Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
2.1. Chemicals and reagents
2.2. Tissue culture
2.3. Cell treatments
2.4. Cytotoxicity assay
2.5. Genotoxicity assay
2.6. Real-time PCR (qPCR)
2.7. Statistical analysis
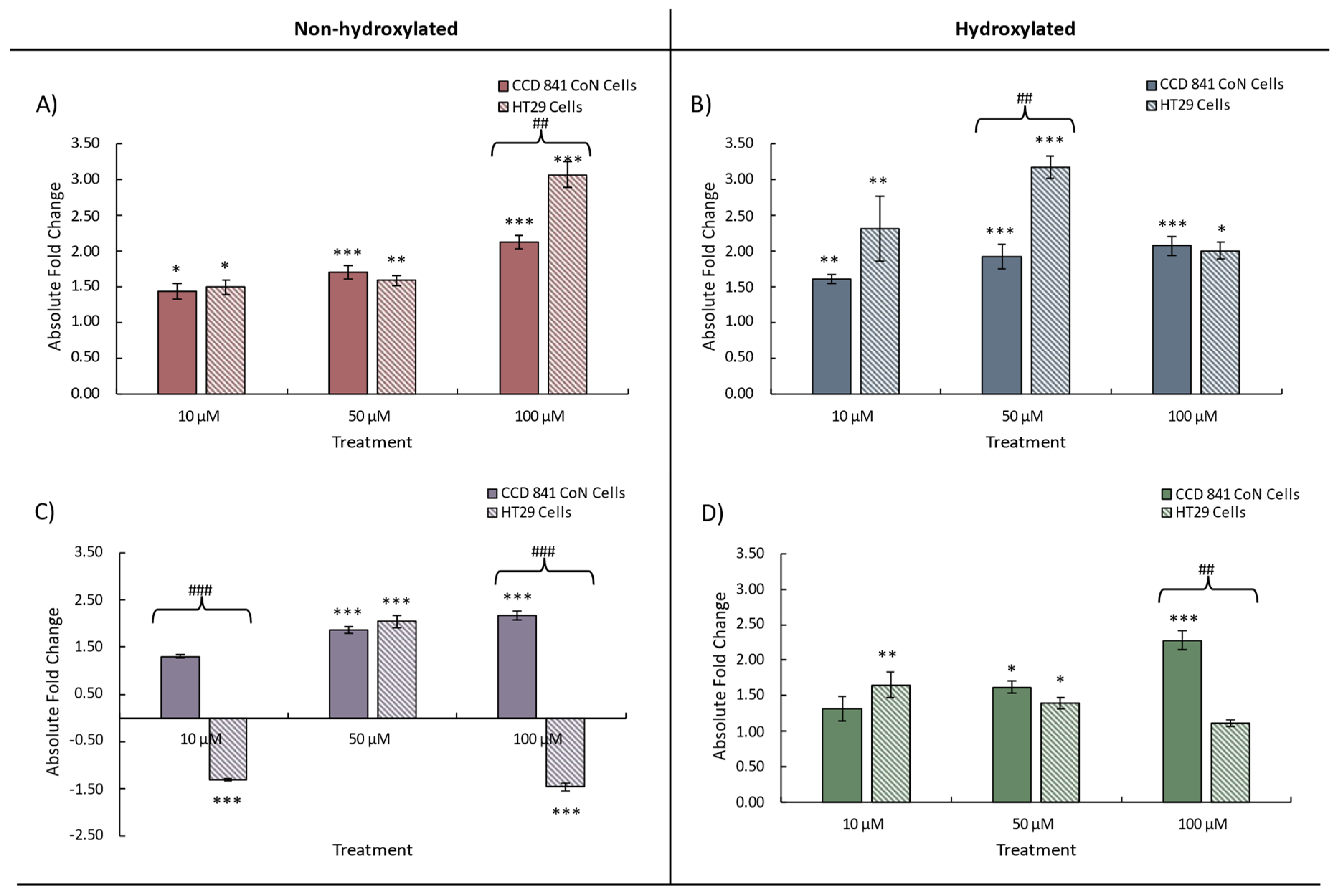
3. Results
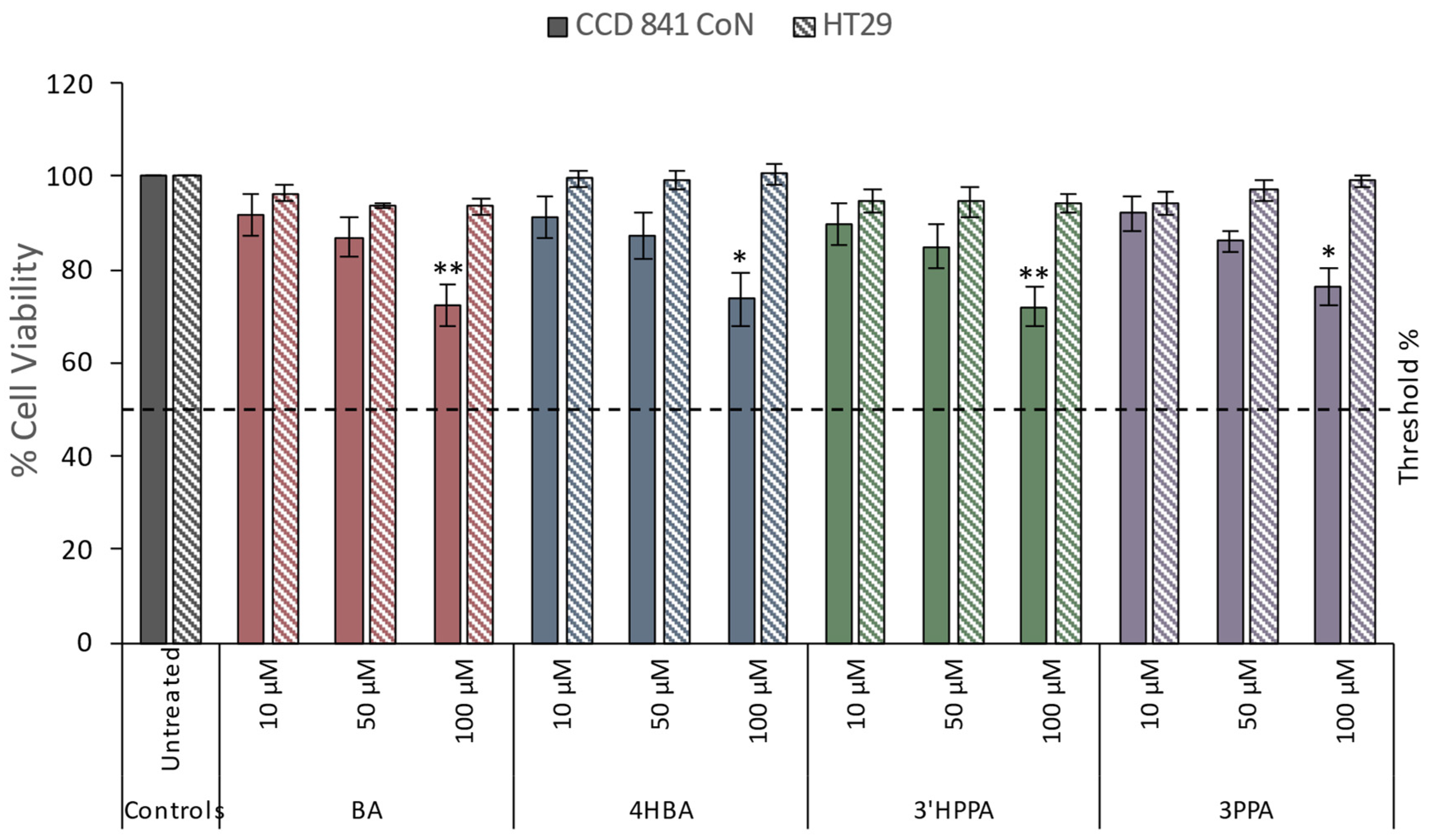
3.1. Cell viability
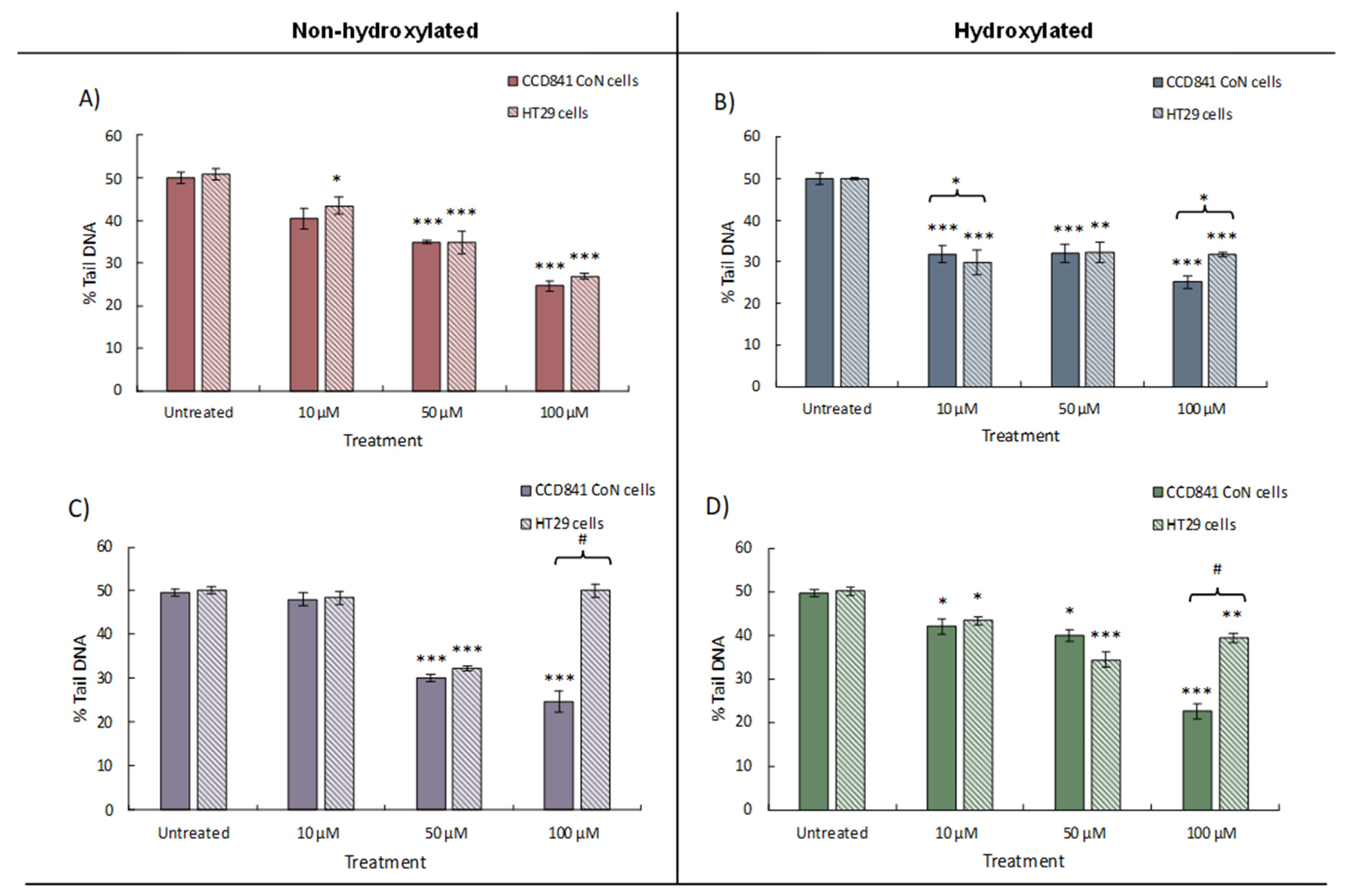
3.2. DNA damage reduction
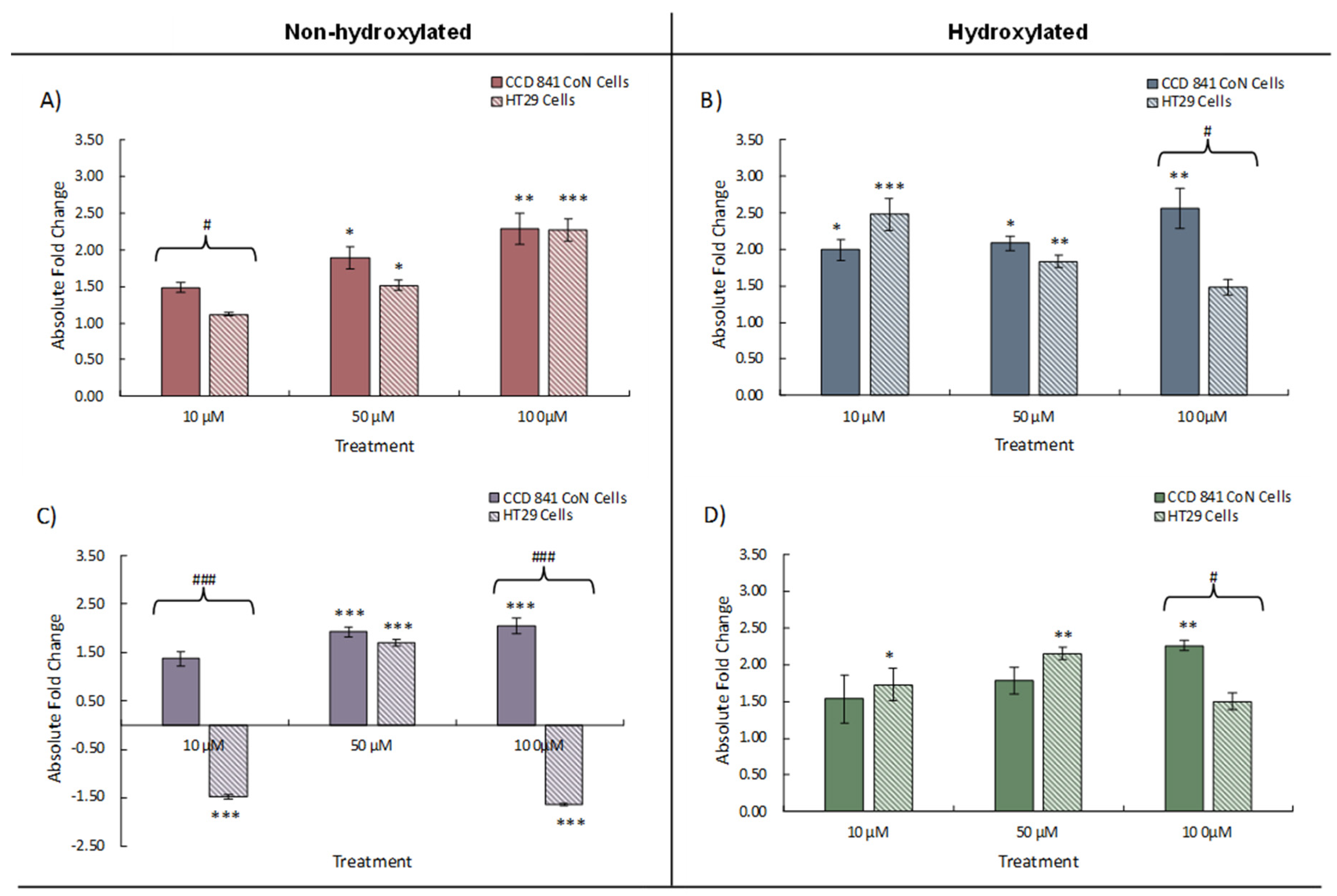
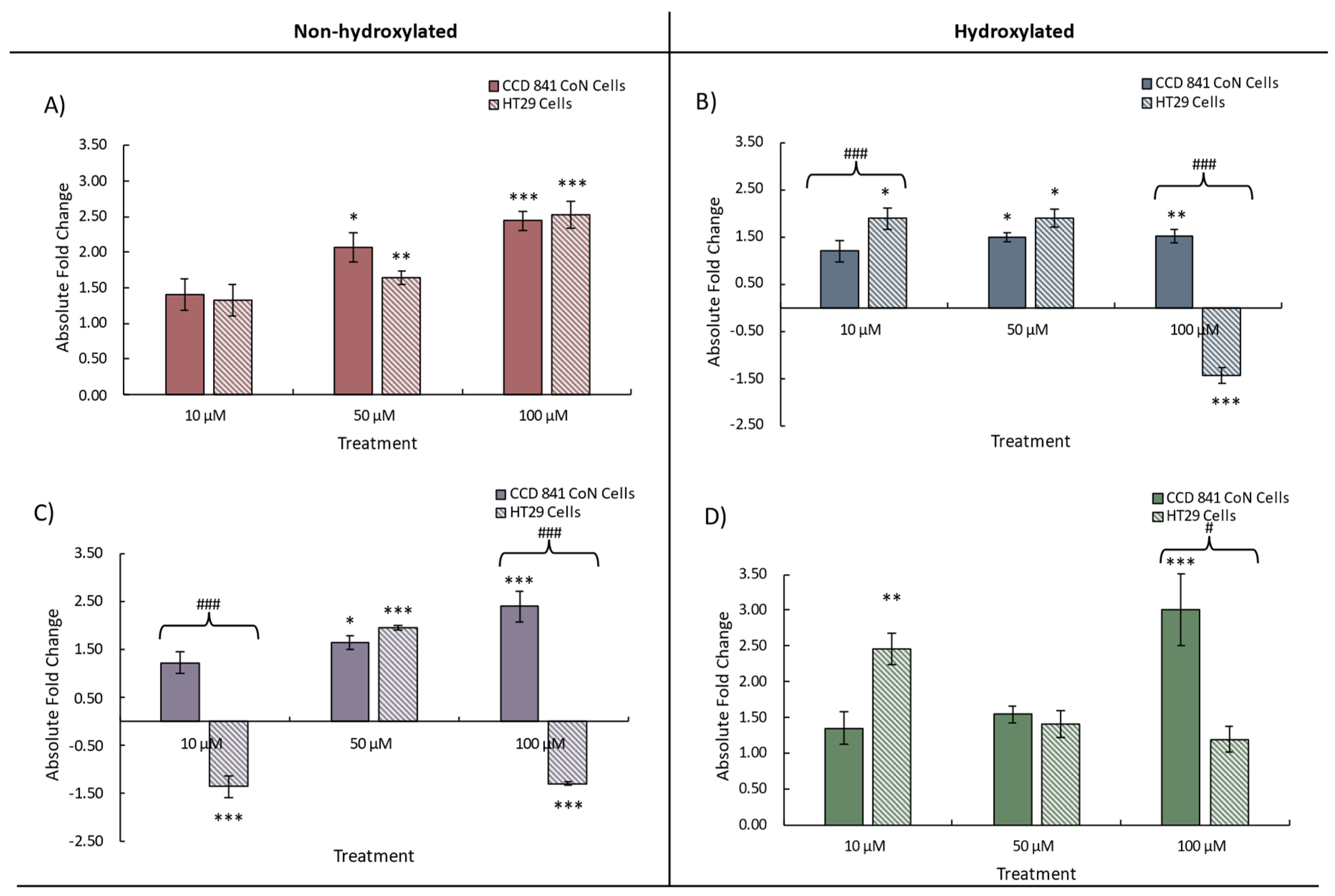
3.3. Modulation of gene expression within the Nrf2-pathway
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Patel, J.; Baptiste, B.A.; Kim, E.; Hussain, M.; Croteau, D.L.; Bohr, V.A. DNA damage and mitochondria in cancer and aging. Carcinogenesis 2020, 41, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Soria-Valles, C.; López-Soto, A.; Osorio, F.G.; López-Otín, C. Immune and inflammatory responses to DNA damage in cancer and aging. Mech Ageing Dev 2017, 165, 10–16. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Gurkar, A.U.; Wang, Y.; Vijg, J.; Hoeijmakers, J.H.J.; Robbins, P.D. Nuclear genomic instability and aging. Annu Rev Biochem 2018, 87, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Heinen, C.D. The mismatch repair-dependent DNA damage response: mechanisms and implications. DNA Repair 2019, 78, 60–69. [Google Scholar] [CrossRef]
- Rashid, S.; Freitas, M.O.; Cucchi, D.; Bridge, G.; Yao, Z.; Gay, L.; Williams, M.; Wang, J.; Suraweera, N.; Silver, A.; McDonald, S.A.C.; Chelala, C.; Szabadkai, G.; Martin, S.A. MLH1 deficiency leads to deregulated mitochondrial metabolism. Cell Death Dis 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E. , Santini, A. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res 2019, 33, 2221. