Submitted:
09 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
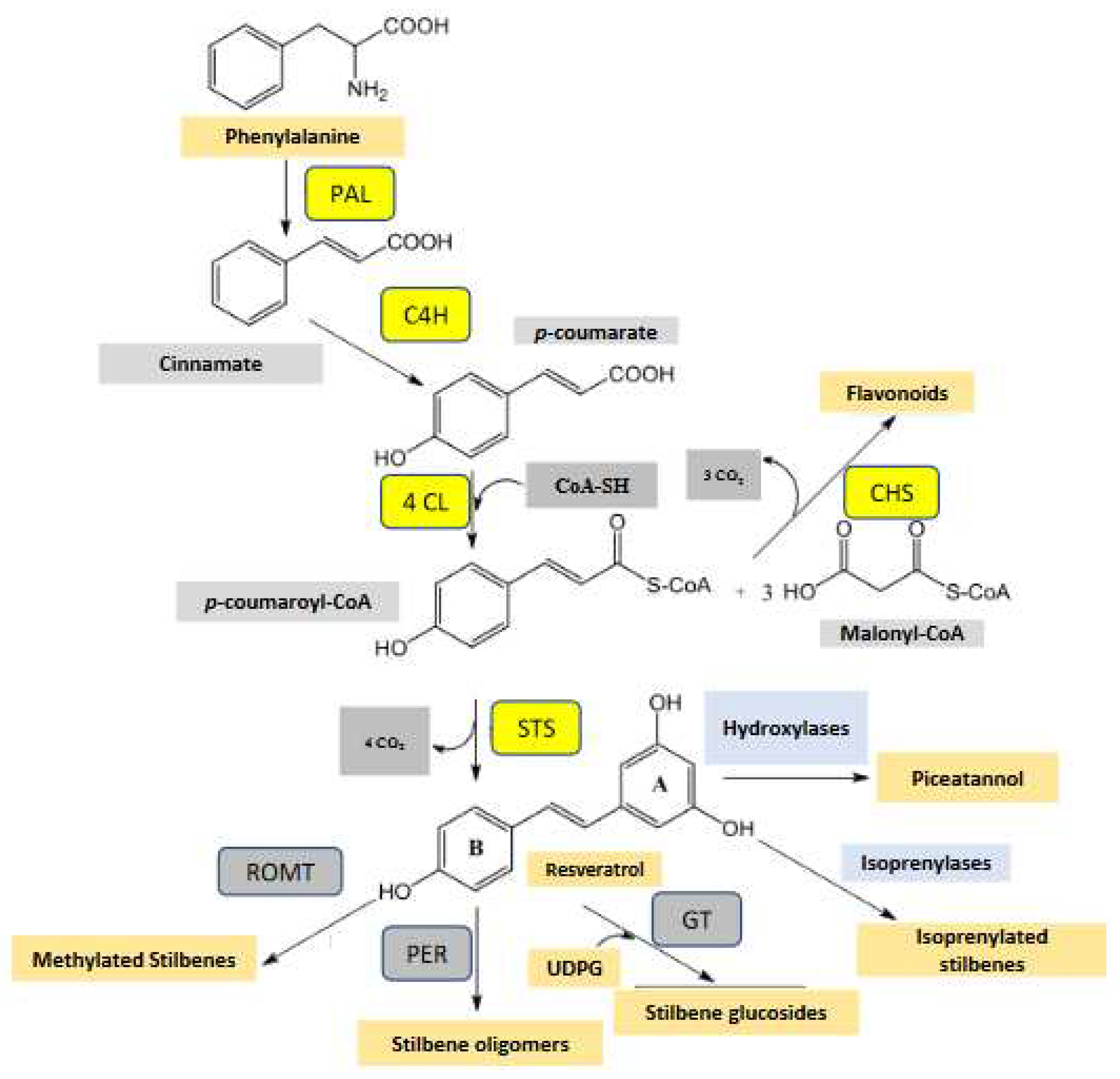
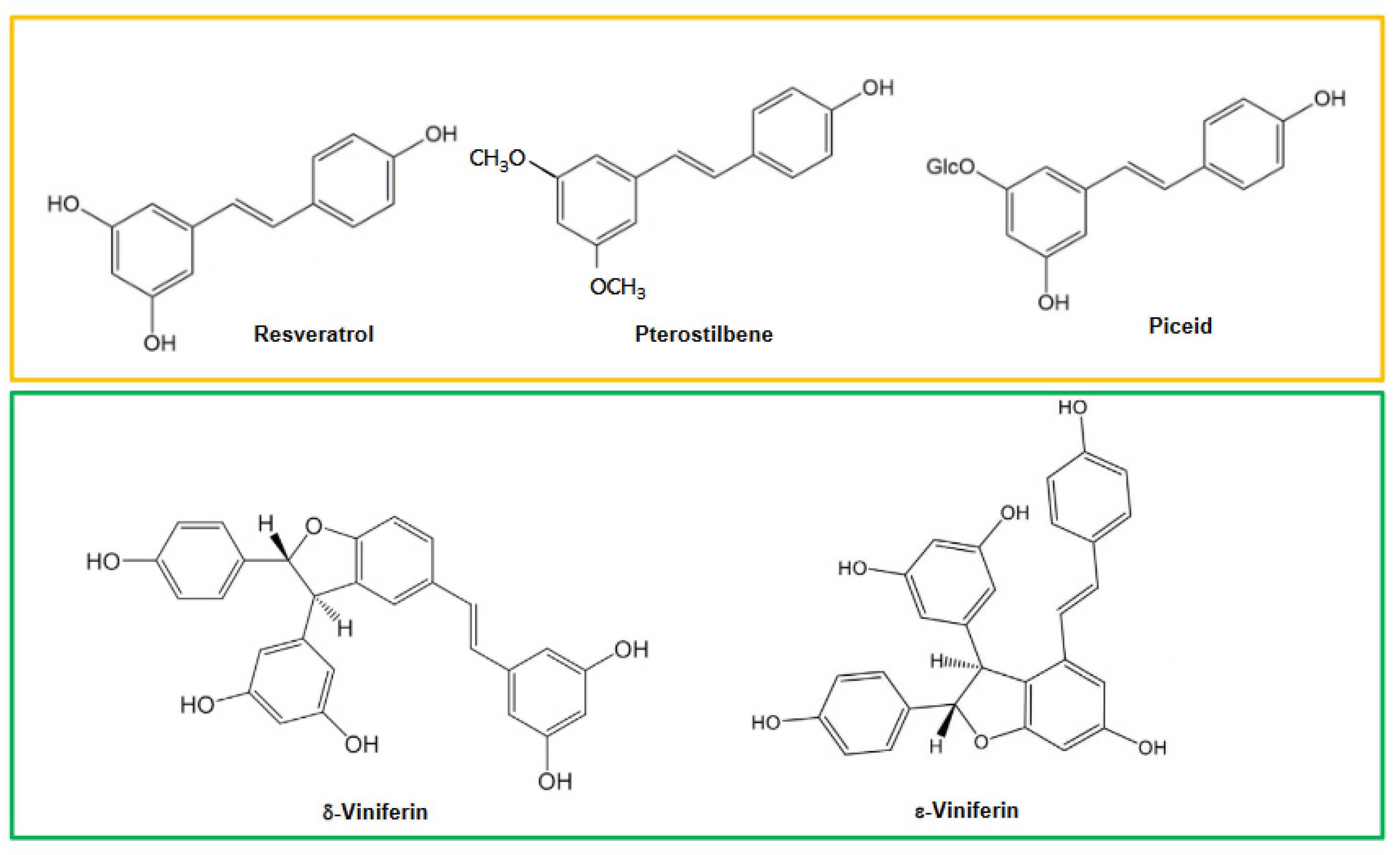
2. Chemistry and antifungal activity of some stilbenes of grapevine: A brief overview
3. Induction of stilbene phytoalexin synthesis by chemicals and possible applications in the vineyard
3.1. Organic and Inorganic Metallic Salts
| Chemicals | Plant Material | Biological Inputs | References |
|---|---|---|---|
| Fosetyl-Al | Potted grapevine (cv Carignan) | Protection towards P. viticola infection. No effect of fosetyl-Al alone on stilbene accumulation but active when applied at the time of infection or shortly after | [61] |
| Fosetyl-Al | cv Riesling (susceptible), V. rupestris (mid-tolerant) and cv Castor (resistant) leaf discs | Total suppression of P. viticola sporulation for pre-infectional applications. No protection towards the susceptible cultivar with post-infectional applications. Induction of stilbene production and priming effect | [62] |
| Synermix (aluminum chloride in combination with a seaweed extract) | Eight-year period experiments in the vineyard (V. vinifera) | Efficacy of 33.6% towards B. cinerea infection vs. Iprodione (42.2%). 70.7% efficacy for the combination Synermix/Iprodione. High AlCl3-induced resveratrol production | [51,64] |
| Copper sulfate | In vitro plantlets and potted grapevine (cv Chardonnay) | Protection against B. cinerea infection (> 60%) and only 38% towards P. viticola associated with an induction of chitinase and β-1,3-glucanases along with stilbene production | [50] |
| Fosetyl-Al | Nine-year period experiments in the vineyard (cvs Albana and Sangiovese) | Reduction of the foliar symptoms of esca decorrelated with stilbene production | [56] |
3.2. Phytohormone derivatives with a stimulating effect on grapevine natural defenses
| Compounds | Plant Material | Biological Inputs | References |
|---|---|---|---|
| Benzathiadiazole | Detached grape bunches (cv Merlot) | Significant decrease in B. cinerea incidence of the grape clusters (87% of them exhibited between 0 and 10% infection). Low stilbene accumulation | [71] |
| Benzathiadiazole | Potted grapevine (cv Grïuner Veltiner) | Reduction of downy mildew disease incidence to 15.8%. Strong activation of PAL and STS genes as well as peroxidases and PR-proteins | [72] |
| Benzathiadiazole | Leaves of grapevine plants (cv Cabernet Sauvignon) | Growth inhibition of P. viticola (60–98% and E. necator (65–75%). Overexpression of the STS gene with high piceid accumulation and significant accumulation of pterostilbene. Overexpression of several genes coding for PR proteins, LOX and GST | [73] |
| Benzathiadiazole vs. Pyrimethanil | Two-year period experiments in the vineyard (cv Semillon) | Decreases of grey mold incidence of respectively 35 and 20% for pyrimethanil and benzathiadiazole in 2014; −29 and −25% in 2015. Upregulation of VvSTS and VvROMT genes with no correlated accumulation of stilbenes | [76] |
| Methyljasmonate | Grapevine plants placed in a confined atmosphere with methyljasmonate (cv Cabernet Sauvignon) | High increase in leaf piceid content and little or no effect on the resveratrol content of grape berries. No test of disease pathogen control |
[78] |
| Methyljasmonate | Potted grapevine of the Barbera variety | Low increase in stilbene phytoalexin potential | [80] |
| Methyljasmonate | Plant cuttings (one application); multiple applications in the vineyard (from floraison to veraison) (cv Cabernet Sauvignon) |
