1. Introduction
Since acid mine drainage (AMD) is a persistent by-product of current and past mining activities, it is currently one of the most serious environmental concerns in South Africa and around the world. AMD presents a challenge for both operating and inactive or abandoned mines, in underground tunnels, shafts, open pits, waste rock piles, and mill tailings [
1,
2,
3,
4]. Although AMD is more severe in closed and abandoned mines where pumps are turned off and the water tables rebound, it is less noticeable when mines are active because the water tables are kept low by pumping [
5,
6,
7]. Besides the environmental impacts, AMD also impacts on sustainability, which includes the ecological, social, and economic concerns. AMD has an impact on the resource extraction industry, which has an impact on economic activity, infrastructure, and people’s livelihoods. This has a significant impact on the climate change policies of developing nations and their efforts to transition to a green economy. New sustainable technologies, efficient management plans, and AMD treatment procedures are currently required.
AMD causes significant environmental problems that are both locally and globally intractable in the short to medium term. It takes the development of effective, innovative, and affordable approaches for addressing and overcoming these issues. Mining for minerals, such as gold, copper, and nickel, has been linked to AMD issues, which could have long-term consequences for streams and biodiversity. Some metals mining effluents contain high levels of poisonous cyanides and heavy metals, which have major human health and environmental consequences [
3,
8,
9]. To remediate AMD, many wastewater treatment technologies have been used, including neutralization [
10], selective precipitation [
11], membrane processes [
12], ion exchange [
13], and biological sulphate removal [
14]. The challenge, however, is that the constituents of AMD, while hazardous, may be collected and turned into valuable materials that can be commercialized. Sulphuric acid, for example, has a major demand in the chemical and metallurgical industries [
15,
16]. While the need for critical minerals and rare earth metals is likely to rise in the future, there is a need to develop novel solutions that combine rare earth metal recovery and AMD remediation [
17,
18]. The financial advantages could then be utilized to offset the entire expense of AMD therapy.
To that purpose, the most recent research on AMD development, prevention, and treatment is summarized and critically reviewed. The main objective of this paper is to review studies on the prevention, prediction, impact of the AMD on the water quality, treatment and potential recovery of valuable by-products from mine drainage. The specific objectives are to (1) briefly present the past impacts and possible future impacts of AMD on water quality in South Africa, (2) discuss the developments on the prediction and prevention of the AMD. (3) review of main large-scale AMD treatment processes applied in South Africa for the removal of the AMD and simultaneously recovering of the valuable by-products.
2. Past Impact of AMD on Water Quality in South Africa
Since mining began, tailings dump from gold mining have become a common sight around mining towns and have been releasing contaminated water for decades. Tailings dumps flourish in the upper catchments of the Blesbokspruit in Springs and the Klip River, where the effects of this pollution are predominant [
19]. The Witwatersrand gold mines have shut down over several years, therefore water began to fill in the voids, as the pumping of the mines had stopped. This accumulated water travelled into neighbouring mines since all the mines are connected. This process forced these neighbouring mines to take on the responsibility of pumping. A subsidy was initiated by the government to help the mines cover the costs involved in pumping the additional high volumes of water which filled up and requires treatment to an acceptable quality. This treatment involves the use of lime to increase the pH levels and the blowing of oxygen or air into the water to trigger oxidation of the iron, which precipitates along with several other heavy metals. The precipitated iron was settled out, separated and discarded on tailings dumps and the remaining water was diverted into local rivers. However, these discharges added to the load of pollution already being carried by the rivers in the mining towns.
The salinity of the Vaal River is indicative of the effects of diffuse and point source pollution coming from the gold mines of the Central and Western basins, which has increased to more than double between the Vaal Dam and the Barrage. This is due to the incoming water from the Klip River and the Blesbokspruit (via the Sukerbos River) [
20]. The bad quality of the water at the Barrage results in the need to periodically release water from the Vaal Dam to reduce the salinity for the users downstream of the Vaal River. During the wet periods this is no problem, but during a drought, this could pose a challenge, when water in the upper Vaal system, which is primarily for Gauteng, must be released for the purposes of dilution [
21]. The void began to fill once the last Goldfield mine shut down and stopped pumping water. The Western Basin started to decant in 2002. When pumping stopped at the Central Basin in 2008, the water levels continue to rise at a rate of 12m per month, to date. Pumping at the Eastern Basin started to slow down at the end of 2010, and eventually stopped early in 2011. The water that decants from the void is of very poor quality, as is noted from the water discharging from the Western basin. The sulphate concentration is typically approximately 3500mg/L, with a pH from 2 to 3. There are also high levels of iron and other heavy metals present in the water. Oxidation occurs when the iron is exposed to air which leaves a bright orange trail of precipitate on riverbeds and banks. It is expected that the Central Basin in Boksburg and the Eastern Basin in Nigel, where the lowest lying shafts are found, will decant in approximately three years, if there are no interventions put into place [
22].
These points of decantation are based on the premise that the water is free flowing through the voids and that mine shafts are the only openings to these voids. However, this may not be the case. For example, in the Western Basin, water was found to be first decanting from a farm borehole, and thereafter from a very old mine shaft which may or may not have been connected to the main void. Many decant points can arise if the flow rate through the void is not enough to allow for the inflow.
