Submitted:
09 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Brief survey of the GPCR superfamily
2. Review of GPCRs displaying documented ligand-free signaling
3. Energetics and dynamics of GPCR activation
4. Agonist and antagonist interactions with GPCRs with consideration of ligand-free signaling
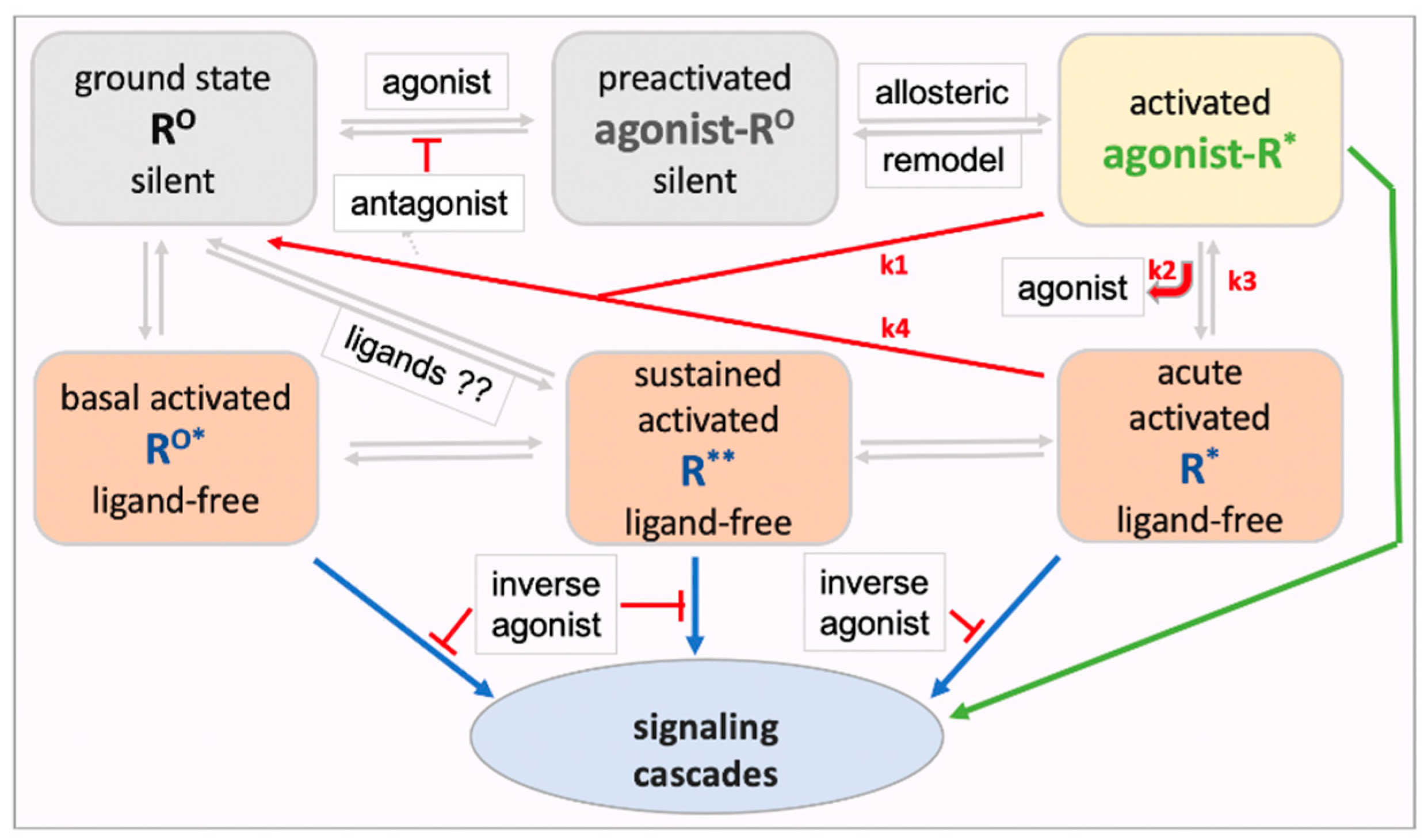
4.1. Dissociation of agonist ligand from the activated receptor with continuing signaling
4.2. Ligand-free signaling of rhodopsin
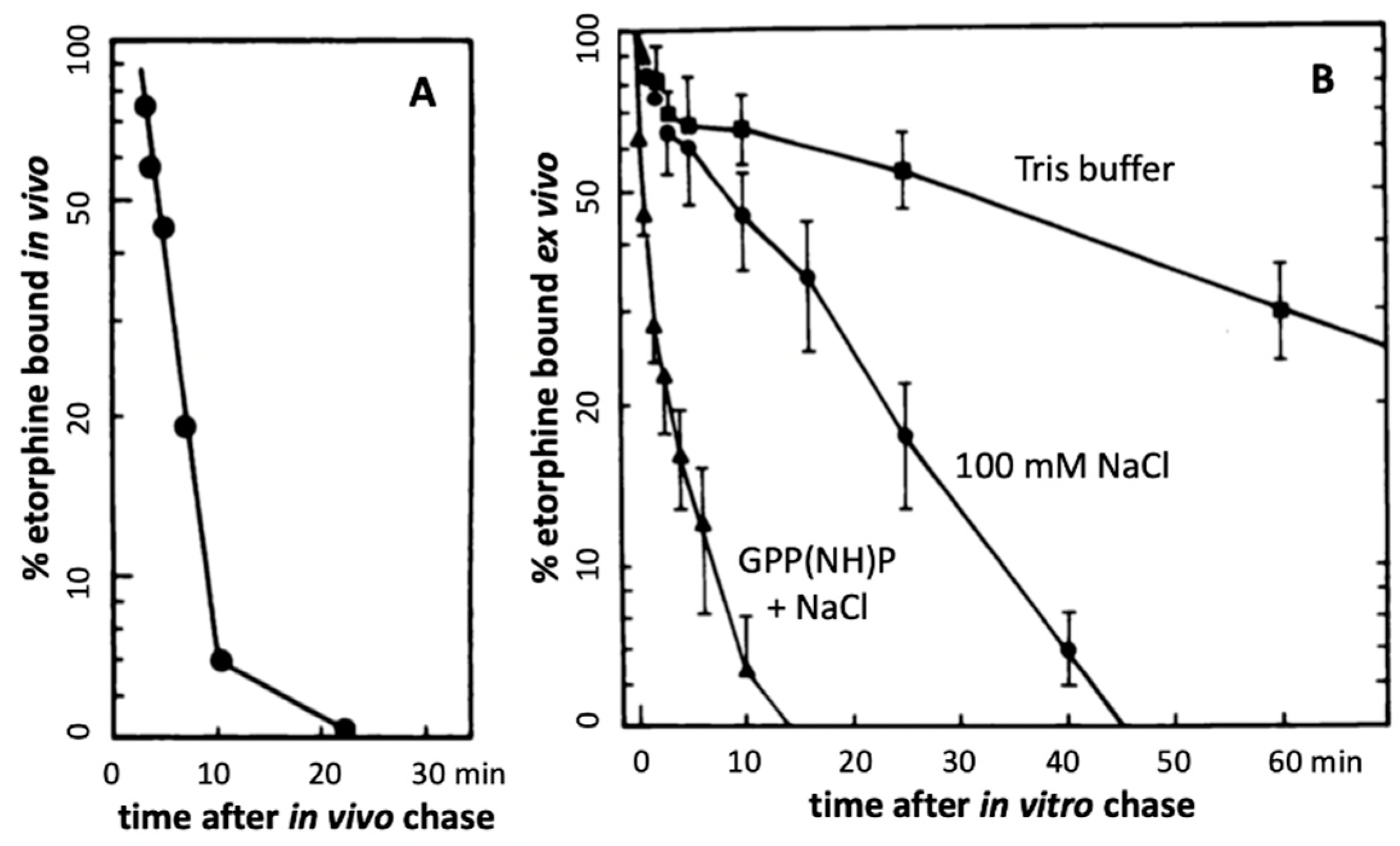
4.3. Etorphine - an ultra-potent μ opioid receptor (MOR) agonist
4.4. Lysergic acid diethylamide (LSD) – an ultra-potent agonist at 5-HT2A
5. Pharmacological significance of activated ligand-free receptor signaling
5.1. Involvement in agonist effects
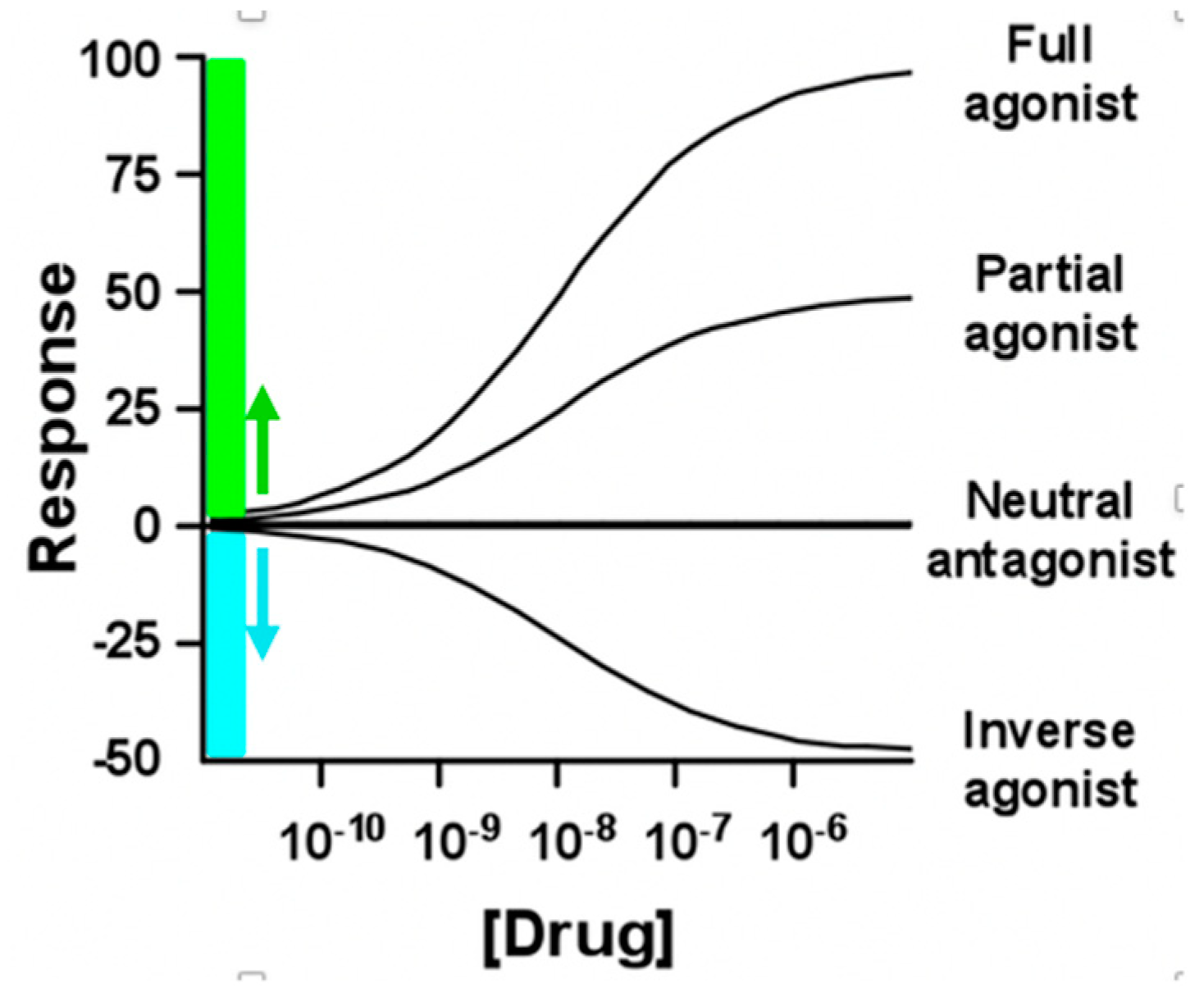
5.2. Pharmacological significance of neutral antagonism and inverse agonism
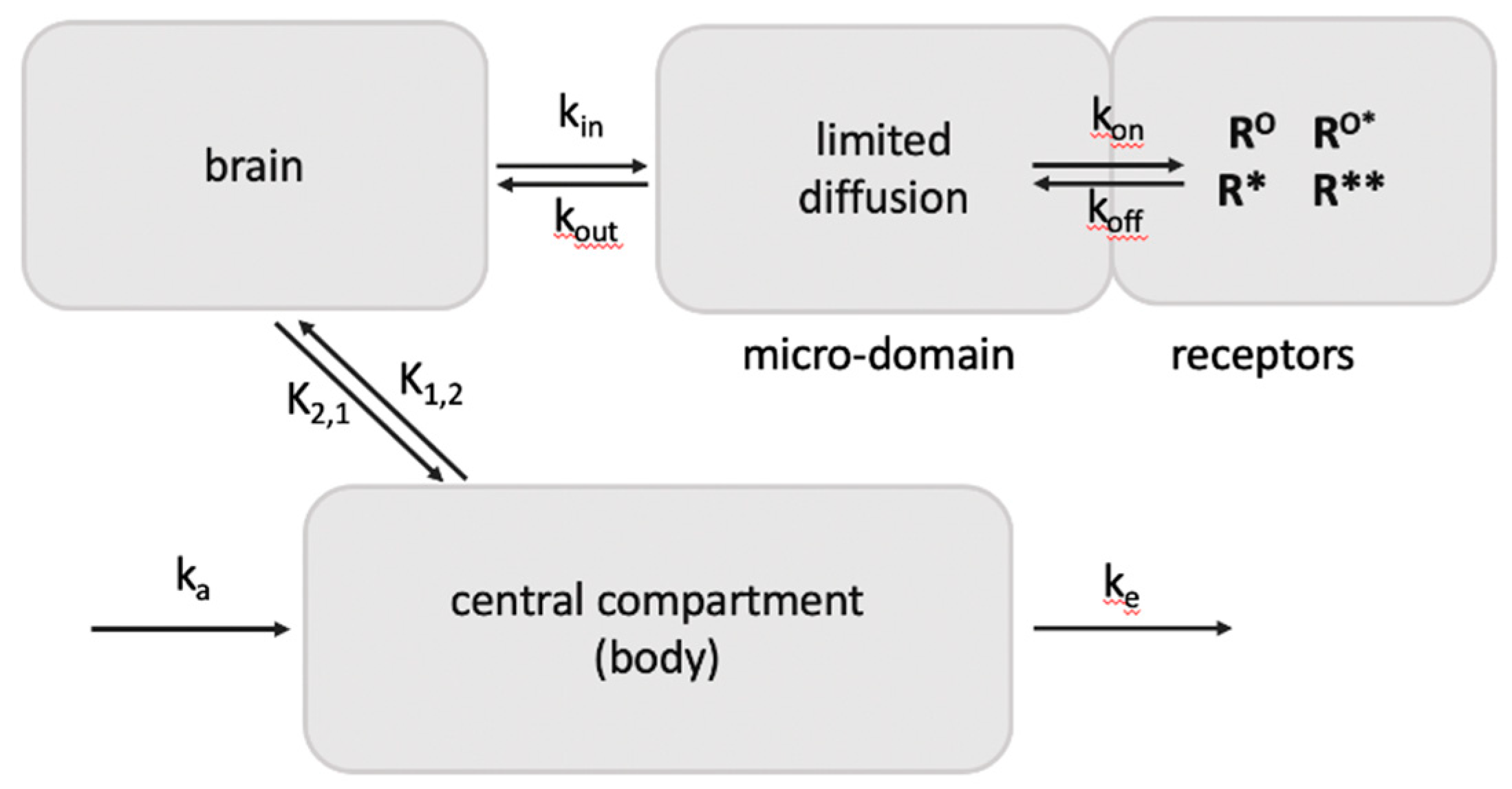
6. Relevance of ligand-free R* signaling to in vivo receptor imaging studies
7. Physiological significance of sustained regulated ligand-free signaling (R** in Figure 1)
8. Opioid dependence and elevated lasting ligand-free MOR** signaling
9. Ligand-free signaling of serotonin receptor 5HT2A
9.1. Physiological and pharmacological relevance
9.2. Psychedelic drugs and 5-HT2A signaling – acute and long-term effect
9.3. Long lasting effects off psychedelics
9.4. Micro-dosing with psychedelics – attempts to separate hallucinogenic from therapeutic effects
10. Growth hormone secretagogue receptor GHSR: high ligand-free signaling
11. Genetic variants that affect ligand-free signaling
12. Summary
11. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. CGTP Collaborators. The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol 2019, 176 (Suppl. 1), S21–S141. [Google Scholar]
- Sriram, K.; Insel, P.A. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol Pharmacol 2018, 93, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Casadó, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, J.P.; Guitart, X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 2014. [Google Scholar] [CrossRef]
- Kurose, H.; Kim, S.G. Pharmacology of antagonism of GPCR. Biol Pharm Bul. 2022, 45, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.M.; Karnik, S.S. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev 2005, 57, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Wingler, L.M.; Lefkowitz, R.J. Conformational basis of G protein-coupled receptor signaling versatility. Trends Cell Biol 2020, 30, 736–747. [Google Scholar]
- Eiger, D.S.; Smith, J.S.; Shi, T.; Stepniewski, T.M.; Tsai, C.F.; Honeycutt, C.; Boldizsar, N.; Gardner, J.; Nicora, C.D.; Moghieb, A.M.; et al. Phosphorylation barcodes direct biased chemokine signaling at CXCR3. Cell Chem Biol 2023, 30, 362–382.e8. [Google Scholar]
- Huang, S.K.; Pandey, A.; Tran, D.P.; Villanueva, N.L.; Kitao, A.; Sunahara, R.K.; Sljoka, A.; Prosser, R.S. Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling. Cell 2021, 184, 1884–1894.e14. [Google Scholar]
- Lu, M.; Zhao, W.; Han, S.; Lin, X.; Xu, T.; Tan, Q.; Wang, M.; Yi, C.; Chu, X.; Yang, W.; Zhu, Y.; Wu, B.; Zhao, Q. Activation of the human chemokine receptor CX3CR1 regulated by cholesterol. Sci Adv 2022, 8, eabn8048. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Prosser, R.S. Dynamics and mechanistic underpinnings to pharmacology of class A GPCRs: an NMR perspective. Am J Physiol Cell Physiol 2022, 322, C739–C753. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Kobilka, B.K. The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Dague, E.; Pons, V.; Roland, A.; Azaïs, J.M.; Arcucci, S.; Lachaize, V.; Velmont, S.; Trevisiol, E.; N'Guyen, D.; Sénard, J.M.; Galés, C. Atomic force microscopy-single-molecule force spectroscopy unveils GPCR cell surface architecture. Commun Biol 2022, 5, 221. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, V.D.; Ittmann, M.; Spencer, D.M. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009, 8, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Bond., R.A.; Ijzerman, A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 2006, 27, 92–96. [Google Scholar] [CrossRef]