Decreases of powdery mildew incidence of 75 and 73% in plant cuttings or in the vineyard, but not for downy mildew. Up-regulation of genes coding for PR-proteins as well as PAL and STS along with increase in stilbene accumulation | [81] |
| Methyljasmonate (MeJa), Benzathiadiazole (BTH), Phosphonates (PHOS) | Plant cuttings (cv Cabernet Sauvignon) |
Respectively 98.5, 97.3 and 85.8% protection towards downy mildew infection with MeJa, BTH and PHOS. High induction of stilbene production decorrelated from VvPAL and VvSTS expression. High overexpression of PR proteins with BTH, lower with MeJa and PHOS | [82] |
| Ethephon (ethylene) | Plant cuttings and leaf discs (cv Cabernet Sauvignon) | Increases in protection (64–70%) against powdery mildew. Up-regulation of defense genes (PAL and STS) along with strong stilbene accumulation (piceid, resveratrol and dimers) | [85] |
| Methyljasmonate/Ethephon | Plant cuttings and cell suspensions (cv Cabernet Sauvignon) | Decreases of 60% of the colonization by downy mildew by methyljasmonate or ethephon alone (only 30% when applied in combination; 85% with fosetyl-Al). Increases in PAL1 and STS along with increase in piceid and resveratrol production in cell suspensions. Inhibition of some PR proteins and LOX gene-induced expression by ethephon | [86] |
3.3. Bio-Elicitors
| Elicitor | Plant Material | Biological Inputs | References | |
|---|---|---|---|---|
| Laminarin | Grapevine in vitro plantlets (cv Chardonnay and Gamay) | 55 to 75% protection against respectively gray mold and downy mildew. Overexpression of genes coding for PR proteins as well as PAL and STS1 genes with concomitant accumulation of phytoalexins (cell suspensions) | [89] | |
| Chitosan partially deacetylated | Grapevine in vitro plantlets (cv Chardonnay) | Total suppression of B. cinerea necrosis on leaves. Overexpression of chitinases, PAL and LOX activities. Direct antifungal activity. No mention of stilbene production | [91] | |
| Chitosan partially deacetylated (20%) | Grapevine in vitro plantlets and potted plants (cv Chardonnay) | 60 to 70% protection against respectively gray mold and downy mildew linked with an increase in chitinase, β-1,3-glucanase activities and augmentation of stilbene content (resveratrol, piceid and ε-viniferin) | [50] | |
| Chitosan + copper sulfate | Potted plants (cv Chardonnay) | 83% protection against downy mildew associated with a potentiation of the phytoalexin response | [50] | |
| Ergosterol | Grapevine plants(cv Ugni Blanc) | 75% protection towards B. cinerea contamination (55% with benzothiadiazole). Overexpression of the WRKY transcription factor, VvST1 and VvLTP1 with correlated resveratrol production | [92] | |
| β-amino-butyric acid | Plant cuttings of cvs Chasselas andSolaris | Strong inhibition of P. viticola sporulation and hyphal growth linked with a priming effect on stilbene accumulation correlated with overexpression of PAL and C4H genes in Solaris and STS in both cultivars | [95] | |
| Bacterial rhamnolipidss | Grapevine in vitro plantlets |
Protection of leaves against B. cinerea infection. Overexpression of PR proteins, LOX, PAL and STS genes | [98] | |
| D-Tagatose (IFP48) | Grapevine in vitro plantlets (cv Chardonnay) | Reduction by 35% of sporangia density of P. viticola associated with a direct anti-oomycete activity. Overexpression of genes encoding PR proteins, LOX9, PAL and STS along with low stilbene production (resveratrol, piceid and dimers) | [104] | |