3. Possible Future Impacts of AMD on Water Quality in South Africa
The Olifants River catchment is in a state of deterioration. There was an idea to install a treatment plant in the heavily polluted Brugspruit area near Witbank, but this had limited efficacy. The main purpose was to address the pH challenge and it did not affect the salinity of the water. A water treatment plant was commissioned in the area (the eMalahleni Water Reclamation Plant) which operates by reverse osmosis. This plant showed that it is possible to treat highly contaminated water to drinking quality standards. The setback is that the cost of the water is approximately three times more than the water being delivered to the area from the Vaal River. This technology, although it can produce drinking water for communities, it can’t be used to improve the overall state of polluted rivers in the area (the plant has a capacity of 20 mL per day, or 0.23 m3/s, and cost in the region of R300 million when built in 2007). The quality of the water of the Olifants River will remain in a state of deterioration in the future. There are large quantities of unmined coal in the Olifants catchment, and several prospective and mining applications require approval. This will lead to more increases in the pollution levels in the future.
Coal mining has been taking place for a vast number of years in the upper Vaal River catchment. Most of these mines are deep and are still quite actively managed. However, it was found that there is a high influx of applications for new mining permits in that catchment, which is alarming. If these permits are approved, then it is without a doubt that the water quality of the Vaal River will decline to the same levels as that in the Olifants River, and the Grootdraai Dam will end up as the Witbank Dam did, with bad water quality. Thereafter the only source of good quality water will lie in the Lesotho Highlands in the Vaal River system. The same fate awaits the Usutu/Pongola and Komati Rivers. Funds have been set aside by the government recently, to tackle the issue of decanting from the gold mines in Witwatersrand which will involve pumping and basic treatment operations being initiated again (addition of lime and removal of iron) in the three currently affected goldfields. This will aid in stopping the uncontrolled decanting in the Western Basin and prevent similar occurrences in the Central and Eastern Basins. However, as much as this intervention will vastly help to improve the Western Basin it will not impact on the water quality in the Vaal River system. Instead, it will just return the system to its original state when the mines were still pumping, treating and releasing water from the mine voids.
Several different technologies have been developed for desalination of contaminated water from local mines. Only one of these has been commercialised and is being implemented which is the reverse osmosis plant in Witbank, which has shown that although reverse osmosis can address the issue, it is not feasible. The financial implications of other technologies have not been demonstrated yet. It is a possibility that although most of the proposed technologies are suitable for treating point sources of contaminated water (e.g., pumped from old mines), it is unlikely that they would be able to treat contaminated water coming from diffuse sources such as waste dumps. For gold mines the water in the void is generally accessible and treatable as a point source. Coal mining is more complex however, and it may not be possible to prevent uncontrolled decanting of AMD from rehabilitated opencast mines. Thus, the water quality in these areas should be expected to decrease.
AMD is a global environmental concern which has major negative impacts on the health and wellbeing of affected people and to the environment itself. There are active or passive-based options for treating AMD, based on its geochemistry. These methods however need long-term treatment and monitoring since AMD formation could remain until sources such as arsenopyrite, pyrite, and pyrrhotite are depleted and/ or converted into non-reactive compounds, which would take decades or even centuries. Thus, there is renewed interest in investigating preventative measures as alternatives since they could restrict the formation of AMD at the source and make them sustainable. Techniques such as oxygen barriers, bactericides, blending with neutralizing materials, and direct passivation of sulfide minerals are all preventative measures which display excellent abilities to prevent AMD formation by restricting oxygen availability, or reactions between acid-producing minerals and oxygenated water.
Since the treatment techniques used for prevention do not need to be continuous, for maintenance as well, they are more sustainable than conventional remediation techniques. However, most prevention techniques especially passivation and microencapsulation are still under experimentation and focus on pure pyrite systems. Results were encouraging for microencapsulation techniques; however, this is restricted to batch, single-metal systems. It is therefore uncertain if they could be used for mine tailings and waste rocks, which has several minerals such as silicates and aluminosilicates, under complex environmental conditions. This indicates that experiments to study the effectiveness of the different microencapsulation techniques using synthetic or real tailings including waste rocks rich in pyrite, should be ongoing. The long-term stability of treated tailings and waste rocks should be investigated in detail under the conditions found in the environment (e.g., drying-wetting cycles). An additional option to consider when looking to restrict AMD formation is to use or recycle mine waste as construction and geopolymer materials. Finding value in and managing mine waste has become highly important.
4. AMD Prevention
The complexity of the treatment system that is required to guarantee that effluent standards will be satisfied depends on a number of variables [
23]. These include the chemical properties of the AMD, the volume of water that needs to be treated, the local climate, the topography, the properties of the sludge, and the anticipated lifespan of the plant [
23]. Various treatment techniques have been developed and can be categorised as either ‘abiotic’ or ‘biotic’, the former of which does not rely on biological activities while the latter does [
5].
Passive treatments involve the passage of mine water through a controlled environment, rather than a receiving water body, where naturally occurring geochemical and biological reactions take place and improve the mine water quality [
24,
25]. Anoxic limestone drains, open limestone channels, limestone leach beds, slag leach beds, diversion wells, limestone sand, and oxidation channels are examples of passive abiotic treatment [
9]. These materials all generate alkalinity which help to neutralise the AMD and raise the pH, while at the same time oxidising and precipitating out metals. Passive biotic treatments, on the other hand, include wetlands and bioreactors where natural biological processes work, either in aerobic or anaerobic conditions, to neutralise the AMD and precipitate the hazardous concentrations of contaminants (e.g., metals) over time [
9,
26]. In line with this, Ramla & Sheridan [
27] proved the efficacy of utilizing indigenous South Africa grass as a suitable organic substrate for sulphate-reducing bacteria to reduce sulphate to sulphides during the passive biotic treatment of AMD. In this experiment, Hyparrhenia hirta grass supplemented with soil containing microbes produced the best outcomes.