- Kleinau, G.; Heyder, N.A.; Tao, Y.X.; Scheerer, P. Structural complexity and plasticity of signaling regulation at the melanocortin-4 receptor. Int J Mol Sci 2020, 21, 5728. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; May, V.; Li, J. PAC1 Receptors: Shapeshifters in Motion. J Mol Neurosci 2019, 68, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, Z.; Rovira, X.; Xue, L.; Roux, S.; Brabet, I.; Xin, M.; Pin, J.P.; Rondard, P.; Liu, J. Allosteric ligands control the activation of a class C GPCR heterodimer by acting at the transmembrane interface. Elife 2021, 10, e70188. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; De Pascali, F.; Richmond, G.W.; Khojah, A.M.; Benovic, J.L. Characterization of a new WHIM syndrome mutant reveals mechanistic differences in regulation of the chemokine receptor CXCR4. J Biol Chem 2022, 298, 101551. [Google Scholar] [CrossRef]
- Pydi, S.P.; Bhullar, R.P.; Chelikani, P. Constitutive activity of bitter taste receptors (T2Rs). Adv Pharmacol 2014, 70, 303–326. [Google Scholar]
- Rediger, A.; Piechowski, C.L.; Yi, C.X.; Tarnow, P.; Strotmann, R.; Grüters, A.; Krude, H.; Schöneberg, T.; Tschöp, M.H.; Kleinau, G.; Biebermann, H. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J Biol Chem 2011, 286, 39623–39631. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Baskaran, R.; Huang, C.Y. Detailed insight on β-adrenoceptors as therapeutic targets. Biomed Pharmacother 2019, 117109039. [Google Scholar] [CrossRef] [PubMed]
- Couty, J.P.; Geshengorn, M.C. G-protein-coupled receptors encoded by human herpesviruses. Trends Pharmacol Sci 2005, 26, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Davis-Poynter, N.; Farrell, H.E. Constitutive signaling by the human cytomegalovirus G protein coupled receptor homologs US28 and UL33 enables trophoblast migration in vitro. Viruses 2022, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.M.; Waldhoer, M.; Lüttichau, H.R.; Schwartz, T.W. Virally encoded 7TM receptors. Oncogene 2001, 20, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Canto, I.; Soh, U.J.; Trejo, J. Allosteric modulation of protease-activated receptor signaling. Mini Rev Med Chem 2012, 12, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Grimsey, N.; Lin, H.; Trejo, J. Endosomal signaling by protease-activated receptors. Methods Enzymol 2014, 535, 389–401. [Google Scholar] [PubMed]
- Wilde, C.; Fischer, L.; Lede, V.; Kirchberger, J.; Rothemund, S.; Schöneberg, T.; Liebscher, I. The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. FASEB J 2016, 30, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, M.; Wang, N.; Wu, Y.; Luo, Z.; Guo, S.; Han, G.W.; Li, S.; Yue, Y.; Wei, X.; Xie, X.; Chen, Y.; Zhao, S.; Wu, J.; Lei, M.; Xu, F. Structural basis of ligand recognition and self-activation of orphan GPR52. Nature 2020, 579, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Sadee, W.; Oberdick, J.; Wang, Z. Biased opioid antagonists as modulators of opioid dependence: Opportunities to improve pain therapy and opioid use management. Molecules 2020, 25, 4163. [Google Scholar] [CrossRef]
- Zhou, B.; Giraldo, J. An operational model for GPCR homodimers and its application in the analysis of biased signaling. Drug Discov Today 2018, 23, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.F.; Dietz, M.S.; Müller, U.; Weghuber, J.; Gatterdam, K.; Wieneke, R.; Heilemann, M.; Lanzerstorfer, P.; Tampé, R. Dynamic in Situ Confinement Triggers Ligand-Free Neuropeptide Receptor Signaling. Nano Lett 2022, 22, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, X.; Bohn, L.M.; Sadée, W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol 2005, 67, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Kobilka, B.K.; and Steyaert, J. Nanobodies to study G protein-coupled receptor structure and function. Annu Rev Pharmacol Toxicol 2017, 57, 19–37 PMID: 27959623. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Berkmann, J.C.; Scheerer, P.; Biebermann, H.; Kleinau, G. Insights into basal signaling regulation, oligomerization, and structural organization of the human G-protein coupled receptor 83. PLoS One 2016, 11, 68260. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.S.; McBride, E.W.; Beinborn, M.; Dunlap, K.; Kopin, A.S. Point mutations in either subunit of the GABAB receptor confer constitutive activity to the heterodimer. Mol Pharmacol 2006, 70, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Thibado, J.K.; Tano, J.Y.; Lee, J.; Salas-Estrada, L.; Provasi, D.; Strauss, A.; Marcelo Lamim Ribeiro, J.; Xiang, G.; Broichhagen, J.; Filizola, M.; Lohse, M.J.; Levitz, J. Differences in interactions between transmembrane domains tune the activation of metabotropic glutamate receptors. Elife 2021, 10, e67027. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Grande, C.; Smith, R.G. apo-Ghrelin receptor (apo-GHSR1a) regulates dopamine signaling in the brain. Front Endocrinol 2014, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Wellman, M.; Abizaid, A. Growth hormone secretagogue receptor dimers: A new pharmacological target. eNeuro 2015, 2, ENEURO.0053-14.2015. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.E.; Ammendrup-Johnsen, I.; Jansen, A.M.; Gether, U.; Madsen, K.L.; Bräuner-Osborne, H. The GPRC6A receptor displays constitutive internalization and sorting to the slow recycling pathway. J Biol Chem 2017, 292, 6910–6926. [Google Scholar] [CrossRef] [PubMed]
