1. Introduction
Aromatic and medicinal plants have the ability to synthesize numerous compounds that are categorized based on their significance for the plant's survival. These compounds are known as primary and secondary metabolites [
1]. Among the products synthesized by these plants are essential oils, which possess distinct antibacterial, antifungal, and food preservation properties against a broad spectrum of species and microbial pathogens [
2]. Several studies have highlighted the potential of essential oils in food preservation, primarily due to their active constituents such as terpenes, terpenoids, carotenoids, coumarins, and curcumins [
3]. These active constituents hold significant importance in the food industry [
4]. Furthermore, volatile compounds, as described by [
5] are organic molecules that readily evaporate at room temperature and contribute to the distinctive odor and taste associated with plants.
The HS-SPME technique offers several advantages, including its simplicity, non-destructive nature, and capability to capture a wide range of volatile compounds, as mentioned by Pellati et al [
6]. It involves utilizing a fiber coated with an appropriate sorbent material, which selectively adsorbs volatile compounds present in the headspace of the sample. Subsequently, the fiber is introduced into the GC-MS system for the separation and identification of the adsorbed compounds.
This technique has proven valuable for volatile compound analysis due to its sensitivity and selectivity, as highlighted by [
7].
To uncover the aromatic mysteries concealed within Thymus vulgaris from the Moroccan terrain, a state-of-the-art technique called headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS) emerges as a remarkable scientific tool. This powerful combination allows for the extraction, separation, and analysis of volatile compounds, providing insights into the intricate tapestry of aromatic molecules [
8].
In this article, we present the methodology employed for HS-SPME and GC-MS analysis, followed by the preliminary characterization of the volatile compounds in Thymus vulgaris from Morocco. The findings of this study contribute to the expanding knowledge base regarding the chemical composition of Thymus vulgaris and pave the way for further research on its potential applications across various industries.
2. Materials and Methods
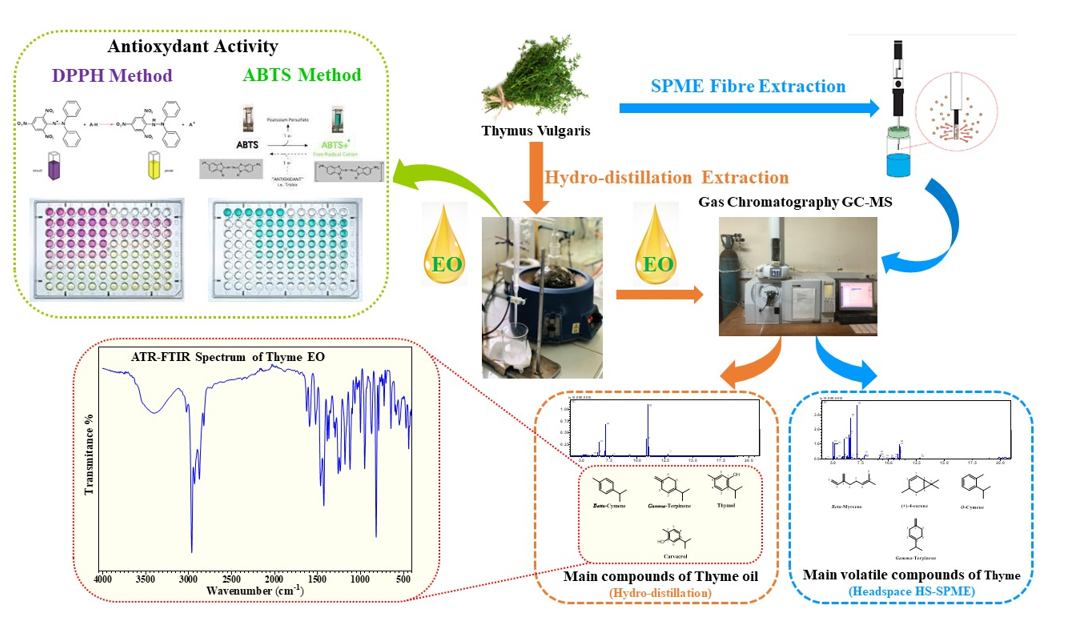
2.1. Plant material and extraction process
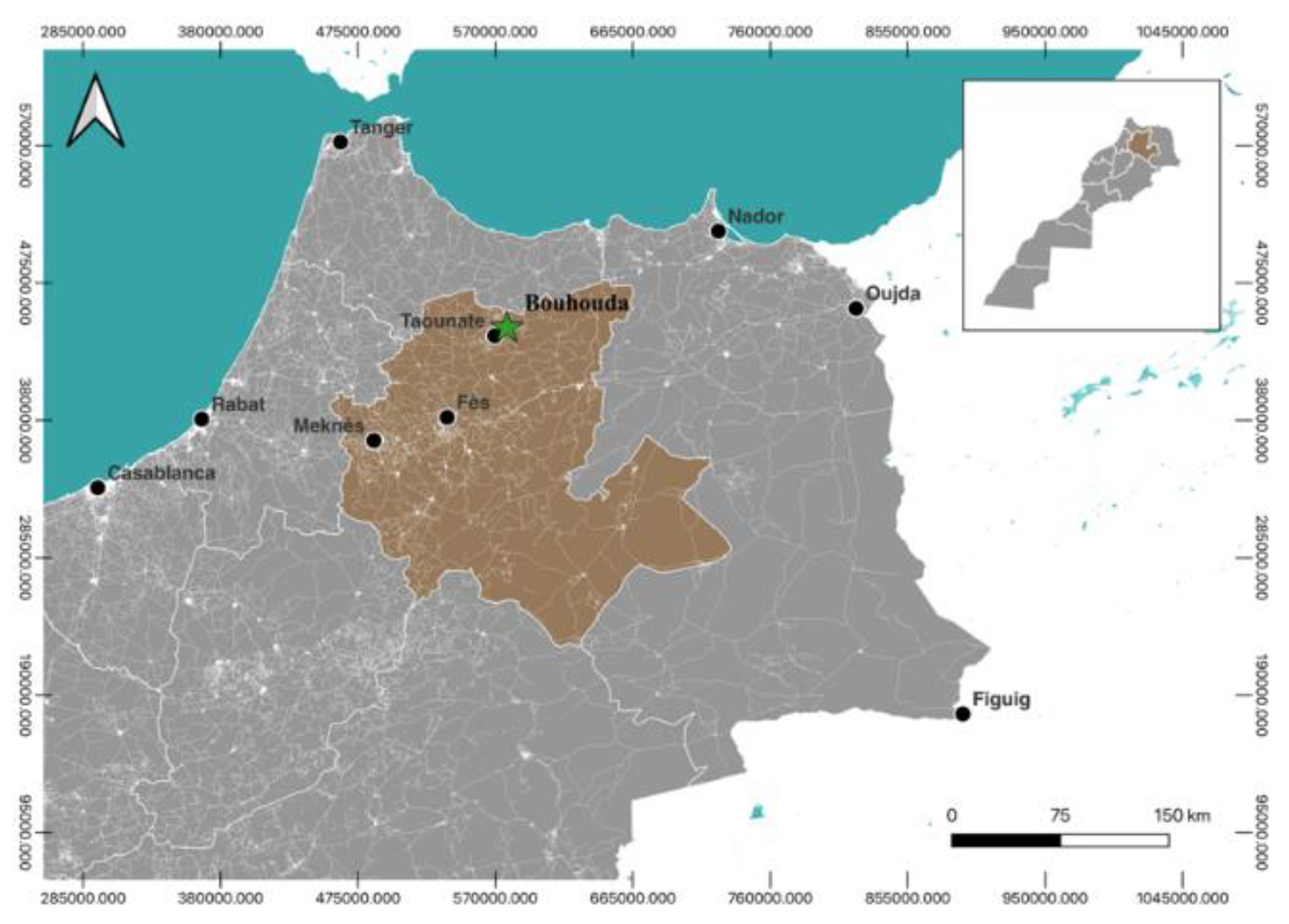
The samples of Thymus vulgaris were collected from the Taounate region, specifically in Plaine Bouhouda (