| D-Tagatose (IFP48) | Grapevine in vitro plantlets (cv Chardonnay) | Reduction by 50% of sporangia density of P. viticola but no effect towards B. cinerea. Overexpression of PR1 and PR2 genes (SA pathway), ERF1 and PR3c genes (JA pathway). Low stilbene production (resveratrol, piceid and dimers) | [105]) | |
4. Control of grapevine diseases by beneficial organisms involving stimulation of phytoalexin synthesis
| Biocontrol Agent | Plant Material | Biological Inputs | References |
|---|---|---|---|
| Uncharacterized soil bacterium B-781 | In vitro grapevine plantlets of V. vinifera (susceptible) and V. rupestris (mid-tolerant) | Complete suppression of gray mold symptoms with increase in resveratrol accumulation | [116] |
| Pseudomonas fluorescens CHA0 and Pseudomonas aeruginosa 7NSK2 | In vitro grapevine plantlets cv Chardonnay | Protection towards gray mold > 20% with P. fluorescens CHA0 and about 35% with and P. aeruginosa 7NSK2. At 3 days post-inoculation with B. cinerea, stilbene concentrations reached very high values in the order of several hundred μg/g FW | [117] |
| Bacillus sutilis, Pantoea agglomerens, Acinetobacter lwoffii and Pseudomonas fluorescens | In vitro grapevine plantlets cv Chardonnay | 35% reduction in gray mold symptoms with B. subtilis, 70% with P. fluorescens and 60% with P. agglomerens and A. lwoffii. Priming effect on phytoalexin accumulation with P. fluorescens and A. lwoffii but not with P. agglomerens and B. subtilis | [118] |
| Trichoderma harzanium strain T39 | Potted grapevine, cv Pinot Noir | 86% reduction in disease symptoms towards downy mildew; induction of PAL and STS genes, no mention of stilbene production | [112] |
| P. fluorescens PTA-CT2 | In vitro grapevine plantlets cv Chardonnay | 60% reduction in gray mold symptoms. Differential expression of PAL and STS genes (higher in leaves than in roots) correlating with accumulation of stilbenes in the two organs | [119] |
| P. fluorescens PTA-CT2 | Two-year-old potted grapevins of the varieties Pinot Noir (susceptible) and Solaris (tolerant) | Reduction in the growth development of P. viticola of 80% in Pinot Noir and only 55% in Solaris, 73–80% for Pinot Noir and 43% for Solaris towards B. cinerea. No induced changes in the basal defenses of the plant with PTA-CT2 alone. PTA-CT2 primed defensive pathways including PAL and STS gene overexpression, which was correlated with increased phytoalexin levels in both varieties | [120] |
| B. subtilis (PTA-271), P. fluorescens (PTA-CT2) and P. agglomerens (PTA-AF2) alone or as binary mixtures | One-year experiment on grapevine plants (cv Chardonnay) in the vineyard including leaves and berries | 80 to 90% reduction in symptoms towards B. cinerea) with PTA-AF2 + PTA-271 on leaves. 93% reduction of B. cinerea symptoms with CT2 + AF2 on berries well correlating with phytoalexin accumulation | [123] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Müller, K.O.; Börger, H. Experimentelle Untersuchungen über die Phytophthora Resistenz der Kartoffel. Arbeit. Biol. Reichsant. Land Forstwirtsch. 1940, 23, 189–231. [Google Scholar]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sanchez, E.; Uddin, M.S.; Bru, R.; Martinez-Marquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Nabavi, S.F.; et al. Phytostilbenes as agrochemicals: biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2021, 28, 1282–1329. [Google Scholar] [CrossRef]