- Segredo, V.; Burford, N.T.; Lameh, J.; Sadée, W. A constitutively internalizing and recycling mutant of the mu-opioid receptor. J Neurochem 1997, 68, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Crilly, S.E.; Puthenveedu, M.A. Compartmentalized GPCR Signaling from Intracellular Membranes. J Membr Biol 2021, 254, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Nash, C.A.; Wei, W.; Irannejad, R.; Smrcka, A.V. Golgi localized β1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCε to regulate cardiac hypertrophy. Elife 2019, 8, e48167. [Google Scholar] [CrossRef] [PubMed]
- Purgert, C.A.; Izumi, Y.; Jong, Y.J.; Kumar, V.; Zorumski, C.F.; O'Malley, K.L. Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J Neurosci 2014, 34, 4589–4598. [Google Scholar] [CrossRef] [PubMed]
- Irannejad, R.; Pessino, V.; Mika, D.; Huang, B.; Wedegaertner, P.B.; Conti, M.; von Zastrow, M. Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol 2017, 13, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Stoeber, M.; Jullié, D.; Lobingier, B.T.; Laeremans, T.; Steyaert, J.; Schiller, P.W.; Manglik, A.; von Zastrow, M.A. Genetically encoded biosensor reveals location bias of opioid drug action. Neuron 2018, 98, 963–976.e5. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; May, V.; Li, J.; Ma, N.; Nivedha, A.K.; Vaidehi, N. Allosteric communication regulates ligand-specific GPCR activity. FEBS J 2021, 288, 2502–2512. [Google Scholar] [PubMed]
- Schafer, C.T.; Fay, J.F.; Janz, J.M.; Farrens, D.L. Decay of an active GPCR: Conformational dynamics govern agonist rebinding and persistence of an active, yet empty, receptor state. Proc. Natl. Acad. Sci. USA 2016, 113, 11961–11966. [Google Scholar] [CrossRef] [PubMed]
- Culhane, K.J.; Gupte, T.M.; Madhugiri, I.; Gadgil, C.J.; Sivaramakrishnan, S. Kinetic model of GPCR-G protein interactions reveals allokairic modulation of signaling. Nat Commun 2022, 2022 13, 1202. [Google Scholar] [CrossRef] [PubMed]
- Unal, H.; Karnik, S.S. Domain coupling in GPCRs: The engine for induced conformational changes. Trends Pharm. Sci. 2012, 33. [Google Scholar] [CrossRef] [PubMed]
- Bondar, A.; Lazar, J. The G protein Gi1 exhibits basal coupling but not preassembly with G protein-coupled receptors. J Biol Chem 2017, 292, 9690–9698. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.E.; Kayser, C.; Maiellaro, I.; Nemec, K.; Möller, J.; Koschinski, A.; Zaccolo, M.; Annibale, P.; Falcke, M.; Lohse, M.J.; Bock, A. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell. 2022, 185, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Elgeti, M.; Hubbell, W.L. DEER analysis of GPCR conformational heterogeneity. Biomolecules 2021, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Irannejad, R.; Tomshine, J.C.; Tomshine, J.R.; Chevalier, M.; Mahoney, J.P.; Steyaert, J.; Rasmussen, S.G.; Sunahara, R.K.; El-Samad, H.; Huang, B.; von Zastrow, M. Conformational biosensors reveal GPCR signalling from endosomes. Nature 2013, 495, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, O.; Torres, R.; Bellocchio, L.G.; Rosenthal, S.J. Membrane nanoscopic organization of D2L dopamine receptor Pprobed by quantum dot tracking. Membranes 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Sadee, W.; McKew, J.C. Ligand-Free Signaling of G-Protein-Coupled Receptors: Relevance to μ Opioid Receptors in Analgesia and Addiction. Molecules 2022, 27, 5826. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Nivedha, A.K.; Vaidehi, N. Allosteric communication regulates ligand-specific GPCR activity. FEBS J. 2021, 288, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Rinken, A.; Veiksina, S.; Kopanchuk, S. Dynamics of ligand binding to GPCR: Residence time of melanocortins and its modulation. Pharmacol Res 2016, 113 Pt B, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.C.; Rosenbaum, J.S.; Kurowski, M.; Sadée, W. 3H-Etorphine Receptor Binding In Vivo: Small Fractional Occupancy Elicits Analgesia. Mol. Pharmacol. 1982, 21, 272–279. [Google Scholar] [PubMed]