Figure 1). The harvesting process was carried out randomly on May 19, 2021. Following the harvest, the plant was air-dried in a shaded area for a duration of 10 days. Subsequently, 200g of the dried plant material was subjected to hydrodistillation using a Clevenger apparatus for a period of 3.5 hours. The resulting essential oil was collected and stored in a glass vial, which was then kept at a temperature of -20 degrees Celsius.
2.2. GC-MS conditions for Headspace HS-SPME
HTA Autosampler Manager multipurpose sampler was used for the HS-SPME procedure with a 50/30μm divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS) filter. 2g of thyme powder was transferred to a 20ml headspace flask.
The sample was incubated for 5min in a heating box at 80°C. The fiber was desorbed at 250°C, followed by isolation and identification by GC-MS Shimadzu GC system (Kyoto, Japan). The GC system was equipped with a capillary column RTX-5, which had a stationary phase of dimethylpolysiloxane containing 5% of a phenyl group (30mx0.25mmx0.25). The mobile phase used was pure helium (99.99%), flowing at a constant rate of 3 mL min
-1. During the analysis, the injection, ion source, and interface temperature were all set at 250°C. The GC oven temperature was initially held at 50°C for 1 minute, then ramped up to 250°C at a rate of 10°C/minute and maintained for 1 minute. Mass spectra were acquired at an electron energy of 70eV within the mass range of 40 to 300m/z. The identification of the components present in the essential oils was performed using a database, likely referring to a reference database containing information about known chemical compounds [
9].
2.3. Attenuated total reflecting-Fourier-transform infrared (ATR-FTIR) analysis
To assess and confirm the chemical composition of the substances in thyme essential oil, the ATR-FTIR method was employed. Spectra were recorded on a Jasco 4700-ATR spectrophotometer (Shimadzu, Japan) in the range of 400-4000 cm-1.
2.4. Scavenging activity of DPPH radical
The DPPH radical scavenging capacity of Thyme essential oil samples was determined using the following method [
10]. A total of 0.5mL of the sample was mixed with 2.5mL of a methanolic solution containing DPPH at a concentration of 0.004%. The mixture was vigorously shaken and then incubated for 1 hour at room temperature in the dark. After incubation, the absorbance of the mixture was measured at 517nm using a Shimadzu UV-1650PC spectrophotometer, with a blank serving as the reference. Ascorbic acid was used as a control in this experiment. The IC50 value, which represents the concentration of the sample required to inhibit 50% of the DPPH° radicals.