- Jeandet, P.; Uddin, M.S.; Clément, C.; Aziz, A.; Jacquard, C; Khan, H.; Shah, M.A.; Ait-Barka, E.; Koffas, M.; Nabavi, S.M.; et al. Production of high molecular-ordered stilbene oligomers: Total synthesis, bio-catalyzed synthesis and production by plant systems. Nat. Prod. Rep. 2023, 30, 1045–1057. [CrossRef]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. BioFactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Clément, C.; Nabavi, S.F.; Habtemariam, S.; Nabavi, S.M.; Cordelier, S. Engineering stilbene metabolic pathways in microbial cells. Biotechnol. Adv. 2018, 36, 2264–2283. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defence mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry 1977, 16, 1193–1196. [Google Scholar] [CrossRef]
- Langcake, P. Disease resistance of Vitis spp. and the production of the stress metabolites resveratrol, ε-viniferin, α-viniferin and pterostilbene. Physiol. Plant Pathol. 1981, 18, 213–226. [Google Scholar] [CrossRef]
- Nabavi, S.; Samec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 39, 107461. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S.; et al. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Qian, Y.; Lynch, J.H.; Guo, L.; Rhodes, D.; Morgan, J.A.; Dudareva, N. Completion of the cytosolic post-chorismate phenylalanine biosynthetic pathway in plants. Nat. Comm. 2019, 10, 15. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fregoni, M.; Trevisan, M.; Mattivi, F.; Vrhovsek, U.; Falchetti, R. The occurrence of the stilbene piceatannol in grapes. Vitis 2002, 41, 133–136. [Google Scholar]
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry, 1979, 18, 1025–1027. [Google Scholar] [CrossRef]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol-O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [PubMed]
- Hall, D.; De Luca, V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 2007, 49, 579–591. [Google Scholar] [CrossRef]
- Härtl, K.; Huang, F. C.; Giri, A. P.; Franz-Oberdorf, K.; Frotscher, J.; Shao, Y.; Hoffmann, T.; Schwab, W. Glucosylation of smoke-derived volatiles in grapevine (Vitis vinifera) is catalyzed by a promiscuous resveratrol/guaiacol glucosyltransferase. J. Agric. Food Chem. 2017, 65, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Calderon, A.A.; Pedreno, M.A.; Munoz, R.; Ros Barcelo, A. Zymographic screening of plant peroxidase isoenzymes oxidizing 4-hydroxystilbenes. Electrophoresis 1990, 11, 507–508. [Google Scholar] [CrossRef]
- Calderon, A.A.; Zapata, J.M.; Pedreno, M.A.; Munoz, R.; Ros Barcelo, A. Levels of 4-hydroxystilbene-oxidizing isoperoxidases related to constitutive disease resistance in in vitro-cultured grapevine. Plant Cell, Tissue Organ Cult. 1992, 29, 63–70. [Google Scholar] [CrossRef]
- Morales, M.; Alcantara, J.; Ros Barcelo, A. Oxidation of trans-resveratrol by a hypodermal peroxidase isoenzyme from gamay rouge grape (Vitis vinifera) berries. Am. J. Enol. Vitic. 1997, 48, 33–38. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. Oxidative dimerisation of 4-hydroxystilbenes in vitro: production of a grapevine phytoalexin mimic. J.C.S. Chem. Comm. 1977, 7, 208–210. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Sanches Silva, A.; Clément, C.; Nabavi, S.F.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M.; Nabavi, S.M. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020, 38, 107461. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J. Phytopathol. 1995, 143, 135–139. [Google Scholar] [CrossRef]
- Bavaresco, L.; Petegolli, D.; Cantu, E.; Fregoni, M.; Chiusa, G.; Trevisan, M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 1997, 36, 77–83. [Google Scholar]
- Dercks, W.; Creasy, L.L. The significance of phytoalexins in the Plasmopara viticola-grapevine interaction. Physiol. Mol. Plant Pathol. 1989, 34, 189–202. [Google Scholar] [CrossRef]