Both passive biotic and abiotic AMD treatment require relatively little resource input, tend to be more useful for AMD flows of less than 2 to 5 ML/d, with low acidity, e.g. <800 mg/L as CaCO3, and which require little metal and sulphate removal [
24,
28,
29]. However, in comparison to active treatments, passive treatments need larger areas of land and additional time to neutralise AMD and precipitate the contaminants. Thus, passive options are more applicable when AMD treatment needs to be accomplished at closed mine sites with low AMD flows as they are a potentially lower cost, longer-term sustainable option. An example of one that has been studied in some detail is the passive system set up to treat AMD mine seepage from a long-abandoned mine near the town of Red Oak in eastern Oklahoma [
26]. Passive systems present the advantages of being self-sustaining, requiring sporadic maintenance and having very low operating and capital costs [
24]. However, the quality of the resultant effluent is poorer than that produced by active treatment systems [
24].
Unlike passive treatments which depend mostly on naturally occurring reactions, active AMD treatment is performed in a constructed plant where processes are controlled and sustained via the continuous input of resources [
30]. It involves the utilisation of alkaline substances to increase the pH of the drainage and precipitate heavy and toxic metals from AMD [
25]. In line with this, the operating and capital costs of sustaining effective and efficient functioning of the plant can be high as it requires a continuous supply of chemicals, electrical and mechanical power sources and the employment of operations and maintenance staff [
30]. Therefore, active AMD treatment is more suited for application at operational mine sites where the necessary resources are more readily available [
31]. Additionally, the efficiency, cost and potential environmental impacts of utilizing an active treatment system are also dependent on the type of neutralizing agent used [
32]. Selection of the neutralizing agent is based on the chemical composition of AMD, site-specific conditions and expected outcomes with the understanding that some level of cost and benefit trade-off will be required. For instance, sodium hydroxide is more effective in AMD treatment than lime but is approximately 1.5 times more expensive and must be handled in line with specific health and safety requirements due to its hazardous nature [
32]. Likewise, anhydrous ammonia requires safe handling and if excessively used can spur nitrification or denitrification in receiving water bodies [
32]. In some cases, the split treatment of AMD may yield the most desirable results e.g. using lime and limestone [
33].
The benefits of active AMD treatment, however, can be considered as great advantages over passive treatment techniques [
34]. These include that active treatment can be applied to all AMD flow rates, it’s fast and effective, it produces good quality effluent with a potential for cost recovery via the sale of product water, metals and by-products and involves a lower cost in the handling and disposal of generated sludge [
30,
32].
Traditional abiotic active treatment of AMD is characterised by the use of alkaline chemicals to neutralise acids, deactivate metals and precipitate salts [
35,
36]. Calcium, sodium, ammonium or magnesium based chemicals that have been, and are, used to neutralise AMD include calcium hydroxide (slaked lime, Ca(OH)
2), calcium carbonate (limestone, CaCO
3), calcium oxide (lime or quick/burnt lime, CaO), sodium carbonate (soda ash, Na
2CO
3), sodium hydroxide (caustic soda, NaOH), magnesium hydroxide (Mg(OH)
2) and ammonium hydroxide (NH
4OH) [
37]. Using calcium hydroxide, calcium oxide or calcium carbonate can result in large amounts of sludge that retain water as calcium will bond with sulphates and then precipitate out of solution, together with the metals (usually as hydroxides), at higher pH levels [
38]. Recycling this sludge is difficult, leaving disposal to landfill or sludge dams as the main option for handling this waste [
38]. Furthermore, some metal hydroxides are amphoteric, which presents the probability for dissolution of potentially harmful chemicals from the sludge both during and after disposal [
39]. Thus, the sludge disposed at landfill sites or sludge dams would need to be properly managed and regularly monitored to ensure that no long term negative environmental impacts occur [
38]. In addition, landfills and sludge dams can occupy large areas of land, especially when considerable amounts of sludge are produced and disposed [
38]. Neutralising chemicals such as magnesium hydroxide, ammonium hydroxide and sodium hydroxide have proven to be comparatively more useful as they tend to precipitate metals (e.g., as hydroxides) while leaving the sulphate in solution. This sulphate can subsequently be treated to produce gypsum, which may be valuable in other economic and industrial sectors.
An area of rising importance in the active abiotic treatment of AMD is the use of waste by-products to treat other wastes. An example of this is the use of calcium containing waste such as dust from cement and lime kilns to neutralise AMD and precipitate metals. Another example is the use of coal combustion by-products to partially treat AMD. Coal based by-products generally are very good adsorbents because they have a high surface area, microporous structure and high surface reactivity [
40]. However, these by-products seem to be best at removing trace concentrations of more toxic metals such as radioactive thorium, uranium, radium and lead. Kaur et al. [
36] also describes the use of an alkaline waste material from the alumina refining industry as a possible alternative neutralising material. The costs to obtain and use one waste to treat another is often much lower, making AMD neutralisation and other treatment processes (e.g., metal extraction) potentially much cheaper. However, the feasibility of these types of options will vary depending on the type, availability and location of the various wastes to the AMD as well as the properties of the AMD. In addition, it must be noted that calcium-based neutralisation chemicals tend to produce considerable quantities of waste sludge which would still need to be dealt with if cement waste or lime kiln dust is used. Other examples of some good absorbents that have shown potential to treat AMD include bauxite and naturally occurring bentonite clay. Bentonite (primarily aluminium phyllosilicate) has been used to neutralise AMD and remove metals. However, the suitability of these type of adsorbents needs to be investigated on a large, long-term scale to prove that they can work as well as the current technologies whilst also being more sustainable and cost effective.
Active biotic AMD treatment involves the use of off-line sulfidogenic bioreactors where hydrogen sulphide produced by sulphate-reducing bacteria (SRB) is used both to add alkalinity to neutralise the acidic waste streams, and to precipitate metals as insoluble sulphide precipitates, which may then be recovered and reprocessed [
5,
41].