2.5. Antioxidant assay using a model β-carotene/linoleate system
The antioxidant activity was determined using the β-carotene bleaching method with some modifications [
11]. Initially, 1ml of a β-carotene solution with a concentration of 0.2 mg mL
-1, solubilized in 1ml of chloroform, was added to a mixture containing 200mg of Tween 20 and 20mg of linoleic acid. The mixture was then subjected to evaporation at 40°C to remove the solvent. After evaporation, the mixture was diluted with 30ml of distilled water and placed in an ultrasonic bath with stirring to form an emulsion. Subsequently, 4ml of this emulsion was transferred to tubes, each containing 0.2ml of the sample dissolved in methanol at various concentrations. The samples were vigorously vortexed and then placed in a water bath set at 45°C. The initial absorbance (A
i) of each sample was measured at 470nm. After a period of 2 hours, the final absorbance (A
f) was measured at the same wavelength (470nm). All samples were analyzed in triplicate, and the results were averaged.
2.6. Radical cation ABTS+. decoloration test
The radical cation ABTS [2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium] was evaluated using a technique described in the literature, with some modifications [
12]. Initially, an aqueous solution of ABTS with a concentration of 7 mM was mixed with an aqueous solution of K
2S
2O
8 at a concentration of 2.4 mM in equal volumes. The resulting mixture was incubated in the dark at room temperature for 16 hours. After incubation, the radical cation solution was diluted with ethanol until it reached an absorbance value of 0.8 at 744 nm. Then, 1mLof this diluted solution was mixed with 1mL of an ethanolic solution containing the examined or reference essential oil at various concentrations. To zero the spectrophotometer, pure ethanol was used as a reference. Absorbances were measured at 734 nm after a 10-minute incubation in the dark at room temperature.
2.7. Statistical analysis