- Engel, S.; Gershengorn, M.C. Thyrotropin-releasing hormone and its receptors--a hypothesis for binding and receptor activation. Pharmacol Ther 2007, 113, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Kleinau, G.; Jaeschke, H.; Mueller, S.; Worth, C.L.; Paschke, R.; Krause, G. Molecular and structural effects of inverse agonistic mutations on signaling of the thyrotropin receptor--a basally active GPCR. Cell Mol Life Sci 2008, 65, 3664–3676. [Google Scholar] [CrossRef] [PubMed]
- Quillan, J.M.; Carlson, K.W.; Song, C.; Wang, D.; Sadée, W. Differential effects of mu-opioid receptor ligands on Ca(2+) signaling. J Pharmacol Exp Ther 2002, 302, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Arden, J.R.; Segredo, V.; Wang, Z.; Lameh, J.; Sadée, W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem 1995, 65, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Scheerer, P.; Hofmann, K.P.; Choe, H.W.; Ernst, O.P. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 2008, 454, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Farrens, D.L. A constitutively activating mutation alters the dynamics and energetics of a key conformational change in a ligand-free G protein-coupled receptor. J Biol Chem 2013, 288, 28207–28216. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.S.; Holford, N.H.G.; Richard, M.L.; Aman, R.A.; Sadee, W. Discrimination of three types of opioid binding sites in rat brain in vivo. Mol. Pharmacol. 1984, 25, 242–248. [Google Scholar] [PubMed]
- Perry, D.C.; Mullis, K.B.; Oie, S.; Sadée, W. Opiate Antagonist Receptor Binding In Vivo: Evidence for a New Receptor Binding Model. Brain Res. 1980, 199, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.S.; Holford, N.H.G.; Sadée, W. In Vivo Receptor Binding of Opioid Drugs at the μ Site. J. Pharmacol. Exp. Ther. 1985, 233, 735–740. [Google Scholar]
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S.; Liechti, M.E. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharm 2021, 46, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Schmid, Y.; Enzler, F.; Gasser, P.; Grouzmann, E.; Preller, K.H.; Vollenweider, F.X.; Brenneisen, R.; Mu ̈ ller, F.; Borgwardt, S.; Liechti, M.E. Acute effects of lysergic acid diethylamide in healthy subjects. Biol. Psychiatry 2015, 78, 544–553. [Google Scholar] [CrossRef]
- Dolder, P.C.; Schmid, Y.; Haschke, M.; Rentsch, K.M.; Liechti, M.E. Pharmacokinetics and concentration-effect relationship of oral LSD in humans. Int J Neuropsychopharmacol 2015, 19, pyv072, Erratum in: Int J Neuropsychopharmacol 2016. [Google Scholar] [CrossRef] [PubMed]
- Dolder, P.C.; Schmid, Y.; Steuer, A.E.; Kraemer, T.; Rentsch, K.M.; Hammann, F.; Liechti, M.E. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet 2017, 56, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.E.; Janssen, P.F.; Niemegeers, C.J. Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol 1989, 163, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E.; Shoichet, B.K.; Skiniotis, G.; Roth, B.L. Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell 2020, 182, 1574–1588.e19. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal structure of an LSD-bound human serotonin receptor. Cell 2017, 168, 377–389.e12. [Google Scholar] [CrossRef] [PubMed]
- Hartig, P.R.; Scheffel, U.; Frost, J.J.; Wagner, H.N.Jr. In vivo binding of 125I-LSD to serotonin 5-HT2 receptors in mouse brain. Life Sci. 1985, 37, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.L.; Hutsell, B.A.; Blough, B.E.; Poklis, J.L.; Negus, S.S. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int J Neuropsychopharmacol 2015, 18, pyv009. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.W.; Liu, D.N.; Yan, L.H.; Cui, X.Y.; Zhang, K.P.; Qi, C.; Chen, J. Nociceptive stimulus modality-related difference in pharmacokinetic-pharmacodynamic modeling of morphine in the rat. Pharmacol Biochem Behav 2006, 85, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Ihmsen, H.; Hering, W.; Geisslinger, G.; Dingemanse, J.; Schwilden, H.; Schüttler, J. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin. Pharmacol. Ther. 1999, 65, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Cocchetto, D.M.; Owens, S.M.; Perez-Reyes, M.; DiGuiseppi, S.; Miller, L.L. Relationship between plasma delta-9-tetrahydrocannabinol concentration and pharmacologic effects in man. Psychopharmacol 1981, 75, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhu, X.; Jusko, N.M.; Krzyzanski, W.; Jusko, W.J. Pharmacodynamic model of slow reversible binding and its applications in pharmacokinetic/pharmacodynamic modeling: review and tutorial. J Pharmacokinet Pharmacodyn 2022, 49, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Michel-Reher, M.B.; Hein, P. A systematic review of inverse agonism at adrenoceptor subtypes. Cells 2020, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Raehal, K.M.; Bilsky, E.J.; Sadée, W. Inverse agonists and neutral antagonists at μ opioid receptor (MOR): Possible role of basal receptor signaling in narcotic dependence. J. Neurochem. 