Table 1 below summarizes various technologies for the treatment of the AMD.
5. AMD Impact
High quantities of dissolved metals and acid constitute AMD, which is extremely harmful to groundwater, streams, and rivers. AMD also damages ecosystems, corrodes infrastructure, and poses a number of environmental challenges for aquatic life. This often results in contaminated water supplies to areas where freshwater is not easily accessible [
42].
For AMD pollutants to affect humans, they need to be exposed and several AMD pollutants are dangerous to humans [
43]. According to Orlović-Leko et al. [
44], heavy metals have an adverse effect on both people and the environment, and they can linger for a very long time in natural ecosystems where they build up at higher and higher levels of the food chain. This will lead to acute and chronic diseases where metabolic functioning is disrupted by accumulation in vital organs and glands [
45].
The water from AMD does terrible damage since it starts out clear and quickly turns brilliant orange when iron oxides and hydroxides precipitate due to the high acidity levels. By becoming embedded on the river, stream, or ocean bed, this fine precipitate, known as ochre, cements substrates that serve as a food supply for benthic creatures, which eventually go extinct [
43]. Fish hence has an effect further up the food chain. Therefore, even if the acidity and heavy metals are mitigated, AMD still has an impact on people and wildlife far downstream due to these indirect consequences.
Heavy metals also contaminate soil which poses serious environmental issues where plant growth is affected by oxidative stress [
46]. According to Li et al. [
47], this causes cellular damage and disturbs homeostasis, which affects the physiology and morphology of plants. Calcium and magnesium are unavailable to plants as well as nitrogen, phosphorus, and potassium when the pH of the soil is low. At low pH, soil particles also release aluminum, iron, and manganese, enhancing their toxicity. Furthermore, low soil pH affects how well plants use nutrients, establish roots, and tolerate drought by reducing the activity of soil organisms that break down organic materials. According to Jiao et al. [
48], heavy metals are accumulated by aquatic creatures like fish both directly from tainted water and indirectly through the food chain. Since they are highly persistent and poisonous at trace levels, cadmium, copper, lead, and zinc have the potential to cause severe oxidative stress in aquatic organisms [
49]. While chronic exposure can cause mortality or stunted growth, limited reproduction, malformations, or lesions, acute exposure to them can directly kill organisms. As a result, aquatic organisms’ typical physiological processes—including ion exchange with the water and respiration—are influenced by the pH of the water.
6. Current Treatments Technologies and Resource Recovery
This section reviews some examples of AMD treatment projects that are either commercially developed and in operation, in the pilot stage or under evaluation are discussed further. There are three steps in the alkali-barium-calcium (ABC) method, which was developed by the Council for Scientific and Industrial Research (CSIR) in South Africa. The first stage is the addition of lime and calcium sulphide to remove metals and acids. The second stage involves treating most of the remaining water with barium carbonate to remove remaining sulphate as barium sulphate. The barium sulphate and some sludge wastes are reduced in a coal fired kiln to recover some of the alkaline compounds used for neutralisation as well as barium and calcium, some of which can be recycled back into the treatment process. This method is a potentially cost-effective treatment for AMD because of potential reuse opportunities from recycling. However, it still produces a significant amount of waste sludge that must be disposed. In addition, this process also involves high capital and operating costs, especially with running a coal fired kiln.
A possible modification/improvement to the above is the Tshwane University of Technology magnesium-barium-alkali (MBA) treatment process. This process uses barium hydroxide for two purposes, that is, to precipitate and remove sulphate as barium sulphate and to precipitate and remove magnesium as magnesium hydroxide.
The CSIR recently developed and patented sustainable AMD treatment technology called Magnesite-Softeners-Reverse-Osmosis-Eutectic (MASRO) Freeze Crystallisation, that uses magnesite slurry to neutralise the AMD and precipitate metal hydroxides. The advantage of using magnesite is that most of the gypsum, which usually forms a large portion of AMD sludge if it is first treated with a calcium compound like lime as a neutralising agent, does not precipitate with the metal hydroxide sludge. This allows for the possibility of easily concentrating and treating the metal hydroxide sludge to remove more of the valuable metals. Following this step, a lime slurry is added to the AMD, and this precipitates a gypsum sludge (70% gypsum) containing brucite (i.e. Mg(OH)
2). Next, the AMD is treated with a soda ash slurry to recover residual calcium (65% as calcium carbonate) and magnesium and finally the remaining water is treated using RO to improve its quality so it can be fit for human consumption. Masindi et al. [
50], published results of a pilot plant of the MASROE process which was designed and built to treat 20kL/d. They also calculated that the direct field costs to treat the AMD by this process was R65.60/kL.
However, if the by-products such as metal hydroxides could be treated to produce iron pigment, gypsum and lime which could be purified to be saleable, then Masindi et al. [
50], calculated that the sale of these could perhaps yield an overall saving of approximately R9.00/kL (it is also noted that the possible sale of treated water did not appear to be accounted for and this may further increase these potential savings). Thus, presuming the above, the direct field costs could possibly be reduced to approximately R56.60/kL (i.e., a ±14% cost saving). However, it must be noted that AMD transportation costs were deemed negligible as it was assumed that the plant would be close to the AMD source. The treatment costs were also calculated presuming that the plant would operate for most of the year (i.e., 95% of the time, 24 hrs/day) and that electrical power and cleaning water were the only required utilities. Treatment costs also excluded any operational labour. In addition, the study did not account for any costs of processing the sludges to prepare iron pigments, gypsum or calcium carbonate of marketable quality. Therefore, it is possible that the costs not accounted for could lead to less of a cost saving than predicted. Until further research is conducted, that these additional costs might potentially increase the overall treatment costs to greater than R65.60/kL. However, one of the key findings from this research was the fact that setting up a process to effectively claim by-products for potential resale is possible if the process is planned and correctly constructed. And also, the removal and resale of valuable components of the sludge such as metals, that would otherwise be potentially toxic if left in the sludge, leads to better environmental protection and potentially has less requirements for newly mined resources.