The obtained results were subjected to descriptive statistical analysis and analysis of variance (ANOVA) using the software "GraphPad Prism 6". All experiments were performed in triplicate, and the data were expressed as the mean ± standard deviation (SD).
3. Results and discussions
3.1. Chemical composition of essential oils by GC-MS and HS-SPME
In this study, we compared the chemical composition of essential oils of
Thymus vulgaris L. grown in the Taounat region by gas chromatography coupled with GC-MS mass spectroscopy and HS-SPME headspace microextraction. The results obtained by GC/MS analysis of essential oils are summarized in (
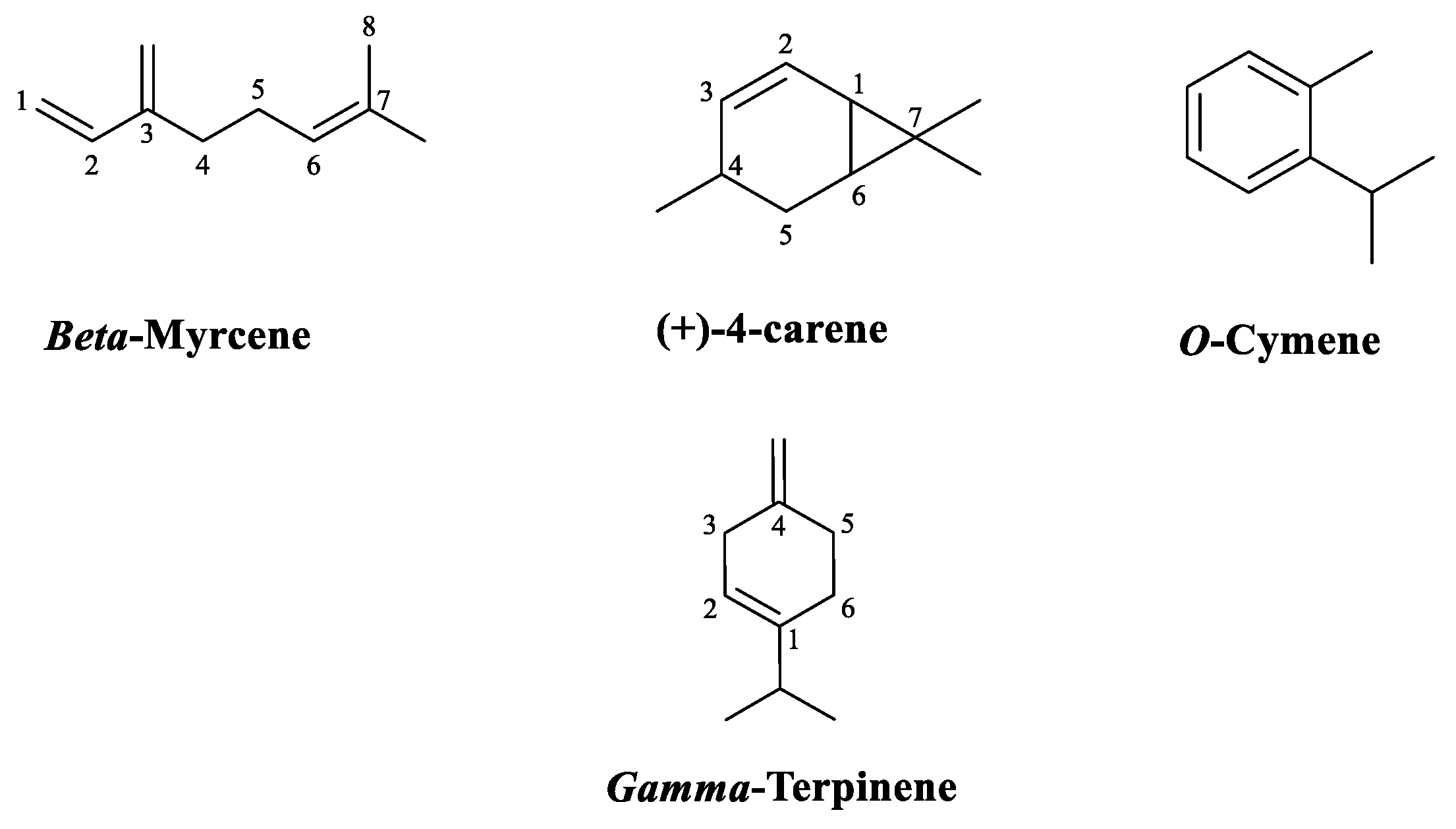
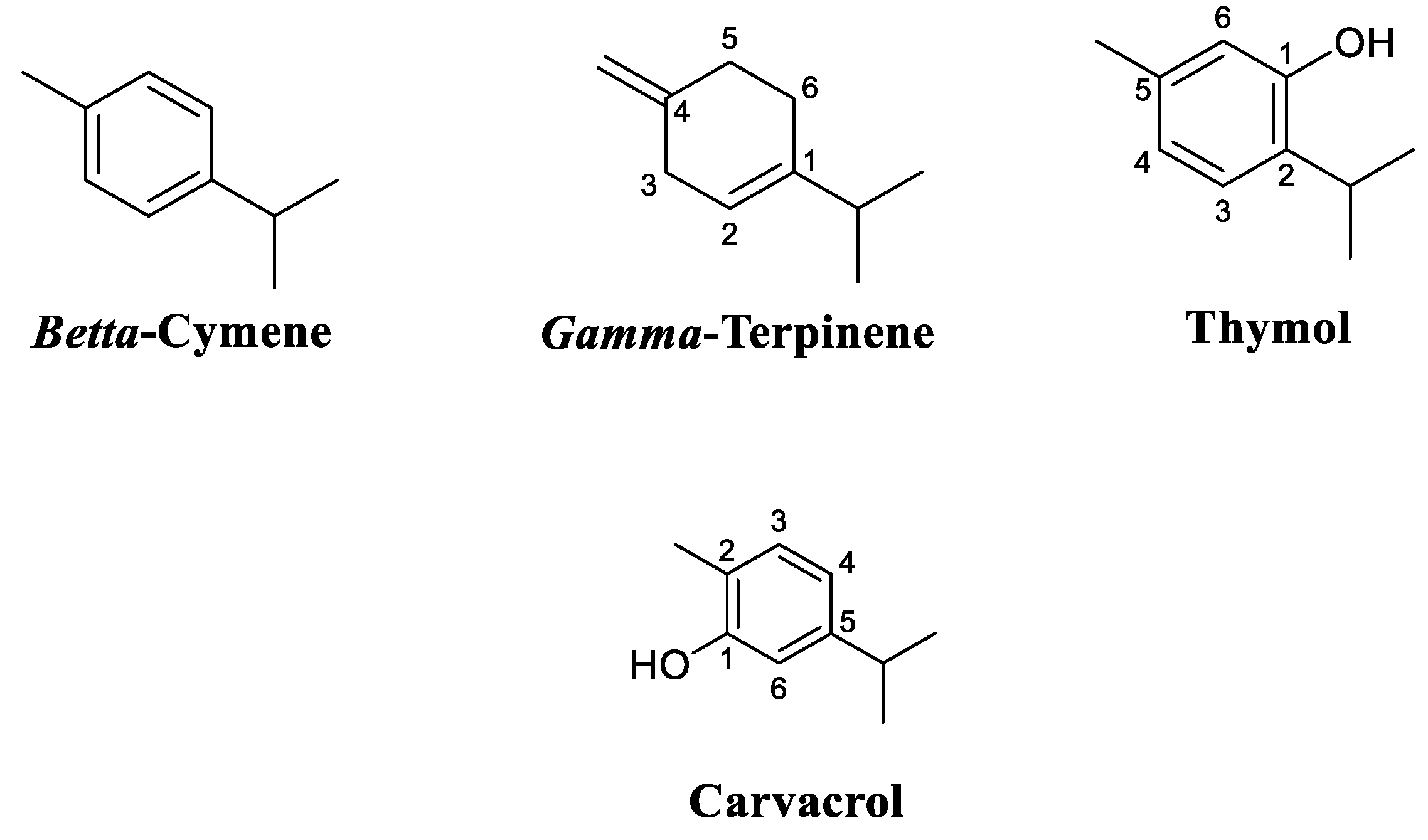
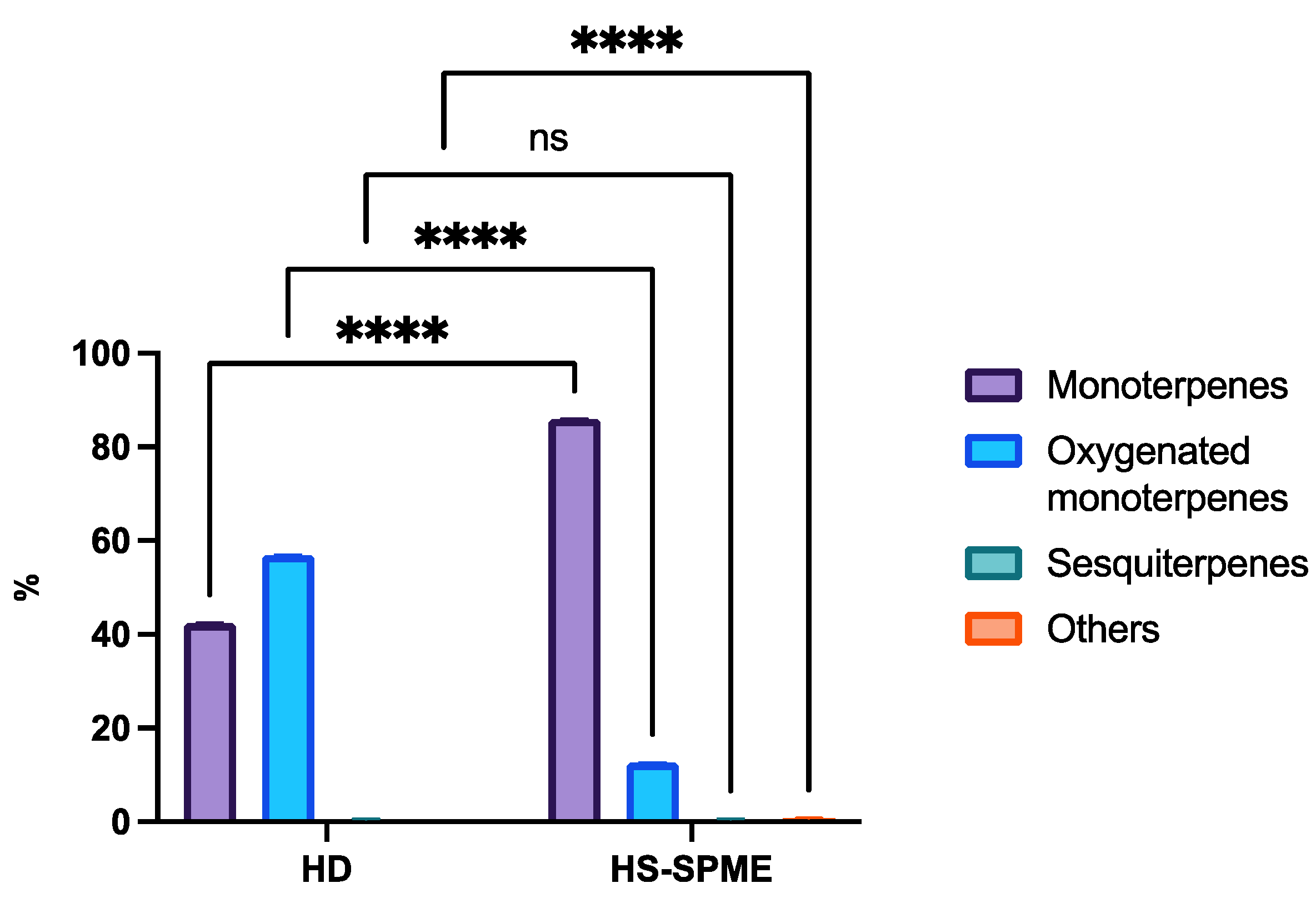
Table 1) where the compounds are listed in order of their retention index on the RTx-5 column. The analysis of the chemical composition of Thyme extract by HS-SPME identified 27 compounds and showed that monoterpene hydrocarbons are the most dominant compounds (85.94%) while oxygenated monoterpenes represented (12.69%). It appears that the main constituents of the essential oil by HS-SPME represent 61.56% of the total chemical composition. These are gamma-terpinene (20.73%), o-cymene (15.04%), 4-carene (14.04%), and beta-myrcene (11.75%). The qualitative analysis of volatile compounds by gas chromatography coupled with GC-MS mass spectroscopy allowed us to identify 11 compounds representing 100% of the total sum of the areas of the peaks of the chromatograms, where oxygenated monoterpenes occupy the first place (56.97%), followed by monoterpenes (42.46%) and sesquiterpenes (0.57%). This oil is characterized by carvacrol (37.63%), thymol (17.35%), gamma-terpinene (24.13%), and beta cymene (10.56%)
(Figure 2 and
Figure 3).
The constituents of the oils from both methods were present in varying proportions. Nevertheless, some components were present only in one or the other. In this respect, alpha terpinene and beta cymene were present only in the Thyme extract by hydrodistillation.