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: the role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary flavonoids for immunoregulation and cancer: food design for targeting disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: a review. Med Res Rev 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.; Touillaud, M.; Katzke, V.; Kühn, T.; Boeing, H.; Förster, J.; Trichopoulou, A.; Valanou, E.; Peppa, E.; Palli, D.; Agnoli, C.; Ricceri, F.; Tumino, R.; de Magistris, M.S.; Peeters, P.H.M.; Bueno-de-Mesquita, H.B.; Engeset, D.; Skeie, G.; Hjartåker, A.; Menéndez, V.; Agudo, A.; Molina-Montes, E.; Huerta, J.M.; Barricarte, A.; Amiano, P.; Sonestedt, E.; Nilsson, L.M.; Landberg, R.; Key, T.J.; Khaw, K.; Wareham, N.J.; Lu, Y.; Slimani, N.; Romieu, I.; Riboli, E.; Scalbert, A. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Gavrilas, L.I.; Cruceriu, D.; Ionescu, C.; Miere, D.; Balacescu, O. Pro-apoptotic genes as new targets for single and combinatorial treatments with resveratrol and curcumin in colorectal cancer. Food Funct 2019, 10, 3717–3726. [Google Scholar] [CrossRef]
- Song, M.; Lee, D.; Chun, K.; Kim, E. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int J Mol Sci 2021, 22, 4376. [Google Scholar] [CrossRef]
- Dobani, S.; Latimer, C.; McDougall, G.J.; Allwood, J.W.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Ternan, N.G.; Pourshahidi, L.K.; Lawther, R.; Tuohy, K.M.; Del Rio, D.; O'Connor, G.; Rowland, I.; Almutairi, T.M.; Crozier, A.; Gill, C.I.R. Ex vivo fecal fermentation of human ileal fluid collected after raspberry consumption modifies (poly)phenolics and modulates geno-protective effects in colonic epithelial cells. Redox Biol 2021, 40, 101862. [Google Scholar] [CrossRef]