The SAVMIN process, developed by Mintek involves five stages of AMD treatment. Firstly, lime is added to precipitate metals. Secondly, using gypsum seed, all remaining gypsum is removed. Thirdly, aluminium hydroxide is added to the remaining AMD water, and this produces ettringite (a calcium-aluminium sulphate mineral) which removes any remaining dissolved calcium and sulphate. Fourthly, the ettringite is removed and remixed with sulphuric acid which causes decomposition into aluminium hydroxide (which is recycled back into the process) and gypsum (some of which is recycled back into the process). Finally, in the fifth stage, the remaining water from stage four is treated with carbon dioxide to lower the pH and remove calcite by precipitation. Advantages of the SAVMIN process include high quality by-products such as metal hydroxides, gypsum and calcite which can potentially be resold to enhance the economic feasibility of this treatment. However, again, it too produces significant amounts of waste sludge that would need to be disposed.
A process developed in the United States of America is the slurry precipitation and recycling reverse osmosis (SPARRO) process. SPARRO uses membrane desalination to treat AMD and produce water at variable recoveries depending on, for instance, its chemical properties. Membrane fouling is and remains a challenge with this process and thus developing membranes to improve their performance may increase the economic feasibility of SPARRO to treat AMD [
41].
Another process developed in South Africa is gypsum – continuous ion exchange (GYP-CIX). It is a continuous fluidised bed ion exchange process designed to remove calcium and sulphate from gypsum saturated waters such as AMD. During the first stage of the GYP-CIX process, cations can be removed from AMD by cation exchange resins. After cation removal, anions are then removed by anion exchange resins. When required, the anion exchange resin is regenerated by lime while the cation exchange resin is regenerated by sulphuric acid. Advantages of this process include calcium and sulphate precipitates of relatively high quality that have the potential to be reused. The use of inexpensive chemicals and efficient water recoveries are further benefits. However, as would seem to be customary, a significant amount of sludge is generated during the renewal of the ion exchange resins, and this typically necessitates an expensive disposal method.
THIOPAQ is a biotechnological approach to AMD treatment. It involves two stages, the first being the addition of hydrogen gas to the AMD to produce sulphide from sulphate which precipitate out metal sulphides. Any excess hydrogen sulphide produced is oxidised to elemental sulphur in the second stage using sulphide oxidising bacteria. Hydrogen gas, used in the first stage of the THIOPAQ process was generated using ethanol and butanol. Recently, however, these chemicals have become quite expensive which has reduced the attractiveness of this approach.
The Rhodes BioSURE process, developed at Rhodes University in South Africa, is a biological treatment used to remove acid from AMD using waste such as sewage sludge or organic wastes. While using these wastes will make treatment cheaper, they are also a potentially limiting reagent if sufficient stock of them is not available. However, the advantages are re-use of sewage sludge or organic waste which would result in lower landfill loads and costs. Interestingly, this process was used by the East Rand Water Care Company in Grootvlei [
41].
A process, developed by Nafasi Water then known as Aveng Water in South Africa and applied at the eMalahleni treatment plant is high pressure reverse osmosis (HiPRO). The recovery of water was generally very good while apart from water, brine and solid waste was also produced. By-products from the solid waste that could potentially be sold were various purities of calcium sulphate and metal sulphates. However, the main challenge was the treatment/disposal of the remaining waste sludge and brine.
Luo et al. [
51] provide details of a treatment that they successfully used to recover metals and produce hydrogen gas using microbial electrolysis cells to treat AMD. This electrolysis technology is one of the more promising to be developed. Through microbiologically assisted electrolysis, these researchers were able to remove copper, nickel and iron from simulated AMD solutions while concurrently producing hydrogen to potentially offset some of the energy inputs during treatment.
Nleya et al. [
34], published their research about the possible production of sulphuric acid from AMD. They summarised that, although not currently economically profitable, methods such as freeze crystallisation and acid retardation may potentially be the most promising technologies for acid recovery. These authors also indicated that the investigation and possible use of lower cost energy sources would assist to make these alternative treatments of AMD more economically viable.
Research details of staged electrochemical treatment in a laboratory to neutralise the pH and remove metals from AMD have recently been published by Brewster et al. [
35]. Briefly, an electrochemical system was set-up and a current applied between a cathode and anode. This caused the pH of the cathodic solution to increase while the anolyte solution pH decreased. The anions, including sulphate, were drawn across a membrane from the cathodic solution into the anolyte solution because of the decrease in pH. The increasing pH in the cathodic solution caused the dissolved metals to concurrently precipitate out at specific pH endpoints. Results indicate that metals like aluminium, iron, manganese, zinc, nickel, lead and others were successfully removed. Advantages of this approach include the use of virtually no chemicals and the production of lower sludge volumes. Other advantages, as indicated by these authors, are the co-precipitation of most AMD metals in a controllable manner which will assist with possible recovery and recycling and the possible recovery and sale of sulphuric acid. Significant disadvantages include high initial capital costs and membrane fouling.