- Malacarne, G.; Vrhovsek, U.; Zulini, L.; Cestaro, A.; Stefanini, M.; Mattivi, F.; Delledonne, M.; Velasco, R.; Moser, C. Resistance to Plasmopara viticola in a grapevine segregating population is associated with stilbenoid accumulation and with specific host transcriptional responses. BMC Plant Biol. 2011, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Zanzotto, A.; de Rosso, M.; Lucchetta, G.; Dalla Vedova, A.; Bavaresco, L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant Pathol. 2016, 93, 112–118. [Google Scholar] [CrossRef]
- Calzarano, F.; D’Agostino, V.; Pepe, A.; Osti, F.; Della Pelle, F.; De Rosso, M.; Flamini, R.; Di Marco, S. Patterns of phytoalexins in the grapevine leaf stripe disease symptoms (esca complex)/grapevine pathosystem. Phytopathol. Mediterr., 2016, 55, 410–426. [Google Scholar]
- Calzarano, F.; Osti, F.; D’Agostino, V.; Pepe, A.; Della Pelle, F.; De Rosso, M.; Flamini, R.; Di Marco, S. Levels of phytoalexins in vine leaves with different degrees of grapevine leaf stripe disease symptoms (Esca complex of diseases). Phytopathol. Mediterr., 2017, 56, 494–501. [Google Scholar]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Richter, H. Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis 2004, 43, 143–145. [Google Scholar]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef]
- Pezet, R.; Pont, V. Mise en evidence de ptérostilbène dans les grappes de Vitis vinifera. Plant Physiol. Biochem. 1988, 26, 603–607. [Google Scholar]
- Alessandro, M.; Di Marco, S.; Osti, F. Cesari, A. bioassays on the activity of resveratrol, pterostilbene and phosphorous acid towards fungi associated with esca of grapevine. Phytoapathol. Mediterr. 2000, 39, 357–365. [Google Scholar]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.F.; Mérillon, J.M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crop Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.F.; Richard, T.; Mérillon, J.M. Stilbenes from Vitis vinifera L. waste: A sustainable tool for controlling Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P.A.G.; Reglinski, T. Biosupression of Botrytis cinerea in grapes. Plant Pathol. 2006, 55, 155–177. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Perrin, D.R.; Cruickshank, I.A.M. Studies on phytoalexins. VII. Chemical stimulation of pisatin formation in Pisum sativum. Aust. J. Biol. Sci. 1965, 18, 803–816. [Google Scholar] [CrossRef]
- Sweigard, J.A.; Matthews, D.E.; VanEtten, H.D. Synthesis of the phytoalexin pisatin by a methyltransferase from pea. Plant Physiol. 1986, 80, 277–279. [Google Scholar] [CrossRef]
- Hanawa, F.; Tahara, S.; Mizutani, J. Antifungal stress compounds from Veratrum grandiflorum leaves treated with cupric chloride. Phytochemistry 1992, 31, 3005–3007. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to fungal pathogens. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Bessis, R.; Joubert, J.M. Induction of phytoalexin (resveratrol) synthesis in grapevine leaves treated with aluminium chloride (AlCl3). J. Agric. Food Chem. 1996, 44, 1979–1981. [Google Scholar] [CrossRef]
- Magarey, P. A.; Wachtel, M. F.; Newton, M. R. Evaluation of phosphonate, fosetyl-Al and several phenylamide fungicides for post-infection control of grapevine downy mildew caused by Plasmopara viticola. Australas. Plant Pathol. 1991, 20, 34–40. [Google Scholar] [CrossRef]
- Gisi, U. Chemical control of downy mildews. In Advances in Downy Mildew Research, Spencer-Phillips, P.T.N.; Gisi, U.; Lebeda, A., Eds, Springer, The Netherlands, 2002, pp. 119–159.
