Submitted:
09 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4 magnetic core
2.2. Synthesis of Fe3O4core-meso SiO2/TiO2 double shell nanoparticles
2.2.1. Coating with mesoporous silica
2.2.2. Titania Coating
2.3. Synthesis of multifunctional Fe3O4core-TiO2/meso SiO2 double shell nanoparticles
2.4. Adsorptive-removal study for methylene blue
3. Results and discussion
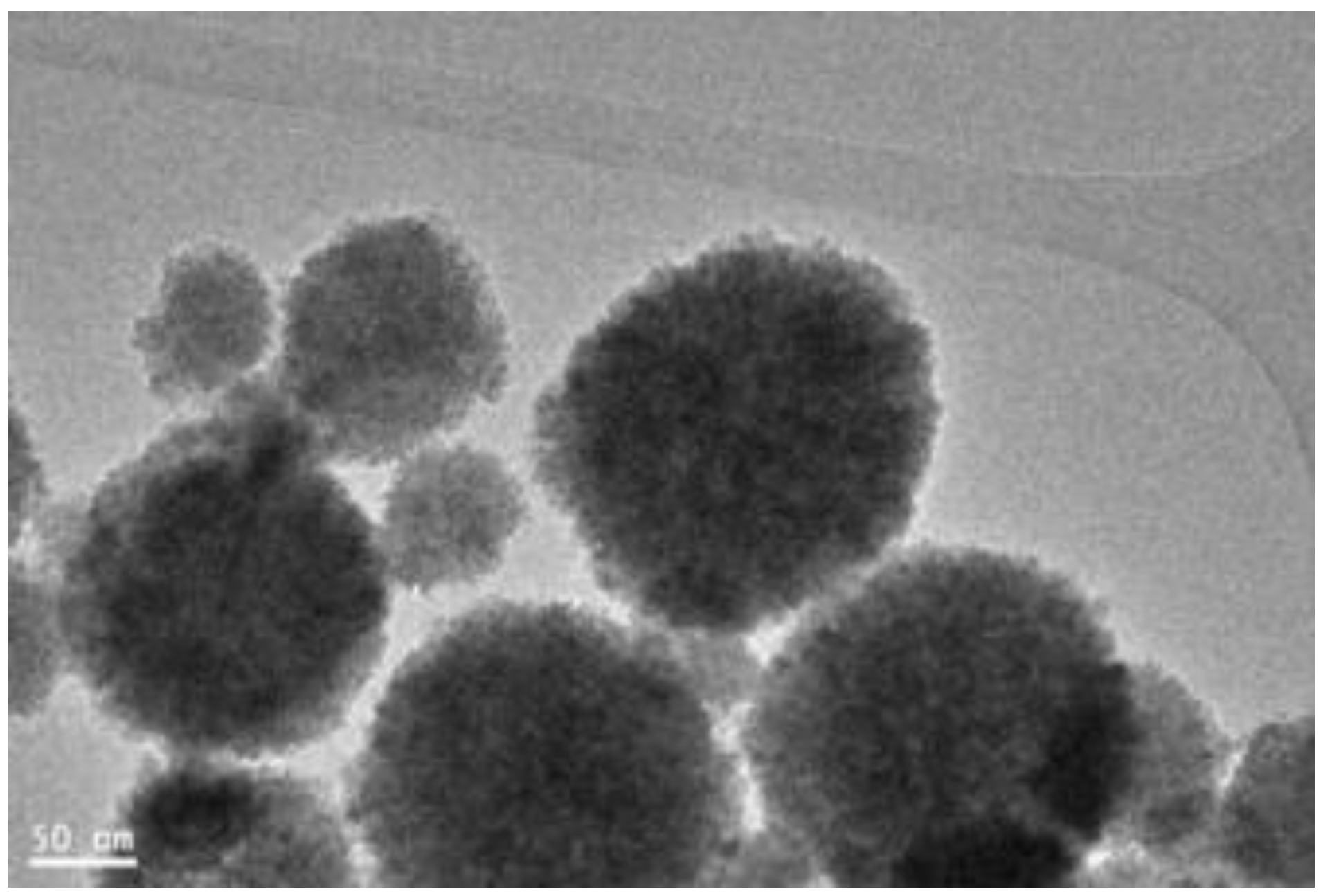
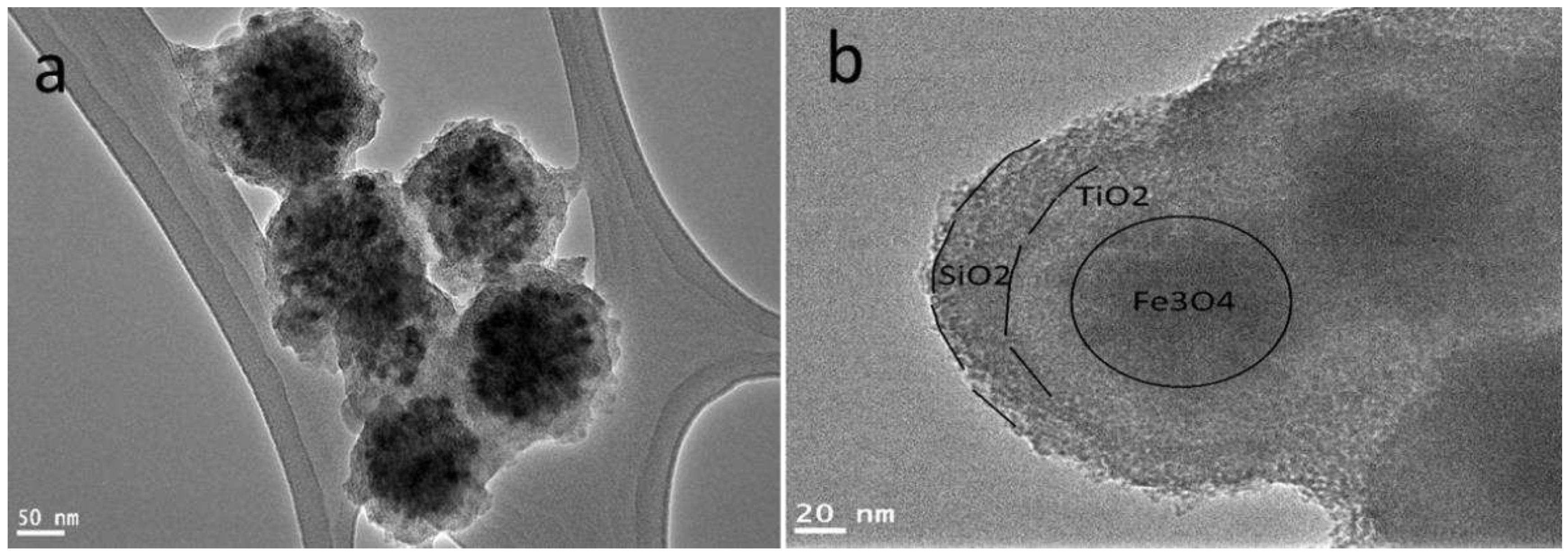