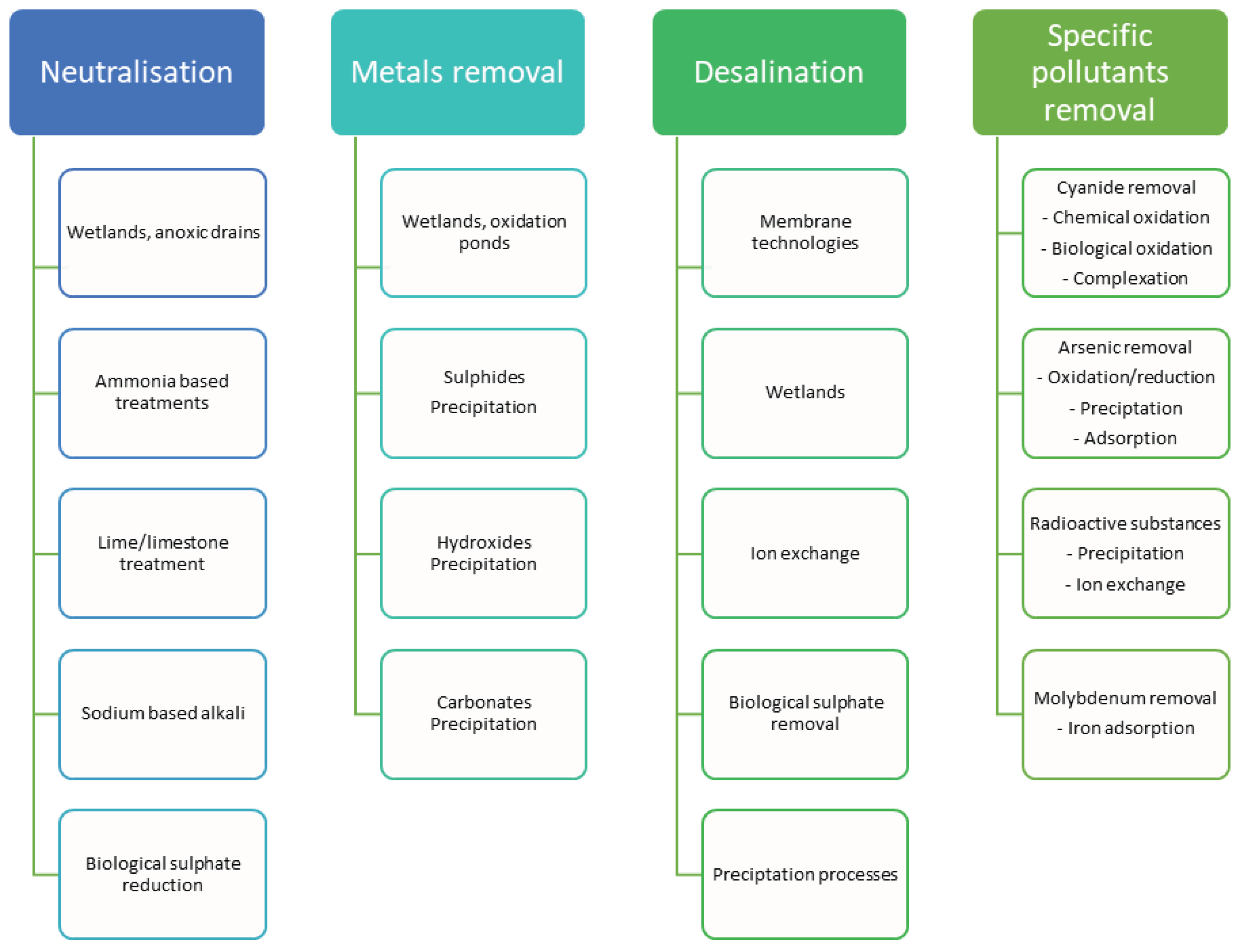
Figure 1 summarises current technologies used for the AMD treatment.
7. Conclusions and Future Prospects
AMD, in South Africa and the rest of the world, is the cause of serious environmental and social concern and requires urgent attention. In this report, the latest literature was reviewed to determine the current and proposed technologies used to treat AMD, the potential market for treatment by-products and support from governments for the application of these technologies.
There are several active, passive, abiotic and biotic treatments that have been investigated and, in some instances, implemented. The most widely used AMD treatment throughout the world is active chemical neutralisation which has been modified recently to be more efficient by continuously recycling most of the sludge, use less neutralisation chemicals, and produce less waste sludge. However, chemical neutralisation remains very expensive and produces significant amounts of potentially toxic waste. AMD occurs at old, abandoned, ownerless mines thus the governments of these countries must pay for AMD treatment. Further, treatment of AMD can last for years, decades or even perpetuity resulting in high, long-term costs. Therefore, there is an urgent need to find treatment improvements of AMD so that it is more environmentally, socially and economically sustainable. Various active and passive treatments have been investigated and generally look promising at lab bench scale and a few at pilot plant scale. However, none of the recent techniques to treat large volumes of AMD has been implemented and proven to work at full scale for an extended period. Thus, the next step remains full scale implementation and successful, long-term operation. Often, however, constraints like costly initial capital expenses are a factor that needs to be overcome. Besides these challenges there is the real potential to produce by-products that could be sold to offset some of the initial capital costs and the on-going operational treatment costs. Some new technologies have successfully reclaimed metals, sulphur-based products such as gypsum and sulphuric acid and other alkali chemicals including calcium carbonate. The market for gypsum, sulphuric acid and iron in the form of iron oxide pigments were studied in this report. All these by-products have shown steady increases in demand globally and this trend is likely to expand. These alternative treatments offer several advantages including the requirement for less new materials because of recycling and a reduction in the amount and treatment and disposal costs of potentially toxic sludge waste. With the possibility of more mining activities being implemented, as well as additional mines coming to the end of their productive lives, the research into more sustainable methods to treat AMD must be intensified. However, emphasis must now be on full-scale implementation of the most promising techniques that recover and sell viable, marketable by-products to significantly offset the cost of traditional AMD treatment technologies and promote the circular economy in South Africa and worldwide.