- Rekanovic, E.; Potocnik, I.; Stepanovic, M.; Milijasevic, S.; Todorovic, B. Field efficacy of fluopicolide and fosetyl-Al fungicide combination (Profiler R©) for control of Plasmopara viticola (Berk. & Curt.) Berl. & Toni. in grapevine. Pestic. i Fitomedicina 2008, 23, 183–187. [Google Scholar]
- Koledenkova, K.; Esmaeel, Q.; Jacquard, C.; Nowak, J.; Clément, C.; Ait-Barka, E. Plasmopara viticola the causal agent of downy mildew of grapevine: From its taxonomy to disease management. Front. Microbiol. 2022, 13, 889472. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Calzarano, F.; Roberti, R.; Veronesi, A.; Amalfitano, C. Effects of grapevine applications of fosetyl-aluminium formulations for downy mildew control on “esca” and associated fungi. Phytopathol. Mediterr., 2011, 50, S285–S299. [Google Scholar] [CrossRef]
- Guest, D.I. Modification of defense responses in tobacco and capsicum following treatment with fosetyl-Al [aluminium tris (o-ethylphosphonate]. Physiol. Plant Pathol. 1984, 25, 125–134. [Google Scholar] [CrossRef]
- Guest, D.I. Evidence from light microscopy of living tissues that fosetyl-Al modifies the defense response in tobacco seedlings following inoculations by Phytphthora nicotianae var. nicotianae. Physiol. Plant Pathol. 1986, 29, 251–261. [Google Scholar] [CrossRef]
- Nemesthoty, G.S.; Guest, D.I. Phytoalexin accumulation, phenylalanine ammonia lyase activity and ethylene biosynthesis in fosetyl-Al treated resistant and susceptible tobacco cultivars infected by Phytophtora nicotianae var nicotianae. Physiol. Mol. Plant Pathol. 1990, 37, 207–219. [Google Scholar] [CrossRef]
- Afek, U.; Sztejnberg, A. Effects of fosetyl-Al and phosphorus acid on scoparone, a phytoalexin associated with resistance of Citrus to Phytophthora citrophthora. Phytopathology 1989, 79, 736–739. [Google Scholar] [CrossRef]
- Raynal, G.; Ravisé, A.; Bompeix, G. Action du tris-O-éthylphosphonate d’aluminium (phosétyl d’aluminium) sur la pathogénie de Plasmopara viticola et sur la stimulation des réactions de défense de la vigne. Ann. Phytopathol. 1980, 12, 163–175. [Google Scholar]
- Dercks, W.; Creasy, L.L. Influence of fosetyl-Al on phytoalexin accumulation in the Plasmopara viticola-grapevine interaction. Physiol. Mol. Plant Pathol. 1989, 34, 203–213. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Adrian, M.; Joubert, J.M.; Yvin, J.C. Use of aluminium chloride as a resveratrol formation elicitor in plants. French Patent N° 95 13 642, PCT Int. Appl. WO 97 18,715, 1997. [Google Scholar]
- Jeandet, P.; Adrian, M.; Breuil, A.C.; Sbaghi, M.; Debord, S.; Bessis, R.; Weston, L.A.; Harmon, R. Chemical induction of phytoalexin synthesis in grapevines: Application to the control of grey mould in the vineyard. Acta Hortic. 2000, 528, 591–596. [Google Scholar] [CrossRef]
- Colby, S.R. Calculating synergistic and antagonistic responses of herbicide combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Wang, R.; Duan, D.; Metzger, C.; Zhu, X.; Riemann, M.; Pla, M.; Nick, P. Aluminium can activate grapevine defense through actin remodeling. Hort. Res. 2022, 9, uhab016. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marin, M.I.; Guerrero, R.F.; Puertas, B.; Garcia-Parrilla, M.C.; Collado, I.G.; Cantos-Villar, E. Impact of preharvest and postharvest treatment combinations on increase of stilbene content in grape. J. Int. Sci. Vigne Vin 2013, 47, 203–212. [Google Scholar] [CrossRef]
- Miliordos, D.-E.; Alatzas, A.; Kontoudakis, N.; Unlubayir, M.; Hatzopoulos, P.; Lanoue, A.; Kotseridis, Y. Benzothiadiazole affects grape polyphenol metabolism and wine quality in two Greek cultivars: Effects during ripening periods over two years. Plants 2023, 12, 1179. [Google Scholar] [CrossRef]
- Friedrich, L.; Lawton, K.; Ruess, W.; Masner, P.; Specker, N.; Gut Rella, M.; Meier, B. Dincher, S.; Staub, T.; Uknes, S. et al. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996, 10, 61–70. [Google Scholar] [CrossRef]
- Görlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 1996, 8, 629–643. [Google Scholar]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef]
- Harm, A.; Kassemeyer, H.H.; Seibicke, T.; Regner, F. Evaluation of chemical and natural resistance inducers against downy mildew (Plasmopara viticola) in grapevine. Am. J. Enol. Vitic. 2011, 62, 184–192. [Google Scholar] [CrossRef]
- Dufour, M.C.; Lambert, C.; Bouscaut, J.; Mérillon, J.M.; Coriot-Costet, M.F. Benzothiadiazole-primed defence responses and enhanced differential expression of defence genes in Vitis vinifera infected with biotrophic pathogens Erysiphe necator and Plasmopara viticola. Plant Pathol. 2013, 62, 370–382. [Google Scholar] [CrossRef]
- Jeandet, P.; Breuil, A.C.; Adrian, M.; Weston, L.A.; Debord, S.; Meunier, P.; Maume, G.; Bessis, R. HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorometry. Anal. Chem. 1997, 69, 5172–5177. [Google Scholar] [CrossRef]