2001, 77, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro, G.; Azzi, M.; deLean, A.; Schiller, P.W.; Bouvier, M. Reciprocal regulation of agonist and inverse agonist signaling efficacy upon short-term treatment of the human delta-opioid receptor with an inverse agonist. Mol. Pharmacol 2005, 67, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Makita, N.; Iiri, T. Inverse agonism: the classic concept of GPCRs revisited [Review]. Endocr J 2016, 63, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, S.; Dighe, S.V.; Madia, P.A.; Yoburn, B.C. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J. Pharmacol. Exp. Ther. 2009, 330, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.C.; Divin, M.F.; Lee, H.; Woods, J.H.; Traynor, J.R. Differential in vivo potencies of naltrexone and 6β-naltrexol in the monkey. J. Pharmacol. Exp. Ther. 2006, 316, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Yancey-Wrona, J.; Dallaire, B.; Bilsky, E.J.; Bath, B.; Burkart, J.; Wenster, L.; Magiera, D.; Yang, X.; Phelps, M.A.; Sadee, W. 6β-Naltrexol, a peripherally selective opioid antagonist that inhibits morphine-induced slowing of gastrointestinal transit: An exploratory study. Pain Med. 2011, 12, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.J.; Somogyi, A.A.; White, J.M. In vivo and in vitro potency studies of 6beta-naltrexol, the major human metabolite of naltrexone. Addict Biol 2002, 7, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Raehal, K.M.; Lowery, J.J.; Bhamidipati, C.M.; Paolino, R.M.; Blair, J.R.; Wang, D.; Sadée, W.; Bilsky, E.J. In vivo characterization of 6β-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol. Exp. Ther. 2005, 313, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, X.; Hu, T.; Qu, C.; Lu, J.; Zhou, Y.; Li, J.; Pei, G. Constitutive activity of serotonin receptor 6 regulates human cerebral organoids formation and depression-like behaviors. Stem Cell Reports 2021, 16, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, X.; Sadee, W. 2007 Different effects of opioid antagonists on mu, delta, and kappa opioid receptors with and without agonist pretreatment. J. Pharmacol. Exp. Ther. 2011, 321, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Muneta-Arrate, I.; Diez-Alarcia, R.; Horrillo, I.; Meana, J.J. Pimavanserin exhibits serotonin 5-HT2A receptor inverse agonism for Gαi1- and neutral antagonism for Gαq/11-proteins in human brain cortex. Eur. Neuropsychopharm. 2020, 36, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chilcoat, H.D.; Amick, H.R.; Sherwood, M.R.; Dunn, K.E. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019, 104, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yokell, M.A.; Zaller, N.D.; Green, T.C.; Rich, J.D. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev 2011, 4, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.; Lewis, J.W.; Macfarlane, I.R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmaco 1977, 60, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Colom, M.; Vidal. ; Zimmer, L. Is There a role for GPCR agonist radiotracers in PET neuroimaging? Front Mol Neurosc 2019, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Mangeant, R.; Dubost, E.; Cailly, T.; Collot, V. Radiotracers for the central serotoninergic system. Pharmaceuticals 2022, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.J.; Stam, N.; Mutel, V.; Vanderheyden, P.M. Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochem Pharmacol 1996, 51, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Crilly, S.E.; Ko, W.; Weinberg, Z.Y.; Puthenveedu, M.A. Conformational specificity of opioid receptors is determined by subcellular location irrespective of agonist. Elife 2021, 20, e67478. [Google Scholar] [CrossRef] [PubMed]