The difference observed between the volatile fraction by HS-SPME and thyme essential oil extracted by hydrodistillation has been reported in the literature. Solid-phase microextraction allows identifying sometimes a large number of compounds or the opposite compared to the classical analysis of essential oils [
13].
Essential oils are a complex mixture of volatile compounds containing polar and non-polar components at different concentrations [
14]. Two or three predominant components are present at high concentrations (20-90%), while the remaining compounds are generally lower [
15].
The terpene fraction is the main group constituting essential oils. Terpenes are characterized by their isoprene units while terpenoids are terpenes that have been biochemically modified by enzymes that substitute oxygen molecules and remove a methyl group [
16]. It is worth noting that monoterpenes (C10) consist of two isoprene molecules (C
5H
8) that present the predominant molecules in EOs, they can reach a percentage of up to 90% of the whole EO and allow a wide variety of structures. Secondly, the aromatic and aliphatic group which is the least abundant in EOs, and its compounds correspond to alcohols, aldehydes, phenols, heterocycles, and methoxy derivatives [
17]. Nevertheless, it should be noted that phenolic compounds ( carvacrol, thymol, eugenol) are one of the most important molecules responsible for the antimicrobial effects of essential oils[
18]. There is evidence that the chemical composition of EOs is highly variable, both qualitatively and quantitatively, depending on factors such as the method of extraction, the season of harvesting, the geographical origin, the plant organ, and the degree of maturity of the plant. This composition was similar to that of leaves grown in Morocco described in the literature [
19]. SPME tends to extract a greater amount of the more volatile monoterpenes than solvent extraction techniques [
20]. These differences are due to the matrix effect in the release of volatile compounds, as each spice has a characteristic plant tissue structure.