- Carregosa, D.; Pinto, C.; Ávila-gálvez, M.Á.; Bastos, P.; Berry, D.; Santos, C.N. A look beyond dietary (poly)phenols: the low molecular weight phenolic metabolites and their concentrations in human circulation. Compr Rev Food Sci Food Saf 2022, 21, 3931. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Chang, W.; Zhou, M. The effects and mechanisms of flavonoids on cancer prevention and therapy: focus on gut microbiota. J Agric Food Chem 2022, 70, 1451–1475. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Calani, L.; Brighenti, F.; Clifford, M.N.; Del Rio, D. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: synthesis, analysis, bioavailability, and bioactivity. Nat Protoc 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: toward prebiotics and further. Front Nutr 2021, 8, 689456. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fontana, M.; Latimer, C.; Ternan, N.G.; Pourshahidi, L.K.; Lawther, R.; O’Connor, G.; Conterno, L.; Gasperotti, M.; Angeli, A.; Lotti, C.; Bianchi, M.; Vrhovsek, U.; Fava, F.; Gobbetti, M.; Gill, C.I.R.; Tuohy, K.M. Ex Vivo Faecal fermentation of human ileal fluid collected after wild strawberry consumption modulates human microbiome community structure and metabolic output and protects against DNA damage in colonic epithelial cells. Mol Nutr Food Res 2021, 66, e2100405. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Brown, E.M.; Nitecki, S.; Pereira-Caro, G.; McDougall, G.J.; Stewart, D.; Rowland, I.R.; Crozier, A.; Gill, C.I. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: potential impact on colonic health. Food Funct 2014, 5, 611–623. [Google Scholar] [CrossRef]