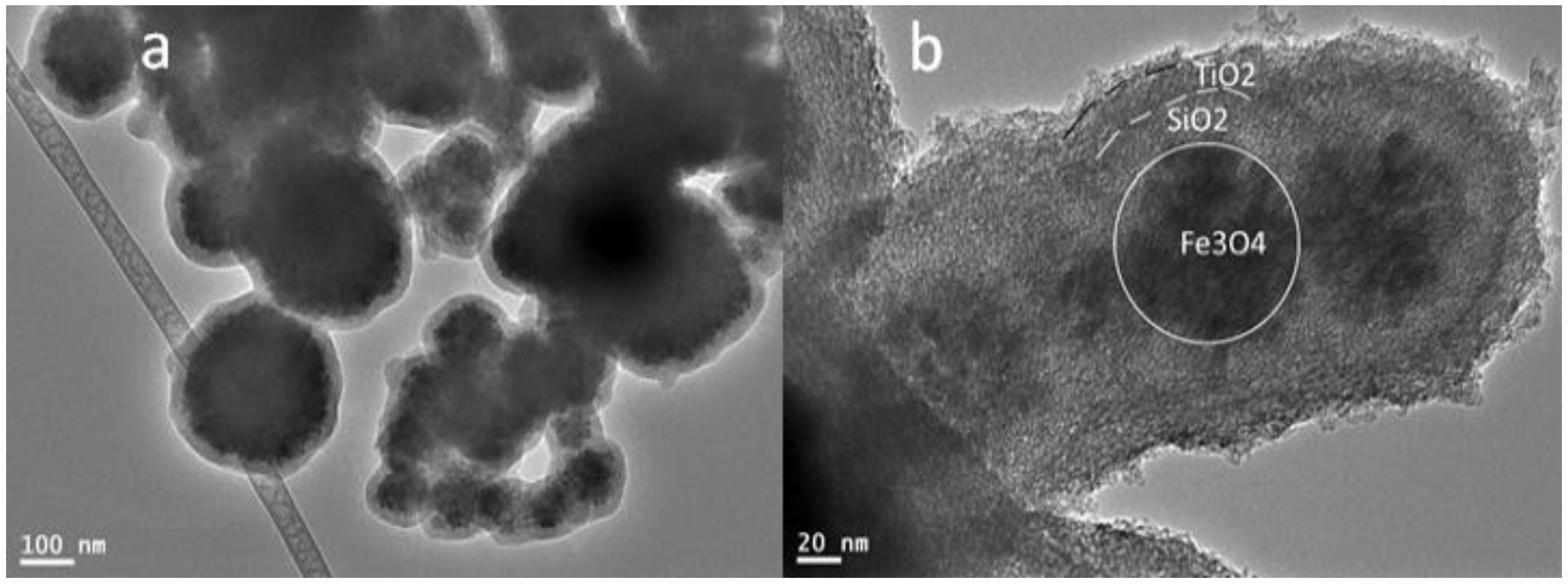
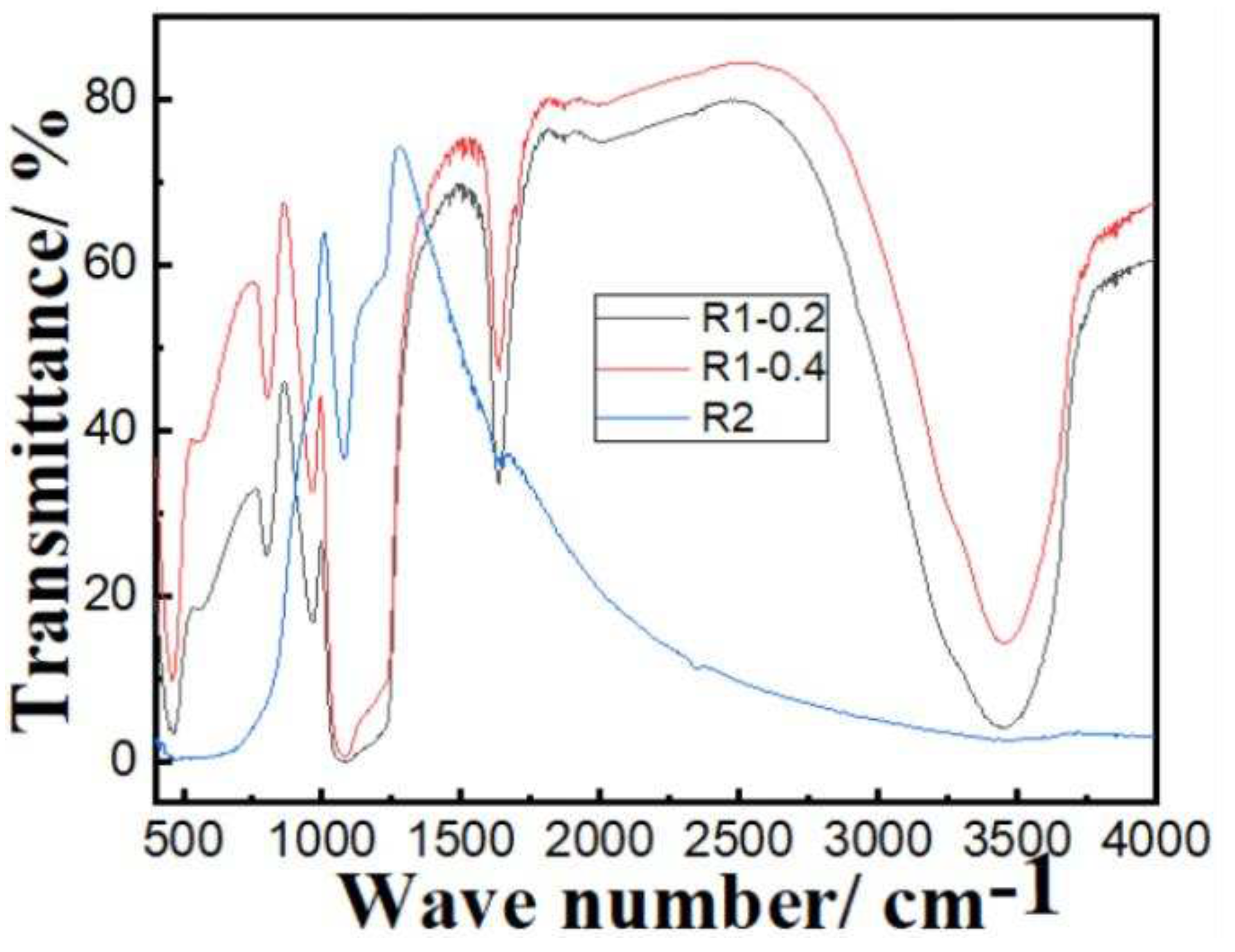
3.1. Characterization of