- Radoux-Mergault, A.; Oberhauser, L.; Aureli, S.; Gervasio, F.L.; Stoeber, M. Subcellular location defines GPCR signal transduction. Sci Adv 2023, 9, eadf6059. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Barros-Álvarez, X.; Zhang, S.; Kim, K.; Dämgen, M.A.; Panova, O.; Suomivuori, C.M.; Fay, J.F.; Zhong, X.; Krumm, B.E.; et al. Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 2022, 110, 3154–3167.e7. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.; Doolen, S.; Donahue, R.R.; Winter, M.K.; Jutras, B.K.L.; He, Y.; Hu, X.; Wieskopf, J.S.; Mogil, J.S.; Storm, D.R.; et al. Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 2013, 341, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Navani, D.M.; Sirohi, S.; Madia, P.A.; Yoburn, B.C. The role of opioid antagonist efficacy and constitutive opioid receptor activity in the opioid withdrawal syndrome in mice. Pharm. Biochem. Behav. 2011, 99, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shoblock, J.R.; Maidment, N.T. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience 2007, 149, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Walwyn, W.; Evans, C.J.; Hales, T.G. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J. Neurosci. 2007, 27, 5092–5104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Prather, P.L. Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol. Pharmacol. 2001, 60, 53–62. [Google Scholar] [CrossRef]
- Liu, J.G.; Prather, P.L. Chronic agonist treatment converts antagonists into inverse agonists at delta-opioid receptors. J. Pharmacol. Exp. Ther. 2002, 302, 1070–1079. [Google Scholar] [CrossRef]
- Belcheva, M.M.; Szùcs, M.; Wang, D.; Sadee, W.; Coscia, C.J. -Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem 2001, 276, 33847–3353. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.; Lau, A.R.; Aten, S.; Schilling, K.; Bales, K.L.; Miller, V.; Fitzgerald, J.; Chen, M.; Hill, K.; Dzwigalski, K.; et al. Pharmacological prevention of neonatal opioid withdrawal in a pregnant guinea pig model. Front. Pharmacol. 2021, 11, 613328. [Google Scholar] [CrossRef] [PubMed]
- Oberdick, J.; Ling, Y.; Phelps, M.A.; Yudovich, M.S.; Schilling, K.; Sadee, W. Preferential delivery of an opioid antagonist to the fetal brain in pregnant mice. J Pharmacol Exp Ther 2016, 358, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.C.; Chavera, T.S.; Jamshidi, R.J.; Berg, K.A.; Clarke, W.P. Constitutive desensitization of opioid receptors in peripheral sensory neurons. J. Pharmacol. Exp. Ther. 2016, 359, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Narvaez, M.; Oflijan, J.; Agnati, L.F.; Fuxe, K. Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes. Biochem Biophys Res Commun 2014, 443, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Bécamel, C.; Chaumont-Dubel, S.; Claeysen, S.; Vandermoere, F.; Marin, P. Novel and atypical pathways for serotonin signaling. Fac Rev 2021, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Toneatti, R.; Shin, J.M.; Shah, U.H.; Mayer, C.R.; Saunders, J.M.; Fribourg, M.; Arsenovic, P.T.; Janssen, W.G.; Sealfon, S.; López-Giménez, J.F.; et al. Interclass GPCR heteromerization affects localization and trafficking. Sci Signal 2020, 13, eaaw3122. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.A.; Harvey, J.A.; Spampinato, U.; Clarke, W.P. Physiological and therapeutic relevance of constitutive activity of 5-HT 2A and 5-HT 2C receptors for the treatment of depression. Prog Brain Res 2008, 172, 287–305. [Google Scholar] [PubMed]
- Aloyo, V.J.; Berg, K.A.; Clarke, W.P.; Spampinato, U.; Harvey, J.A. Inverse agonism at serotonin and cannabinoid receptors. Prog. Mol. Biol. Transl. Sci. 2010, 91, 1–40. [Google Scholar] [PubMed]
- Kantrowitz, J.T. Targeting serotonin 5-HT2A receptors to better treat schizophrenia: Rationale and current approaches. CNS Drugs 2020, 34, 947–959. [Google Scholar] [CrossRef] [PubMed]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr Top Behav Neurosci 2018, 36, 45–73. [Google Scholar] [PubMed]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 2016, 26, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.L.; Lynch, H.F.; Grossman, L.A.; Cohen, I.G. Pressing regulatory challenges for psychedelic medicine. Science 2023, 380, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.A.D.; D'Souza, D.C. The therapeutic potential of psychedelics. Science 2022, 378, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Slocum, S.T.; DiBerto, J.F.; Roth, B.L. Molecular insights into psychedelic drug action. J Neurochem 2022, 162, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; de Manincor, M.; Perkins, D.; Sarris, J. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacol 2021, 238, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.; Vargas, M.V.; Duim, W.C.; Grodzki, A.C.G.; Lein, P.J.; Olson, D.E. Transient stimulation with psychoplastogens is sufficient to initiate neuronal growth. ACS Pharmacol Transl Sci 2020, 4, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kolaczynska, K.E.; Luethi, D.; Trachsel, D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of 4-alkoxy-3,5-dimethoxy-phenethylamines (mescaline derivatives) and related amphetamines. Front Pharmacol 12, 794254. [CrossRef] [PubMed]