Author Contributions
Conceptualization, J.B. and N.R.; formal analysis, J.B. and N.R.; investigation, J.B. and N.R.; resources, J.B.; writing—original draft preparation, J.B. and N.R.; writing—review and editing, J.B. and N.R.; visualization, J.B. and N.R.; supervision, J.B.; project administration, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge the support received for this study from Council for Scientific and Industrial Research (CSIR).
Conflicts of Interest
Declare conflicts of interest.
References
- Baloyi, J.; Seadira, T.; Raphulu, M.; Ochieng, A. Preparation, Characterization and Growth Mechanism of Dandelion-like TiO2 Nanostructures and Their Application in Photocatalysis towards Reduction of Cr(VI). Mater Today Proc 2015, 2, 3973–3987. [Google Scholar] [CrossRef]
- Seadira, T.; Baloyi, J.; Raphulu, M.; Moutloali, R.; Ochieng, A. Acid Mine Drainage Treatment Using Constructed Wetland; 2014. 2014.
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A Review of Treatment Technologies for the Mitigation of the Toxic Environmental Effects of Acid Mine Drainage (AMD). Process Safety and Environmental Protection 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Nepfumbada, C.; Tavengwa, N.T.; Masindi, V.; Foteinis, S.; Chatzisymeon, E. Recovery of Phosphate from Municipal Wastewater as Calcium Phosphate and Its Subsequent Application for the Treatment of Acid Mine Drainage. Resour Conserv Recycl 2023, 190, 106779. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Science of The Total Environment 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Daraz, U.; Li, Y.; Ahmad, I.; Iqbal, R.; Ditta, A. Remediation Technologies for Acid Mine Drainage: Recent Trends and Future Perspectives. Chemosphere 2023, 311, 137089. [Google Scholar] [CrossRef]
- Larochelle, T.; Noble, A.; Ziemkiewicz, P.; Hoffman, D.; Constant, J. A Fundamental Economic Assessment of Recovering Rare Earth Elements and Critical Minerals from Acid Mine Drainage Using a Network Sourcing Strategy. Minerals 2021, Vol. 11, Page 1298 2021, 11, 1298. [Google Scholar] [CrossRef]
- Azapagic, A. Developing a Framework for Sustainable Development Indicators for the Mining and Minerals Industry. J Clean Prod 2004, 12, 639–662. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable Resolutions for Environmental Threat of the Acid Mine Drainage. Science of The Total Environment 2020, 717, 137211. [Google Scholar] [CrossRef]
- Iakovleva, E.; Mäkilä, E.; Salonen, J.; Sitarz, M.; Wang, S.; Sillanpää, M. Acid Mine Drainage (AMD) Treatment: Neutralization and Toxic Elements Removal with Unmodified and Modified Limestone. Ecol Eng 2015, 81, 30–40. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Shekarian, Y.; Rezaee, M. Selective Precipitation of Rare Earth and Critical Elements from Acid Mine Drainage - Part I: Kinetics and Thermodynamics of Staged Precipitation Process. Resour Conserv Recycl 2023, 188, 106654. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Rieger, A.; Steinberger, P.; Pelz, W.; Haseneder, R.; Härtel, G. Optimization Study for Treatment of Acid Mine Drainage Using Membrane Technology. 2010, 45, 2004–2016. [CrossRef]
- Felipe, E.C.B.; Batista, K.A.; Ladeira, A.C.Q. Recovery of Rare Earth Elements from Acid Mine Drainage by Ion Exchange. Environmental Technology (United Kingdom) 2021, 42, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- van Rooyen, M.; van Staden, P.J.; du Preez, K.A. Sulphate Removal Technologies for the Treatment of Mine-Impacted Water. J South Afr Inst Min Metall 2021, 121, 1–7. [Google Scholar] [CrossRef]
- Mondaca, S.L.; Leiva, C.A.; Acuña, C.A.; Serey, E.A. Flow Enhancement of Mineral Pastes to Increase Water Recovery in Tailings: A Matlab-Based Imaging Processing Tool. Sci Program 2020, 2020. [Google Scholar] [CrossRef]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A Critical Review of Prevention, Treatment, Reuse, and Resource Recovery from Acid Mine Drainage. J Clean Prod 2021, 329, 129666. [Google Scholar] [CrossRef]
- Hassas, B.V.; Rezaee, M.; Pisupati, S. V Precipitation of Rare Earth Elements from Acid Mine Drainage by CO2 Mineralization Process. Chemical Engineering Journal 2020, 399, 125716. [Google Scholar] [CrossRef]
- Cicek, Z. Selective Recovery of Rare Earth Elements from Acid Mine Selective Recovery of Rare Earth Elements from Acid Mine Drainage Treatment Byproduct Drainage Treatment Byproduct Recommended Citation Recommended Citation; 2023.
- McCarthy, T.S. The Impact of Acid Mine Drainage in South Africa. S Afr J Sci 2011, 107. [Google Scholar] [CrossRef]
- Naidoo, S. Social Constructions of Water Quality in South Africa: A Case Study of the Blesbokspruit River in the Context of Acid Mine Drainage Treatment. Social Constructions of Water Quality in South Africa: A case study of the Blesbokspruit River in the Context of Acid Mine Drainage Treatment 2022, 1–219. [CrossRef]
- Lourenco, M.; Curtis, C. The Influence of a High-Density Sludge Acid Mine Drainage (AMD) Chemical Treatment Plant on Water Quality along the Blesbokspruit Wetland, South Africa. Water SA 2021, 47. [Google Scholar] [CrossRef]
- Scott, R. Flooding of the Central and East Rand Gold Mines. WRC Report 486/1/95; Pretoria, 1995.