Fe3O4core-double shell
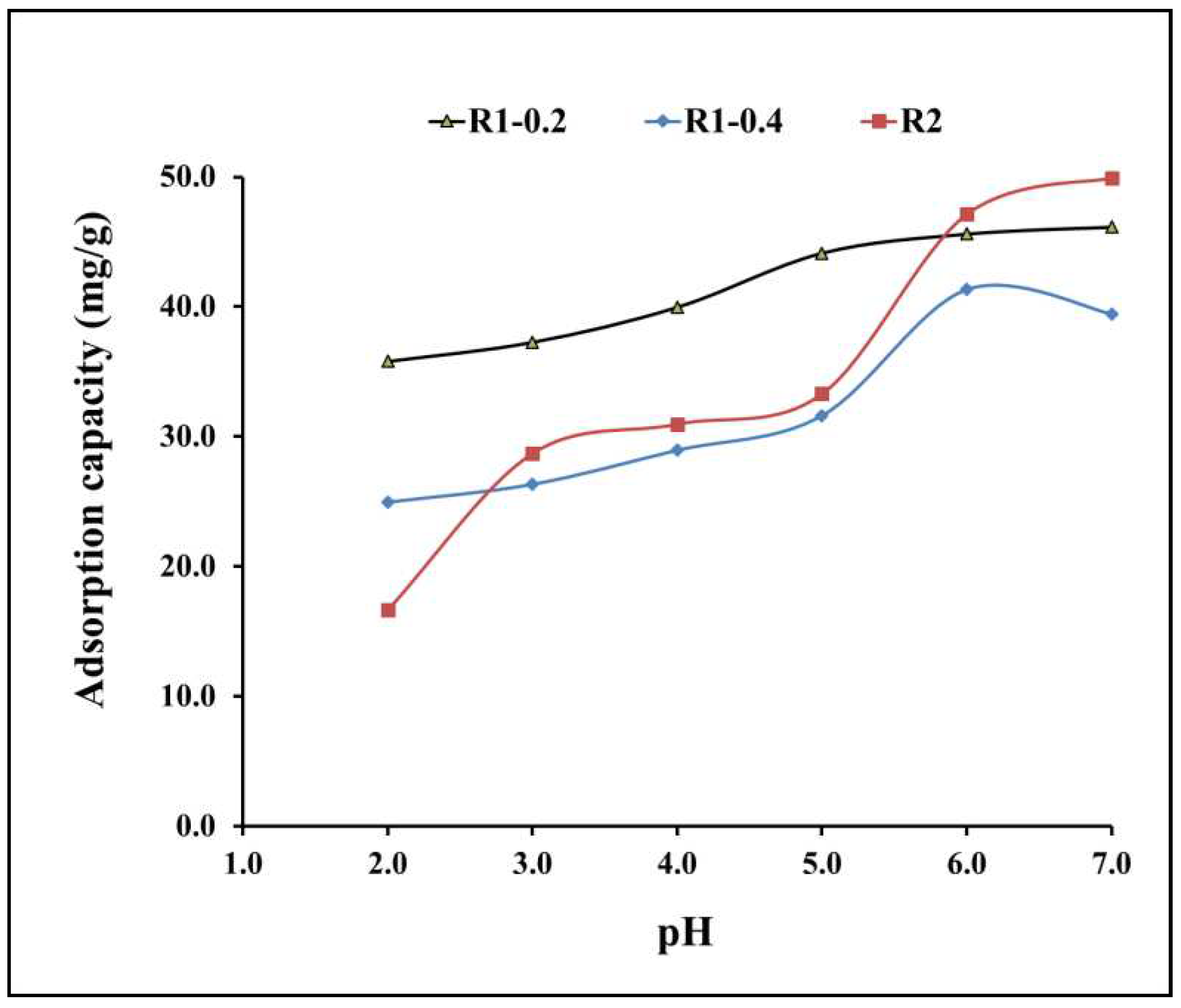
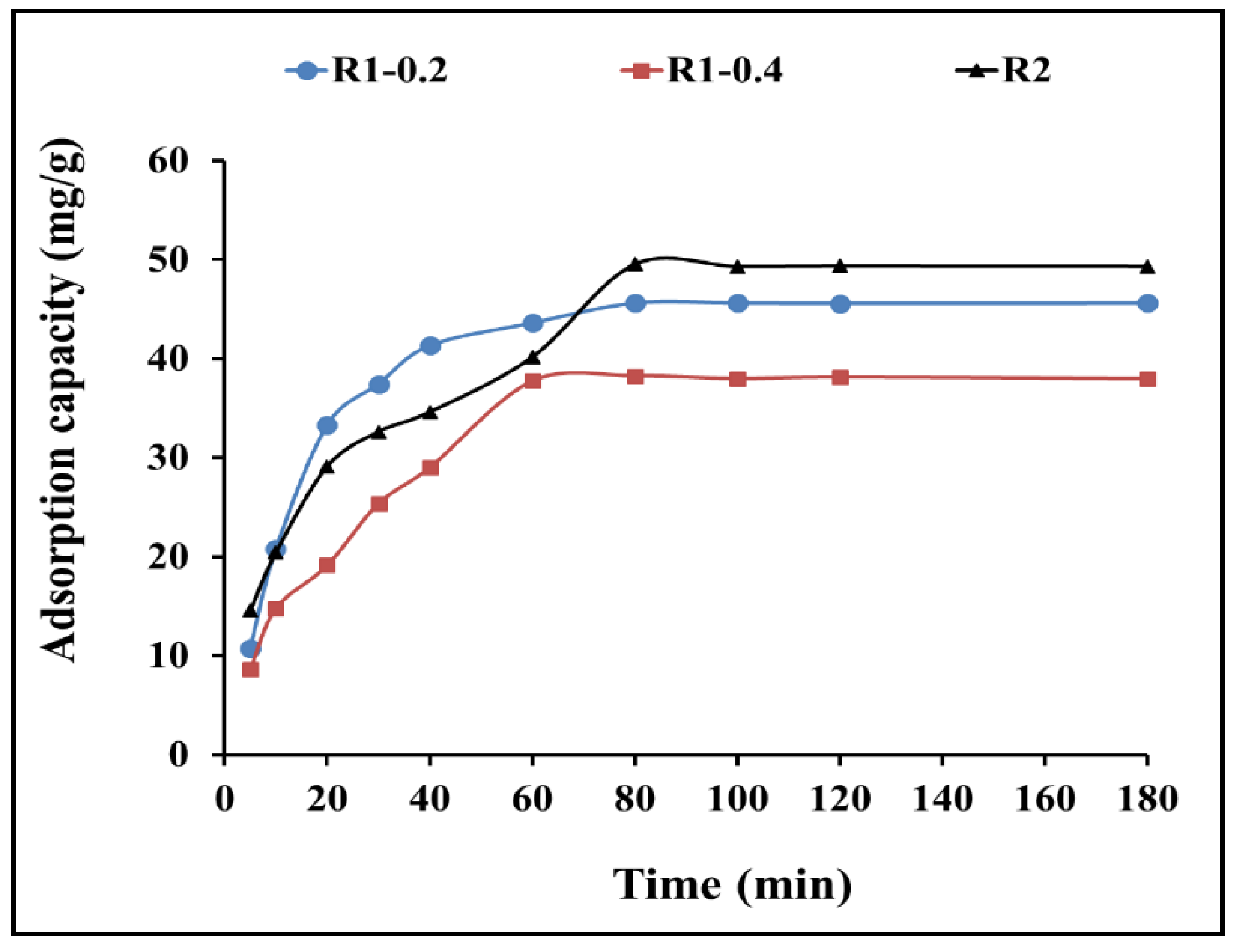