- McDougall, G.J.; Allwood, J.W.; Pereira-Caro, G.; Brown, E.M.; Verrall, S.; Stewart, D.; Latimer, C.; McMullan, G.; Lawther, R.; O’Connor, G.; Rowland, I.; Crozier, A.; Gill, C.I. Novel colon-available triterpenoids identified in raspberry fruits exhibit antigenotoxic activities in vitro. Mol Nutr Food Res 2017, 61, 1600327. [Google Scholar] [CrossRef]
- Jin, H.; Tan, X.; Liu, X.; Ding, Y. Study of effect of tea polyphenols on microsatellite instability colorectal cancer and its molecular mechanism. Int J Colorectal Dis 2010, 25, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shan, S.; Li, H.; Shi, J.; Zhang, X.; Li, Z. Reversal effects of bound polyphenol from foxtail millet bran on multidrug resistance in human hct-8/fu colorectal cancer cell. J Agric Food Chem 2018, 66, 5190–5199. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic targeting of the Nrf2 signaling pathway in cancer. Molecules 2021, 26, 1417. [Google Scholar] [CrossRef]
- Islam, M.A.; Medha, M.M.; Nahar, A.U.; Al Fahad, M.A.; Siraj, M.A. Cancer protective role of selected dietary polyphenols via modulating Keap1/Nrf2/ARE and interconnected signaling pathways. Nutr Cancer 2023, 75, 1065–1102. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic Biol Med 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent advances of natural polyphenols activators for keap1-Nrf2 signaling pathway. Chem Biodivers 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Prasad, R.; Katiyar, S.K. Polyphenols from green tea inhibit the growth of melanoma cells through inhibition of class I histone deacetylases and induction of DNA damage. Genes Cancer 2015, 6, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Bishayee, A.; Yousefi, B. Polyphenols: Major regulators of key components of DNA damage response in cancer. DNA Repair 2019, 82, 102679. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: a comprehensive review. Cell Mol Biol Lett 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Gill, C.I.R.; Carafa, I.; Tuohy, K.M.; De Peña, M.-P.; Cid, C. Digestion and colonic fermentation of raw and cooked Opuntia ficus-indica Cladodes impacts bioaccessibility and bioactivity. J Agric Food Chem 2019, 67, 2490. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 2012, 13, 134. [Google Scholar] [CrossRef]
- Rashid, M.H.; Babu, D.; Siraki, A.G. Interactions of the antioxidant enzymes NAD(P)H: Quinone oxidoreductase 1 (NQO1) and NRH: Quinone oxidoreductase 2 (NQO2) with pharmacological agents, endogenous biochemicals and environmental contaminants. Chem Biol Interact 2021, 345, 109574. [Google Scholar] [CrossRef]
- Zhang, A.; Suzuki, T.; Adachi, S.; Naganuma, E.; Suzuki, N.; Hosoya, T.; Itoh, K.; Sporn, M.B.; Yamamoto, M. Distinct Regulations of HO-1 Gene Expression for Stress Response and Substrate Induction. Mol Cell Biol 2021, 41, e0023621. [Google Scholar] [CrossRef]
- Izquierdo-Vega, J.A.; Morales-González, J.A.; SánchezGutiérrez, M.; Betanzos-Cabrera, G.; Sosa-Delgado, S.M.; Sumaya-Martínez, M.T.; Morales-González, Á.; Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Evidence of some natural products with antigenotoxic effects. Part 1: Fruits and polysaccharides. Nutrients 2017, 9, 102. [Google Scholar] [CrossRef]
- Patra, S. , Pradhan, B., Nayak, R., Behera, C., Das, S., Patra, S., Efferth, T., Jena, M., and Bhutia, S. Dietary polyphenols in chemoprevention and synergistic effect in cancer: clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Coșarcă, S.; Moacă, E.; Tanase, C.; Muntean, D.; Pavel, I.; Dehelean, C. Spruce