Understanding the volatile compounds present in Thymus vulgaris from Morocco holds great significance for the scientific community and industries involved in producing herbal extracts, essential oils, and flavoring agents. This knowledge can help in the selection of appropriate chemotypes for targeted applications, such as the development of natural preservatives or therapeutics [
15].
The preliminary characterization of volatile compounds of Thymus vulgaris from Morocco by headspace solid-phase microextraction (HS-SPME) and GC-MS has demonstrated the richness and diversity of compounds present in this plant. The results highlight the presence of potentially beneficial bioactive compounds for human health.
The variation in volatile profiles according to geographical origin suggests an influence of environmental conditions and local agricultural practices on the chemical composition of thyme. However, further studies are needed for a more in-depth characterization of the compounds.
Figure 4 shows the different classes of chemical compounds found in the essential oil of
Thymus vulgaris L. using different techniques (HD and HS-SPME), the results show that monoterpenes represent 84.94% of Thymus vulgaris L EO, while they represent only 42.46% using hydrodistillation (statistically significant difference P<0.05). similarly, for oxygenated monoterpenes, it was found that they constituted 56.97% using hydrodistillation, and 12.69% using the HS-SPME technique (statistically significant difference P<0.05), while sesquiterpenes represented 0.57% in both methods, and finally, the HS-SPME technique enabled the identification of other compounds not detected by analyzing the essential oil, representing 0.80% of the compounds identified.
3.2. Attenuated total reflecting-Fourier-transform infrared (ATR-FTIR) analysis
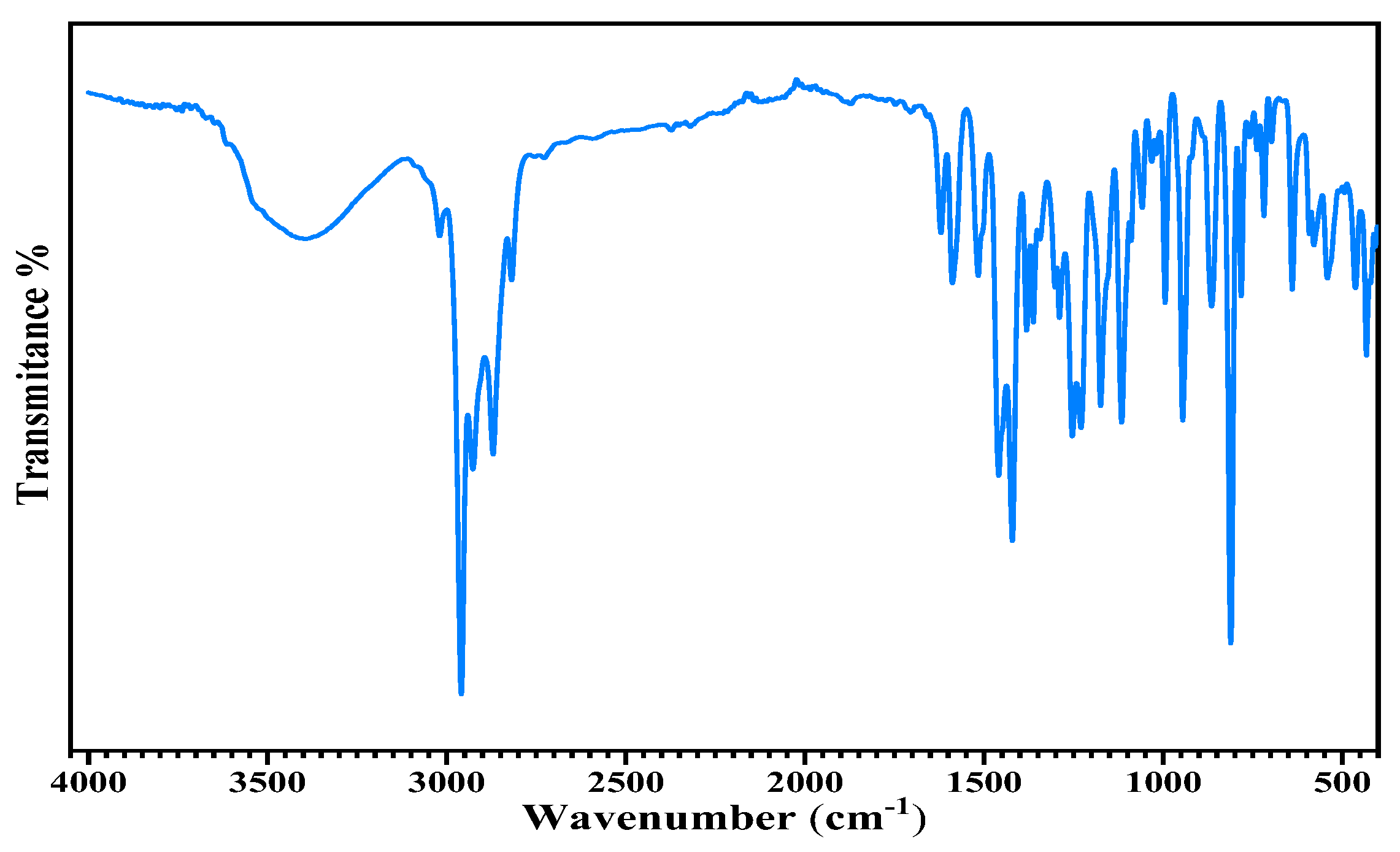
The ATR-FTIR spectrum of Thyme essential oil is presented in (
Figure 5). The spectrum showed an adsorption band between 3500 and 3200 cm-1 which corresponds to hydroxyl (OH) stretching vibrations. The presence of an intense band may indicate the presence of compounds such as phenols and alcohols in the essential oil. The peak observed around 2960 cm-1 is generally attributed to stretching vibrations of the CH bond of methyl groups (CH3) present in terpenoids. These terpenoids are volatile compounds commonly found in essential oils, including Thyme essential oil. The adsorption band at 1660cm-1 is generally associated with the stretching vibrations of the carbonyl bond (C=O). In this case, this band may be due to the presence of compounds such as thymol or carvacrol, which are phenols characteristic of this oil. Bands between 1400 and 1600cm-1 are often attributed to the bending vibrations of aromatic groups present in phenolic compounds such as thymol. Adsorption bands between 900 and 1200cm-1 are due to CO bond vibrations, which may indicate the presence of monoterpenes. A similar observation has been made by other authors [
21,
22,
23] .
