right.
Figure 2.
Individual CoP trajectories of the FD (a) and FI (b) participants; the period analyzed is 30 sec. The plots labelled “control”, “left”, “midline” and “right” show trajectories recorded without the stimulation, with the left, midline and right tES, respectively. The value of the ellipse area is marked in green (mm2).
Figure 2.
Individual CoP trajectories of the FD (a) and FI (b) participants; the period analyzed is 30 sec. The plots labelled “control”, “left”, “midline” and “right” show trajectories recorded without the stimulation, with the left, midline and right tES, respectively. The value of the ellipse area is marked in green (mm2).
Figure 3.
Differences between the length of the center of pressure trajectory along the frontal axis in the experimental and control conditions, in mm. The outlier points, which are either below the lower whisker line or above the upper whisker line are not shown. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05; #p=0.06 compared to control.
Figure 3.
Differences between the length of the center of pressure trajectory along the frontal axis in the experimental and control conditions, in mm. The outlier points, which are either below the lower whisker line or above the upper whisker line are not shown. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05; #p=0.06 compared to control.
Figure 4.
Differences in ellipse area between experimental and control conditions, in mm2 . The outlier points that lie either below the lower whisker line or above the upper whisker line are not shown. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05 compared to control.
Figure 4.
Differences in ellipse area between experimental and control conditions, in mm2 . The outlier points that lie either below the lower whisker line or above the upper whisker line are not shown. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05 compared to control.
Figure 5.
Differences between the root mean square deviation of the center of pressure along the frontal axis in the experimental and control conditions, in mm. The outlier points that lie either below the lower whisker line or above the upper whisker line are not shown. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05 compared to control.
Figure 5.
Differences between the root mean square deviation of the center of pressure along the frontal axis in the experimental and control conditions, in mm. The outlier points that lie either below the lower whisker line or above the upper whisker line are not shown. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05 compared to control.
Figure 6.
Differences in root mean square deviation of the center of pressure along the sagittal axis between experimental and control conditions, in mm. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05 compared to control.
Figure 6.
Differences in root mean square deviation of the center of pressure along the sagittal axis between experimental and control conditions, in mm. (a) FD participants; (b) FI participants; (c) all participants; *p < 0.05 compared to control.
Figure 7.
Differences in the center of pressure parameters obtained during stimulation of the left and right roots of the spinal cord. Results of the FD participants.
Figure 7.
Differences in the center of pressure parameters obtained during stimulation of the left and right roots of the spinal cord. Results of the FD participants.
Table 1.
Postural parameters of the FI, FD participants. N is the number of recorded episodes, tests and retests are considered for each participant.
Table 1.
Postural parameters of the FI, FD participants. N is the number of recorded episodes, tests and retests are considered for each participant.
| Participants |
Ellipse Area, mm2
|
RMSDfront, mm |
RMSDsag, mm |
| FD (N = 16) |
151 [86; 231]*
|
3.1 [2.0; 3.7]*
|
3.1 [2.3; 4.0]*
|
| FI (N = 16) |
56 [46; 102] |
1.6 [1.6; 2.6] |
2.4 [2.1; 2.7] |
| All (N = 32) |
93 [50; 148] |
2.2 [1.6; 3.2] |
2.6 [2.1; 3.3] |
Table 2.
Current intensities of the tES. Number of participants displayed (n).
Table 2.
Current intensities of the tES. Number of participants displayed (n).
| Participants |
Left Dorsal Roots, mA |
Midline, mA |
Right Dorsal Roots, mA |
| FD (n = 8) |
21 ± 6 |
26 ± 13 |
21 ± 6 |
| FI (n = 8) |
20 ± 11 |
22 ± 12 |
19 ± 10 |
| All (n = 16) |
20 ± 11 |
24 ± 12 |
20 ± 10 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).