and beech bark aqueous extracts: source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci Technol 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Moura, C.; Noguti, J.; Jesus, G.; Ribeiro, F.; Garcia, F.; Gollucke, A.; Aguiar, O.; Ribeiro, D. Polyphenols as a chemopreventive agent in oral carcinogenesis: putative mechanisms of action using in-vitro and in-vivo test systems. Eur J Cancer Prev 2013, 22, 467–472. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Cáceres-Jimenez, S.; Bresciani, L.; Mena, P.; Almutairi, T.M.; Dobani, S.; Pourshahidi, L.K.; Gill, C.I.R.; Moreno Rojas, J.M.; Clifford, M.N.; Crozier, A. Excretion by subjects on a low (poly)phenol diet of phenolic gut microbiota catabolites sequestered in tissues or associated with catecholamines and surplus amino acids. Int J Food Sci Nutr 2023, 74, 532–543. [Google Scholar] [CrossRef]
- Brown, E.M.; McDougall, G.J.; Stewart, D.; Pereira-Caro, G.; González-Barrio, R.; Allsopp, P.; Magee, P.; Crozier, A.; Rowland, I.; Gill, C.I.R. Persistence of anticancer activity in berry extracts after simulated gastrointestinal digestion and colonic fermentation. PLOS ONE 2012, 7, e49740. [Google Scholar] [CrossRef]
- Pearce, S.C.; Coia, H.G.; Karl, J.P.; Pantoja-Feliciano, I.G.; Zachos, N.C.; Racicot, K. Intestinal in vitro and ex vivo models to study host-microbiome interactions and acute stressors. Front Physiol 2018, 9, 1584. [Google Scholar] [CrossRef]
- Zoetemelk, M.; Rausch, M.; Colin, D.J.; Dormond, O.; Nowak-Sliwinska, P. Short-term 3d culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep 2019, 9, 7103. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Z.; Wang, Y.; Zhang, L.; Chua, N.; Dai, L.; Chen, J.; Ho, C.L. Evaluation of the anti-inflammatory and anti-oxidative effects of therapeutic human lactoferrin fragments. Front Bioeng Biotechnol 2021, 9. [Google Scholar] [CrossRef]