- Barker, S.A.; McIlhenny, E.H.; Strassman, R. A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955-2010. Drug Test Anal 2012, 4, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.G.; Liu, T.; Huff, S.; Sheler, B.; Barker, S.A.; Strassman, R.J.; Wang, M.M.; Borjigin, J. Biosynthesis and extracellular concentrations of N,N-dimethyltryptamine (DMT) in mammalian brain. Sci Rep 2019, 9, 9333. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Gumpper, R.H. Psychedelics as transformative therapeutics. Am J Psychiatry. 2023, 180, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Family, N.; Hendricks, P.S.; Williams, L.T.; Luke, D.; Krediet, E.; Maillet, E.L.; Raz, S. Safety, tolerability, pharmacokinetics, and subjective effects of 50, 75, and 100 µg LSD in healthy participants within a novel intervention paradigm: A proof-of-concept study. J Psychopharmacol 2022, 36, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Wenzel-Seifert, K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 2002, 366, 381–416. [Google Scholar] [CrossRef] [PubMed]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef] [PubMed]
- Rantamäki, T.; Hendolin, P.; Kankaanpää, A.; Mijatovic, J.; Piepponen, P.; Domenici, E.; Chao, M.V.; Männistö, P.T.; Castrén, E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharm 2007, 32, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A. Administration of N,N-dimethyltryptamine (DMT) in psychedelic therapeutics and research and the study of endogenous DMT. Psychopharm 2022, 239, 1749–1763. [Google Scholar] [CrossRef] [PubMed]
- Polito, V.; Liknaitzky, P. The emerging science of microdosing: A systematic review of research on low dose psychedelics (1955-2021) and recommendations for the field. Neurosci Biobehav Rev 2022, 139, 104706. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, Y.; Zhang, W. The growth hormone secretagogue receptor: its intracellular signaling and regulation. Int J Mol Sci 2014, 15, 4837–4855. [Google Scholar] [CrossRef] [PubMed]
- Davis, J. Hunger, ghrelin and the gut. Brain Res 2018, 1693 Pt B, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mukhopadhyay, S.; Mitra, A. Therapeutic potential of GHSR-1A antagonism in alcohol dependence, a review. Life Sci 2022, 291, 120316. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.P.; Mustafá, E.R.; Barrile, F.; Cassano, D.; De Francesco, P.N.; Raingo, J.; Perello, M. The intriguing ligand-dependent and -independent actions of the growth hormone secretagogue receptor on reward related behaviors. Neurosci Biobehav Rev 2021, 120, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Mear, Y.; Enjalbert, A.; Thirion, S. GHS-R1a constitutive activity and its physiological relevance. Front Neurosci 2013, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Pantel, J.; Legendre, M.; Cabrol, S.; Hilal, L.; Hajaji, Y.; Morisset, S.; Nivot, S.; Vie-Luton, M.-P.; Grouselle, D.; de Kerdanet, M.; Kadiri, A.; Epelbaum, J.; Le Bloc, Y.; Amselem, S. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 2006, 116, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Torz, L.J.; Osborne-Lawrence, S.; Rodriguez, J.; He, Z.; Cornejo, M.P.; Mustafá, E.R.; Jin, C.; Petersen, N.; Hedegaard, M.A.; Nybo, M.; et al. Metabolic insights from a GHSR-A203E mutant mouse model. Mol Metab 2020, 39, 101004. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.P.; Castrogiovanni, D.; Schiöth, H.B.; Reynaldo, M.; Marie, J.; Fehrentz, J.A.; Perello, M. Growth hormone secretagogue receptor signalling affects high-fat intake independently of plasma levels of ghrelin and LEAP2, in a 4-day binge eating model. J Neuroendocrinol 2019, 31, e12785. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Abdalla, M.M. Ghrelin - physiological functions and regulation. Eur Endocrinol. 2015, 11, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kazius, J.; Wurdinger, K.; van Iterson, M.; Kok, J.; Bäck, T.; et al. GPCR NaVa database: natural variants in human G protein-coupled receptors. Hum Mutat 2008, 29, 39–44. [Google Scholar] [CrossRef]
- Pándy-Szekeres, W.; Caroli, J.; Marmybekov, A.; Kermani, A.A.; Kserü, G.M.; Kooistra, A.J.; Gloriam, D.E. GPCRdb in 2023: state-specific structure models using AlphaFold2 and new ligand resources. Nucleic Acids Research 2023, 51, D395–D402. [Google Scholar] [CrossRef]
- Chen, G.; Way, J.; Armour, S.; Watson, C.; Queen, K.; Jayawickreme, C.K.; Chen, W.J.; Kenakin, T. Use of constitutive G protein-coupled receptor activity for drug discovery. Mol Pharmacol 2000, 57, 125–134. [Google Scholar] [PubMed]