3.3. Antioxidant activity
Many complementary methods have been used to evaluate the antioxidant activity of Thymus vulgaris essential oil from the Taounat region. However, a single technique cannot evaluate all possible mechanisms that characterize an antioxidant. In this regard, three tests were performed: DPPH free radical scavenging, β-carotene bleaching test, and ABTS+- cation decoloration test. The effectiveness of the essential oil and ascorbic acid in scavenging the DPPH radical is represented by the IC50 value; it corresponds to the concentration required to inhibit 50% of the initial free radical concentration. The lower the IC50 value, the more appreciable the compound's antioxidant activity. The antioxidant activity of essential oils is attributed to their chemical composition. However, it can be due to one of the majority constituents, other minority constituents, or synergy between them [
24]. Our essential oil contains monoterpenes at fairly high levels. An in the previous study reported that oils with a monoterpene predominance showed́ a rather modest activity [
25]. (Ruberto and Baratta et al. 2000) attributed the modest antioxidant potential of essential oil to the presence of oxygenated monoterpenes including 1,8-cineole which is considered a weak antioxidant with a fairly high IC50 of 9.360 mg mL
-1 [
26]. Using free radical scavenging test (DPPH) method and beta-carotene bleaching assay (
Table 2), the antiradical potential of tested thyme essential oil was lower than that of ascorbic acid with IC50 of (0.016±0.001 mg mL
-1; 0.82±0.003 mg mL
-1) respectively. The IC50 of thyme EO was estimated to be (0.51±0.11 mg mL
-1; 2.58±0.10 mg mL
-1) respectively. Using the ABTS method, Thymus vulgaris essential oil showed high antioxidant activity (IC50=0.013±0.004 mg mL
-1) compared to synthetic antioxidants whose IC50=0.05±0.008 mg mL
-1).
The essential oil of Thyme consisting of sesquiterpenes and monoterpenes showed very good values for the ABTS test, it may be due to the usefulness of this method for investigations on lipophilic antioxidants [
27]. The lack of antioxidant activity for this essential oil in the DPPH radical reduction and beta carotene bleaching assay may be due to the fact that terpene compounds are not capable of donating a hydrogen atom and the low solubility they provide in the reaction medium of the assay, as this assay uses methanol as solvent. In general, the essential oil of thyme tested from the Taounat region showed a modest antioxidant potential with all three methods.
4. Conclusion
T. Vulgaris was found to produce a higher yield of EO, suggesting that the qualification of this species is economical for the food industry. Thymol and carvacrol are the primary components that define the quality of Thymus vulgaris. These constituents have a significant role in flavoring and seasoning food. In addition, medicinal plants that have a high capacity for antioxidation help to prevent the cells from being damaged by free radicals. The results of DPPH analysis, beta-carotene bleaching, and ABTS+ cation decoloration test indicated that thyme essential oil had high antioxidant activity; this is consistent with their thymol and carvacrol contents. These species were also found to be rich in phenolic compounds and flavonoids. The information gained from these results may be useful for the cultivation of some Thymus species. Moreover, thymol and carvacrol, the primary components of the Eos of the studied Thymus species, can be considered to be natural sources of antioxidants that have value in food and medicinal applications. Future investigations could be directed to the aromatization of olive oil with essential oils to protect them from oxidation to provide new information.
Author Contributions
Conceptualization, Y.Belbachir. and H.El Farissi.; methodology, Y.Belbachir., H. El Farissi, A. Beraich; S.E. Azizi. and I.Ziani.; investigation, A. Talhaoui and A. El Bachiri.; resources, Y.Belbachir.; data curation, H. El Farissi.; writing-original draft preparation, Y. Belbachir. and H. El Farissi.; writing-review and editing, H.El Farissi. and A. Talhaoui. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No data was used for the research described in the article.