- Randah, A.; Daniel, C.; Rowland, I.R. Acai fruit as a source of bioactive phytochemicals. Nutrients 2018, 10, 496. [Google Scholar] [CrossRef]
- Alqurashi, R.M.; Alarifi, S.N.; Walton, G.E.; Costabile, A.F.; Rowland, I.R.; Commane, D.M. In vitro approaches to assess the effects of açai (Euterpe oleracea) digestion on polyphenol availability and the subsequent impact on the faecal microbiota. Food Chem 2017, 234, 190–198. [Google Scholar] [CrossRef]
- Lee, U.-J.; Sohng, J.K.; Kim, B.-G.; Choi, K.-Y. Recent trends in the modification of polyphenolic compounds using hydroxylation and glycosylation. Curr Opin Biotechnol 2023, 80, 102914. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Wang, D.-S.; Chang, T.-S. Improving free radical scavenging activity of soy isoflavone glycosides daidzin and genistin by 3′-hydroxylation using recombinant Escherichia coli. Molecules 2016, 21, 1723. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Kostić, K.; Brborić, J.; Delogu, G.; Simić, M.R.; Samardžić, S.; Maksimović, Z.; Dettori, M.A.; Fabbri, D.; Kotur-Stevuljević, J.; Saso, L. Antioxidant activity of natural phenols and derived hydroxylated biphenyls. Molecules 2023, 28, 2646. [Google Scholar] [CrossRef]
- Mou, S.; Zhou, Z.; He, Y.; Liu, F.; Gong, L. Curcumin inhibits cell proliferation and promotes apoptosis of laryngeal cancer cells through Bcl-2 and PI3K/Akt, and by upregulating miR-15a. Mol Med Rep 2017, 14, 4937–4942. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yoshida, H.; Matsuda, S.; Ryoke, T.; Ohta, K.; Ohmori, M. The therapeutic potential of epigallocatechin 3 gallate against human oral squamous cell carcinoma through inhibition of cell proliferation and induction of apoptosis: In vitro and in vivo murine xenograft study. Clin Oral Investig 2016, 20, 1139–1148. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Ju, M.-K.; Kwon, K.-R.; Huh, Y.S.; Han, Y.-K.; Lee, S.H. Antioxidant mechanism of polyphenol-rich Nymphaea nouchali leaf extract protecting DNA damage and attenuating oxidative stress-induced cell death via Nrf2-mediated heme-oxygenase-1 induction coupled with ERK/p38 signaling pathway. Biomolecules 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Silva, M.M.; Rocha, C.R.R.; Kinker, G.S.; Pelegrini, A.L.; Menck, C.F.M. The balance between NRF2/GSH antioxidant mediated pathway and DNA repair modulates cisplatin resistance in lung cancer cells. Sci Rep 2019, 9, 17639. [Google Scholar] [CrossRef]
- Darband, S.G.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci 2020, 253, 117584. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural products as modulators of Nrf2 signaling pathway in neuroprotection. Int J Mol Sci 2023, 24, 3748. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Liu, Q.; Quiles, J.L.; Filosa, R.; Kamal, M.A.; Wang, F.; Kai, G.; Zou, X.; Teng, H.; Xiao, J. Protective effects of raspberry on the oxidative damage in HepG2 cells through Keap1/Nrf2-dependent signalling pathway. Food Chem Toxicol 2019, 133, 110781. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: bioactivity beyond antioxidant capacity. Trends Food Sci Technol 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp Mol Med 2019, 51, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