- Duke, S.O. Benefits of resveratrol and pterostilbene to crops and their potential nutraceutical value to mammals. Agriculture 2022, 12, 368. [Google Scholar] [CrossRef]
- Bellée, A.; Cluzet, S.; Dufour, M.-C.; Mérillon, J.-M.; Corio-Costet, M.-F. Comparison of the impact of two molecules on plant defense and on efficacy against Botrytis cinerea in the vineyard: A plant defense inducer (benzothiadiazole) and a fungicide (pyrimethanil). J. Agric. Food Chem. 2018, 66, 3338–3350. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Tisserant, L.P.; Crouzet, J.; Courot, E. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. C.R. Chimie 2016, 19, 1062–1070. [Google Scholar] [CrossRef]
- Larronde, F.; Gaudillière, J.P.; Krisa, S.; Descendit, A.; Deffieux, G.; Mérillon, J.M. Airborne methyl jasmonate induces stilbene accumulation in leaves and berries of grapevine plants. Am. J. Enol. Vitic. 2003, 54, 63–66. [Google Scholar] [CrossRef]
- Creasy, L.L.; Coffee, M. Phytoalexin production potential of grape berries. J. Am. Soc. Hortic. Sci. 1988, 113, 230–234. [Google Scholar] [CrossRef]
- Vezzulli, S.; Civardi, S.; Ferrari, F.; Bavaresco, L. Methyljasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. Am. J. Enol. Vitic. 2007, 58, 530–533. [Google Scholar] [CrossRef]
- Belhadj, A.; Saigne, C.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.-F.; Mérillon, J.-M. Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator, J. Agric. Food Chem. 2006, 2006 54, 9119–9125. [Google Scholar] [CrossRef]
- Burdziej, A.; Bellée, A.; Bodin, E.; Fonayet, J.V.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.-F. Three types of elicitors induce grapevine resistance against downy mildew via common and specific immune responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef]
- Smillie, R.; Grant, B.R.; Guest, D.I. The mode of action of phosphite: Evidence for both direct and indirect modes of action on three Phytophthora spp. in plants. Phytopathology 1989, 79, 921–926. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, A.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.-F.; Mérillon, J.-M. Ethephon elicits protection against Erysiphe necator in grapevine. J. Agric. Food Chem. 2008, 56, 5781–5787. [Google Scholar] [CrossRef] [PubMed]
- Faurie, B.; Cluzet, S.; Corio-Costet, M.F.; Mérillon, J.-M. Methyl jasmonate/ethephon cotreatment synergistically induces stilbene production in Vitis vinifera cell suspensions but fails to trigger resistance to Erysiphe necator. J. Int. Sci. Vigne Vin 2009, 43, 99–110. [Google Scholar] [CrossRef]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Bailleul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef] [PubMed]
- Gil-Munoz, R.; Fernandez-Fernandez, J.I.; Crespo-Villegas, O.; Garde-Cerdan, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bezier, A.; Lambert, B.; Joubert, M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef]
- Stasinska-Jakubas, M.; Hawrylak-Nowak, B. Protective, biostimulating, and eliciting effects of chitosan and its derivatives on crop plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Couderchet, M.; Vernet, G.; Aziz, A. Chitosan stimulates defense reactions in grapevine leaves and inhibits development of Botrytis cinerea. Eur. J. Plant Pathol. 2006, 114, 405–413. [Google Scholar] [CrossRef]
- Laquitaine, L.; Gomès, E.; François, J.; Marchive, C.; Pascal, S.; Hamdi, S.; Atanassova, R.; Delrot, S.; Coutos-Thévenot, P. Molecular basis of ergosterol-induced protection of grape against Botrytis cinerea: Induction of type I LTP promoter activity, WRKY, and stilbene synthase gene expression. Mol. Plant Microb. Interact. 2006, 19, 1103–1112. [Google Scholar] [CrossRef]
- Jakab, G.; Cottier, V.; Toquin, V.; Rigoli, G.; Zimmerly, L.; Metraux, J.P.; Mauch-Mani, B. Beta-aminobutyric acid-induced resistance in plants. Eur. J. Plant Pathol. 2001, 107, 29–37. [Google Scholar] [CrossRef]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Métraux, J.P.; Mauch-Mani, B. Dissecting the β-aminobutyric acid–induced priming phenomenon in Arabidopsis. Plant Cell 2005, 17, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, A.R.; Hamiduzzaman, M. Md; Gindro, K.; Neuhaus, J.M.; Mauch-Mani, B. Beta-aminobutyric acid-induced resistance in grapevine against downy mildew: Involvement of pterostilbene. Eur. J. Plant Pathol. 2008, 122, 185–195. [Google Scholar] [CrossRef]
- Monnier, N.; Furlan, A.; Botcazon, C.; Dahi, A.; Mongelard, G.; Cordelier, S.; Clément, C.; Dorey, S.; Sarazin, C.; Rippa, S. Rhamnolipids from Pseudomonas aeruginosa are elicitors triggering Brassica napus protection against Botrytis cinerea without physiological disorders. Front. Plant Sci. 2018, 9, 1170. [Google Scholar] [CrossRef]
- Robineau, M; Le Guenic, S.; Sanchez, L.; Chaveriat, L.; Lequart, V.; Joly, N.; Calonne, M.; Jacquard, C.; Declerck, S.; Martin, P. et al. Synthetic mono-rhamnolipids display direct antifungal effects and trigger an innate immune response in tomato against Botrytis cinerea. Molecules 2020 25, 3108.