Acknowledgments
This work comes within the framework of valorization of the bioresources within the laboratory of Environment and Applied Chemistry (LCAE), Physical Chemistry of Natural Resources and Processes team, Faculty of Sciences, Oujda, Morocco. The authors would like to express their gratitude to the Director of the Analysis Platform at the Faculty of Sciences in Oujda for generously providing access to the necessary facilities and materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tripathi NN, Kumar N Putranjiva roxburghii oil—A potential herbal preservative for peanuts during storage. Journal of Stored Products Research 2007, 43, 435–442. [CrossRef]
- Sonker N, Pandey AK, Singh P Efficiency of Artemisia nilagirica (Clarke) Pamp. essential oil as a mycotoxicant against postharvest mycobiota of table grapes: Artemisia nilagirica oil as a mycotoxicant for table grapes. J Sci Food Agric 2015, 95, 1932–1939. [CrossRef] [PubMed]
- Gormez A, Bozari S, Yanmis D, et al. The Use of Essential Oils of Origanum rotundifolium as Antimicrobial Agent Against Plant Pathogenic Bacteria. Journal of Essential Oil Bearing Plants 2016, 19, 656–663. [Google Scholar] [CrossRef]
- Pandey AK, Mohan M, Singh P, et al. Chemical composition, antibacterial and antioxidant activity of essential oil of Eupatorium adenophorum Spreng. from Eastern Uttar Pradesh, India. Food Bioscience 2014, 7, 80–87. [Google Scholar] [CrossRef]
- Pandey AK, Kumar P, Singh P, et al. (2017) Essential Oils: Sources of Antimicrobials and Food Preservatives. Front Microbiol 7. Front Microbiol 7.
- Pellati F, Prencipe FP, Benvenuti S Headspace solid-phase microextraction-gas chromatography–mass spectrometry characterization of propolis volatile compounds. Journal of Pharmaceutical and Biomedical Analysis 2013, 84, 103–111. [CrossRef]
- Plutowska B, Chmiel T, Dymerski T, et al. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chemistry 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Soleimani M, Daryasari AP, Ghorbani A, et al. Analysis of the Volatile Compounds in Thymus vulgaris L. Using Improved HS-SPME-GC-MS and Comparison with Conventional Methods. Journal of Essential Oil Bearing Plants 2014, 17, 1233–1240. [Google Scholar] [CrossRef]
- Loukili EH, Abrigach F, Bouhrim M, et al. Chemical Composition and Physicochemical Analysis of Opuntia dillenii Extracts Grown in Morocco. Journal of Chemistry 2021, 2021, 1–11. [Google Scholar]
- Sánchez-Vioque R, Rodríguez-Conde MF, Reina-Ureña JV, et al. In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.). Industrial Crops and Products 2012, 39, 149–153. [Google Scholar] [CrossRef]
- Conforti F, Statti G, Uzunov D, et al. Comparative Chemical Composition and Antioxidant Activities of Wild and Cultivated Laurus nobilis L. Leaves and Foeniculum vulgare subsp. piperitum (Ucria) Coutinho Seeds. Biological & Pharmaceutical Bulletin 2006, 29, 2056–2064. [Google Scholar]
- Dieng SIM, Fall AD, Diatta-Badji K, et al. Evaluation de l’activité antioxydante des extraits hydro-ethanoliques des feuilles et écorces de Piliostigma thonningii Schumach. Int J Bio Chem Sci 2017, 11, 768. [Google Scholar] [CrossRef]
- Manssouri M, Ansari A, Znini M, et al. CARACTERISATION DES COMPOSES VOLATILS D’AMMODAUCUS LEUCOTRICHUS DU MAROC PAR MICROEXTRACTION EN PHASE SOLIDE DANS L’ESPACE DE TETE (HS-SPME) ET PAR HYDRODISTILLATION COUPLE A LA CG-SM. 7.
- Raut JS, Karuppayil SM A status review on the medicinal properties of essential oils. Industrial Crops and Products 2014, 62, 250–264. [CrossRef]
- Pavela R Essential oils for the development of eco-friendly mosquito larvicides: A review. Industrial Crops and Products 2015, 76, 174–187. [CrossRef]
- Burt S Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology 2004, 94, 223–253. [CrossRef] [PubMed]
- Dhifi W, Bellili S, Jazi S, et al. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Khan A, Shoaib Amjad M, Saboon GC-MS analysis and biological activities of Thymus vulgaris and Mentha arvensis essential oil. Turkish Journal of Biochemistry 2019, 44, 388–396. [CrossRef]
- Ed-Dra A, Nalbone L, Filali FR, et al. Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability 2021, 13, 4594. [Google Scholar] [CrossRef]
- Rohloff J Monoterpene Composition of Essential Oil from Peppermint ( Mentha × piperita L. ) with Regard to Leaf Position Using Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry Analysis. J Agric Food Chem 1999, 47, 3782–3786. [Google Scholar] [CrossRef]
- Tarhan İ, Çelikten Ş, Kestek HM, et al. Development of a new and rapid FTIR method using chemometric modeling techniques for the determination of lavandin adulteration in lavender essential oil. Vibrational Spectroscopy 2023, 127, 103559. [Google Scholar] [CrossRef]
- Gudi G, Krähmer A, Krüger H, et al. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy on Intact Dried Leaves of Sage ( Salvia officinalis L.): Accelerated Chemotaxonomic Discrimination and Analysis of Essential Oil Composition. J Agric Food Chem 2015, 63, 8743–8750. [Google Scholar] [CrossRef]
- Kayabaş A, Yıldırım E Biochemical Fingerprints of Some Endemic Plants Growing in Gypsum Soils: Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopic Study. ejb 2021, 0, 0–0.
- Wang W, Wu N, Zu YG, et al. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chemistry 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Gachkar L, Yadegari D, Rezaei M, et al. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chemistry 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Ruberto G, Baratta MT Antioxidant activity of selected essential oil components in two lipid model systems. Food Chemistry 2000, 69, 167–174. [CrossRef]
- Andrade MA, das Graças Cardoso M, De Andrade J, et al. Chemical composition and antioxidant activity of essential oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).