- Luzum, J.A.; Sweet, K.M.; Binkley, P.F.; Schmidlen, T.J.; Jarvis, J.P.; Christman, M.F.; Sadee, W.; Kitzmiller, J.P. CYP2D6 genetic variation and beta-blocker maintenance dose in patients with heart failure. Pharm Res 2017, 34, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Hainer, V.; Aldhoon Hainerová, I.; Kunešová, M.; Taxová Braunerová, R.; Zamrazilová, H.; Bendlová, B. Melanocortin pathways: suppressed and stimulated melanocortin-4 receptor (MC4R). Physiol Res 2020, 69, S245–S254. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, P.; Schöneberg, T. The relevance of genomic signatures at adhesion GPCR loci in humans. Handb Exp Pharmacol 2016, 234, 179–217. [Google Scholar] [PubMed]
- Unal, H.; Karnik, S.S. Constitutive activity in the angiotensin II type 1 receptor: discovery and applications. Adv Pharmacol 2014, 70, 155–174. [Google Scholar] [PubMed]
- Xu, B.; Chakraborty, R.; Eilers, M.; Dakshinamurti, S.; O'Neil, J.D.; Smith, S.O.; Bhullar, R.P.; Chelikani, P. High-level expression, purification and characterization of a constitutively active thromboxane A2 receptor polymorphic variant. PLoS One 2013, 8, e76481. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Pediani, J.D.; Marsango, S.; Jolly, R.; Stoneman, M.R.; Biener, G.; Handel, T.M.; Raicu, V.; Milligan, G.J. Chemokine receptor CXCR4 oligomerization is disrupted selectively by the antagonist ligand IT1t. Biol Chem 2021, 296, 100139. [Google Scholar] [CrossRef] [PubMed]
- Vassart, G.; Costagliola, S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol 2011, 7, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Helfinger, L.; Tate, C.G. Expression and purification of the human thyroid-stimulating hormone receptor. Methods Mol Biol 2022, 2507, 313–325. [Google Scholar] [PubMed]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog Retin Eye Res 2018, 62, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Patchett, A.A.; Nargund, R.P.; Tata, J.R.; Chen, M.H.; Barakat, K.J.; Johnston, D.B.; Cheng, K.; Chan, W.W.; Butler, B.; Hickey, G.; et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci USA 1995, 92, 7001–7005. [Google Scholar] [CrossRef] [PubMed]
- Arang, N.; Gutkind, J.S. G Protein-Coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett 2020, 594, 4201–4232. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S. An insight into GPCR and G-proteins as cancer drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, B.D.; Hamm, H.E. Roles of G-protein-coupled receptor signaling in cancer biology and gene transcription. Curr Opin Genet Dev 2007, 17, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Bongers, B.J.; Gorostiola González, M.; Wang, X.; van Vlijmen, H.W.T.; Jespers, W.; Gutiérrez-de-Terán, H.; Ye, K.; IJzerman, A.P.; Heitman, L.H.; van Westen, G.J.P. Pan-cancer functional analysis of somatic mutations in G protein-coupled receptors. Sci Rep 2022, 12, 21534. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Moyung, K.; Corriden, R.; Carter, H.; Insel, P.A. GPCRs show widespread differential mRNA expression and frequent mutation and copy number variation in solid tumors. PLoS Biol 2019, 17, e3000434. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Chen, J.; Wu, B.; Liu, J.; Guo, Y.; Li, M.; Pu, X. Multi-omics integration analysis of GPCRs in pan-cancer to uncover inter-omics relationships and potential driver genes. Comput Biol Med 2023, 161, 106988. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat Rev Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, S.; Peng, S.B. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol 2005, 27, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.; Proia, R.L. The role of G protein-coupled receptors in lymphoid malignancies. Cell Signal 2017, 39, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jespers, W.; de Waal, J.J.; Wolff, K.A.N.; van Uden, L.; IJzerman, A.P.; van Westen, G.J.P.; Heitman, L.H. Cancer-related somatic mutations alter adenosine A1 receptor pharmacology-A focus on mutations in the loops and C-terminus. FASEB J 2022, 36, e22358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J. Hedgehog signaling mechanism and role in cancer. Semin Cancer Biol 2022, 85, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Z.; Liu, Z.; Song, C. Overcoming the emerging drug resistance of smoothened: an overview of small-molecule SMO antagonists with antiresistance activity. Future Med Chem 2018, 10, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.H.; Aktary, Z.; Larue, L.; Delmas, V. Targeting GPCRs and their signaling as a therapeutic option in melanoma. Cancers 2022, 14, 706. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