- Varnier, A. L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.H.; Kauffmann, S.; Pugin, A. et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.B.; Van Den Ende, W. Sugars and plant innate immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, M. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Mijailovic, N.; Nesler, A.; Perazzolli, M.; Ait Barka, E.; Aziz, A. Rare sugars: Recent advances and their potential role in sustainable crop protection. Molecules 2021, 26, 1720. [Google Scholar] [CrossRef]
- Chahed, A.; Lazazzara, V.; Moretto, M.; Nesler, A.; Corneo, P.E.; Ait-Barka, E.; Pertot, I.; Puopolo, G.; Perazzolli, M. The differential growth inhibition of Phytophthora spp. caused by the rare sugar tagatose is associated with species-specific metabolic and transcriptional changes. Front. Microbiol. 2021, 12, 711545. [Google Scholar] [CrossRef]
- Corneo, P.E.; Jermini, M.; Nadalini, S.; Giovannini, O.; Nesler, A.; Perazzolli, M.; Pertot, I. Foliar and root applications of the rare sugar tagatose control powdery mildew in soilless grown cucumbers. Crop Prot. 2021, 149, 105753. [Google Scholar] [CrossRef]
- Mijailovic, N.; Nesler, N.; Perazzolli, M.; Aziz, A.; Ait-Barka, E. Foliar application of a tagatose-based product reduces downy mildew symptoms through induction of grapevine resistance and anti-oomycete action. Agronomy 2022, 12, 498. [Google Scholar] [CrossRef]
- Mijailovic, N.; Richet, N.; Villaume, S.; Nesler, N.; Perazzolli, M.; Ait-Barka, E. Aziz, A. D-tagatose-based product triggers sweet immunity and resistance of grapevine to downy mildew, but not to gray mold disease. Plants 2022, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, J.A.; Heil, M.; Ahman, I.; Björkman, C. Optimizing crops for biocontrol of pests and disease. Trends Plant Sci. 2015, 20, 698–712. [Google Scholar] [CrossRef]
- Stenberg, J.A. A conceptual framework for integrated pest management Trends Plant Sci. 2017, 22, 759-769.
- Bakker, P.A.H.M.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The soil-borne legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W. et al. The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 2022, 13, 2717–2741. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases –What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Ait-Barka, E. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Perazzoli, M.; Moretto, M.; Fontana, P.; Ferrarini, A.; Velasco, R.; Moser, C.; Delledonne, M.; Pertot, I. Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genomics 2012, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Arras, G.; d’Hallewin, G.; Molinu, M.G.; Dore, A.; Venditti, T.; Fois, M.; Lima, G.; Agabbio, M. Induction of phytoalexin biosynthesis in orange fruit by the biocontrol yeast Rhodotorula glutinis. Comm. Appl. Biol. Sci. Ghent University 2006, 71, 915–921. [Google Scholar]
- Van Peer, R.; Niemann, G.J.; Schippers, B. Induced resistance and phytoalexin accumulation in biological control of fusarium wilt of carnation by Pseudomonas sp strain WCS417r. Phytopathology 1991, 81, 728–734. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A. Priming of camalexin accumulation in induced systemic resistance by beneficial bacteria against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000. J. Exp.Bot. 2022, 73, 3743–3757. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Chereyathmanjiyil, A.; Masih, I.; Chapuis, L.; Benoît, A. Biological control of Botrytis cinerea causing grey mould disease of grapevine and elicitation of stilbene phytoalexin (resveratrol) by a soil bacterium. FEMS Microbiol. Lett. 1998, 165, 65–70. [Google Scholar] [CrossRef]
- Verhagen, B.W.M. Verhagen, B.W.M., Trotel-Aziz, P.; Couderchet, M., Höfte, M.; Aziz, A. Pseudomonas spp.-induced resistance to Botrytis cinerea is associated with induction and priming of defence responses in grapevine. J. Exp. Bot. 2010, 61, 249-260.
- Verhagen, B.W.M.; Trotel-Aziz, P.; Jeandet, P.; Baillieul, F. M.; Aziz, A. Improved resistance against Botrytis cinerea by grapevine-associated bacteria that induce a prime oxidative burst and phytoalexin induction. Phytopathology 2011, 101, 768–777. [Google Scholar] [CrossRef]
- Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A. Pseudomonas fluorescens PTA-CT2 triggers local and systemic immune response against Botrytis cinerea in grapevine. Mol. Plant Microb. Interact. 2015, 28, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, S.; Trotel-Aziz, P.; Rabenoelina, F.; Schwarzenberg, A.; Nguema-Ona, E.; Clément, C.; Aziz, A. Strengthening grapevine resistance by Pseudomonas fluorescens PTA-CT2 relies on distinct defense pathways in susceptible and partially resistant genotypes to downy mildew and gray mold diseases. Front. Plant Sci. 2019, 10, 1112. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Trotel-Aziz, P.; Quantinet, D.; Biagianti, S.; Aziz, A. Biological control of Botrytis cinerea by selected grapevine-associated bacteria and stimulation of chitinase and β-1,3-glucanase activities under field conditions. Eur. J. Plant Pathol. 2007, 118, 43–57. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Quantinet, D.; Couderchet, M.; Aziz, A.; Trotel-Aziz, P. Differential induction of grapevine resistance and defense reactions against Botrytis cinerea by bacterial mixtures in vineyards. BioControl 2013, 58, 117–131. [Google Scholar] [CrossRef]
- Aziz, A.; Verhagen, B.; Magnin-Robert, M.; Couderchet, M.; Clément, C.; Jeandet, P.; Trotel-Aziz, P. Effectiveness of beneficial bacteria to promote systemic resistance of grapevine to gray mold as related to phytoalexin production in vineyards. Plant Soil 2016, 405, 141–153. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4’-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