- Markovic, R.; Bessho, M.; Masuda, N.; Stevanovic, Z.; Bozic, D.; Trujic, T.A.; Gardic, V. New Approach of Metals Removal from Acid Mine Drainage. Applied Sciences 2020, 10, 5925. [Google Scholar] [CrossRef]
- Humphries, M.S.; McCarthy, T.S.; Pillay, L. Attenuation of Pollution Arising from Acid Mine Drainage by a Natural Wetland on the Witwatersrand. S Afr J Sci 2017, 113, 9. [Google Scholar] [CrossRef]
- Seervi. , V.; H.L, Y.; S.K, S.; A, J. Overview of Active and Passive Systems for Treating Acid Mine Drainage. IARJSET 2017, 4, 131–137. [Google Scholar] [CrossRef]
- Porter, C.M.; Nairn, R.W. Ecosystem Functions within a Mine Drainage Passive Treatment System. Ecol Eng 2008, 32, 337–346. [Google Scholar] [CrossRef]
- Ramla, B.; Sheridan, C. The Potential Utilisation of Indigenous South African Grasses for Acid Mine Drainage Remediation. Water SA 2015, 41, 247. [Google Scholar] [CrossRef]
- Qian, G.; Li, Y. Acid and Metalliferous Drainage–A Global Environmental Issue. Journal of Mining and Mechanical Engineering 2019, 1. [Google Scholar] [CrossRef]
- Dama-Fakir, P.; Sithole, Z.; van Niekerk, A.M.; Dateling, J.; Maree, J.P.; Rukuni, T.; Mthombeni, T.; Ruto, S.; Zikalala, N.; Hughes, C.; et al. Mine Water Treatment Technology Selection Tool: Users’ Guide (TT 711/17); 2017.
- Bwapwa, J.K. A Review of Acid Mine Drainage in a Water-Scarce Country: Case of South Africa. Environmental Management and Sustainable Development 2017, 7, 1. [Google Scholar] [CrossRef]
- Trumm, D. Selection of Active and Passive Treatment Systems for AMDflow Charts for New Zealand Conditions. New Zealand Journal of Geology and Geophysics 2010, 53, 195–210. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr Pollut Rep 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J Clean Prod 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Nleya, Y.; Simate, G.S.; Ndlovu, S. Sustainability Assessment of the Recovery and Utilisation of Acid from Acid Mine Drainage. J Clean Prod 2016, 113, 17–27. [Google Scholar] [CrossRef]
- Brewster, E.T.; Freguia, S.; Edraki, M.; Berry, L.; Ledezma, P. Staged Electrochemical Treatment Guided by Modelling Allows for Targeted Recovery of Metals and Rare Earth Elements from Acid Mine Drainage. J Environ Manage 2020, 275, 111266. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Jones, B.W.; Millar, G.J. Alternative Neutralisation Materials for Acid Mine Drainage Treatment. Journal of Water Process Engineering 2018, 22, 46–58. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid Mine Drainage from Coal Mining in the United States An Overview. J Hydrol (Amst) 2020, 588, 125061. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid Mine Drainage: Prevention, Treatment Options, and Resource Recovery: A Review. J Clean Prod 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U. Bin; Hijab, M.; Mackey, H.; McKay, G. Production and Applications of Activated Carbons as Adsorbents from Olive Stones. Biomass Convers Biorefin 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid Mine Drainage: Challenges and Opportunities. J Environ Chem Eng 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Ruihua, L.; Lin, Z.; Tao, T.; Bo, L. Phosphorus Removal Performance of Acid Mine Drainage from Wastewater. J Hazard Mater 2011, 190, 669–676. [Google Scholar] [CrossRef]
- Kumari, M.; Bhattacharya, T. A Review on Bioaccessibility and the Associated Health Risks Due to Heavy Metal Pollution in Coal Mines: Content and Trend Analysis. Environ Dev 2023, 46, 100859. [Google Scholar] [CrossRef]
- Orlović-Leko, P.; Farkaš, B.; Galić, I. A SHORT REVIEW OF ENVIRONMENTAL AND HEALTH IMPACTS OF GOLD MINING; 2022; Vol. 4.
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, Vol. 14, Page 2385 2022, 14, 2385. [Google Scholar] [CrossRef]
- Oggeri, C.; Laker, M.C. Environmental Impacts of Gold Mining—With Special Reference to South Africa. Mining 2023, Vol. 3, Pages 205-220 2023, 3, 205–220. [Google Scholar] [CrossRef]
- Li, S.; Yu, L.; Jiang, W.; Yu, H.; Wang, X. The Recent Progress China Has Made in Green Mine Construction, Part I: Mining Groundwater Pollution and Sustainable Mining. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A Review of Acid Mine Drainage: Formation Mechanism, Treatment Technology, Typical Engineering Cases and Resource Utilization. Process Safety and Environmental Protection 2023, 170, 1240–1260. [Google Scholar] [CrossRef]
- Zhu, M.; Li, B.; Liu, G. Groundwater Risk Assessment of Abandoned Mines Based on Pressure-State-Response—The Example of an Abandoned Mine in Southwest China. Energy Reports 2022, 8, 10728–10740. [Google Scholar] [CrossRef]
- Masindi, V.; Osman, M.S.; Shingwenyana, R. Valorization of Acid Mine Drainage (AMD): A Simplified Approach to Reclaim Drinking Water and Synthesize Valuable Minerals – Pilot Study. J Environ Chem Eng 2019, 7, 103082. [Google Scholar] [CrossRef]
- Luo, H.; Liu, G.; Zhang, R.; Bai, Y.; Fu, S.; Hou, Y. Heavy Metal Recovery Combined with H2 Production from Artificial Acid Mine Drainage Using the Microbial Electrolysis Cell. J Hazard Mater 2014, 270, 153–159. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).