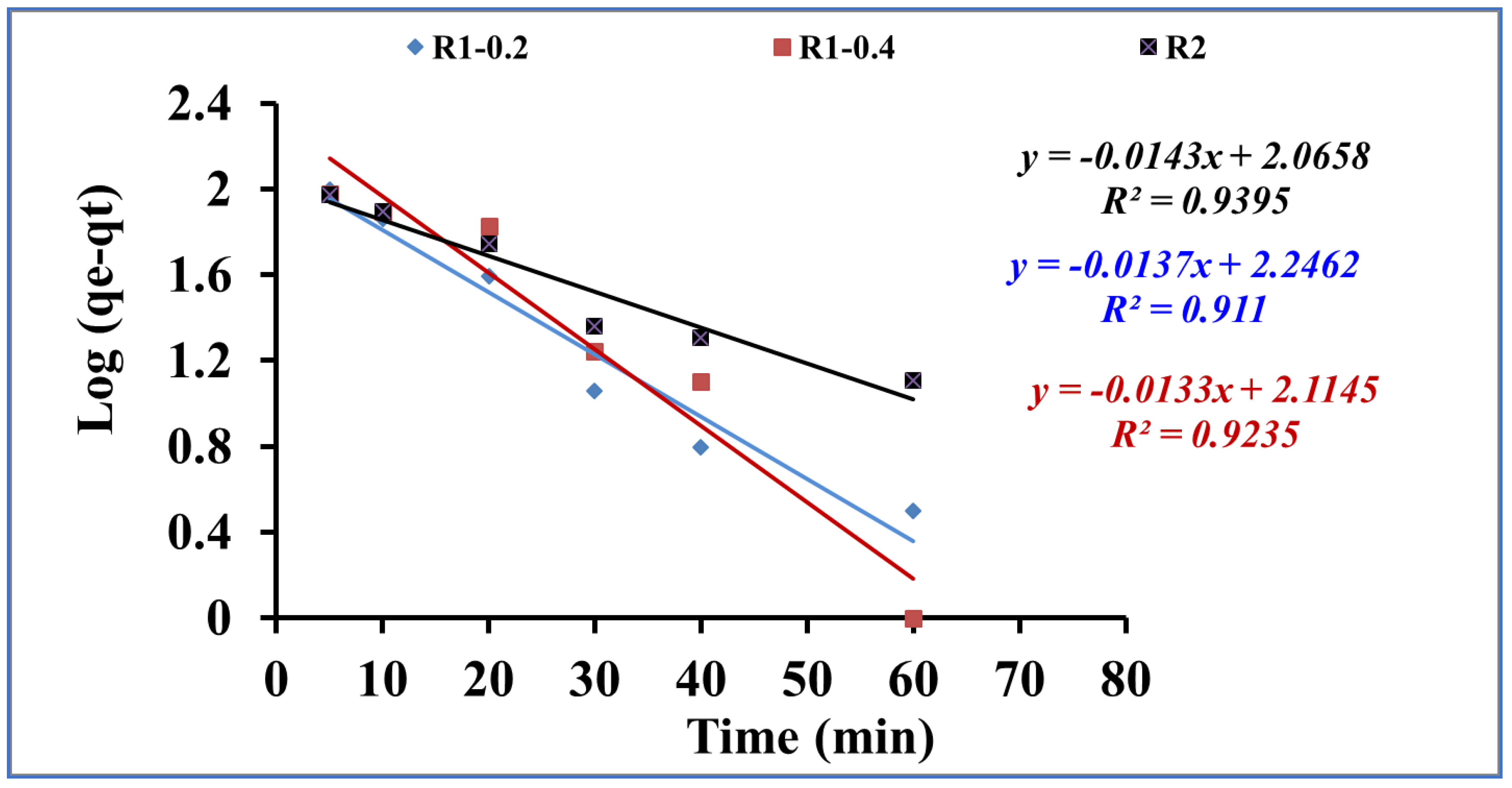
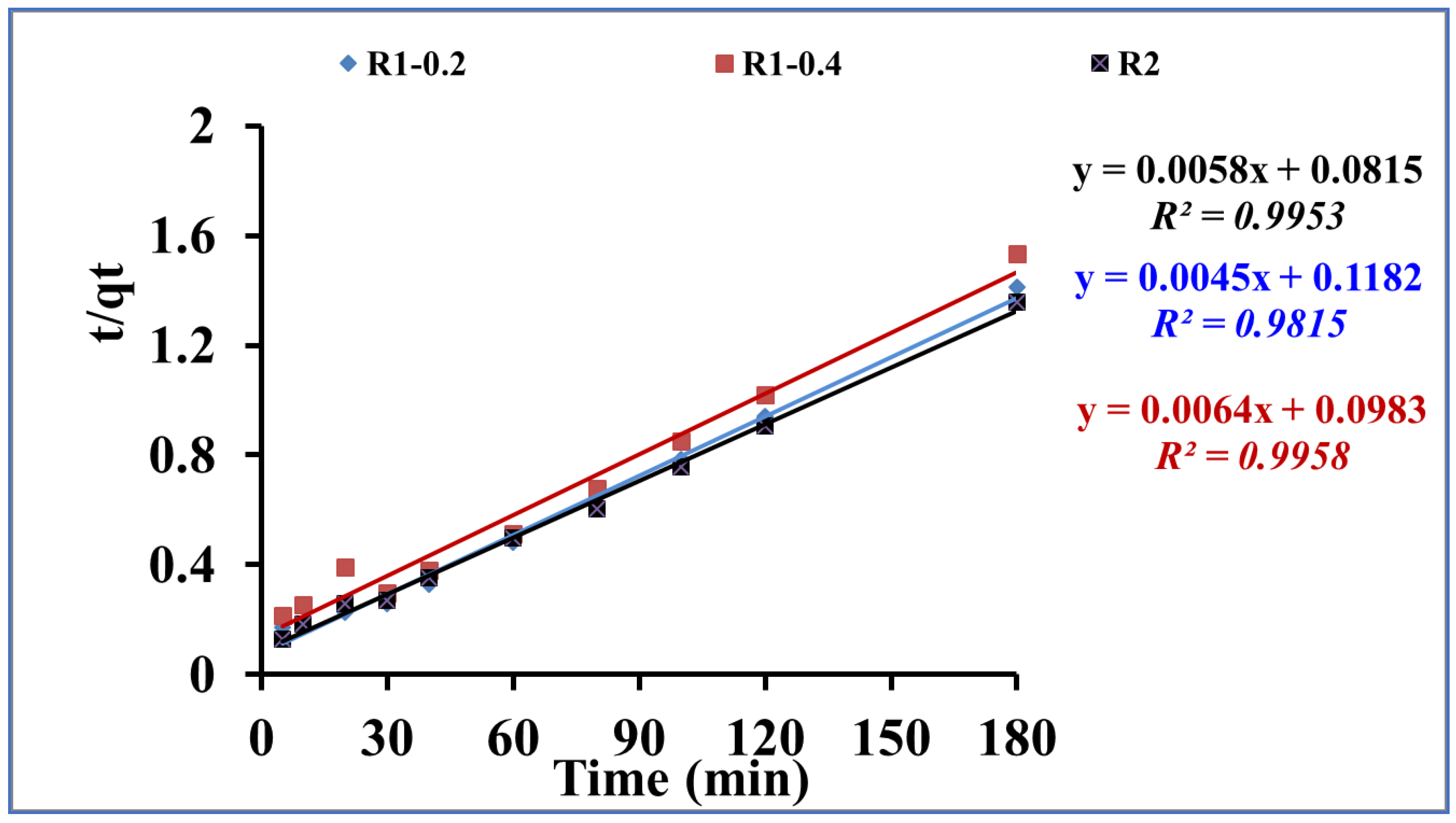
3.2. Adsorptive-remediation investigation
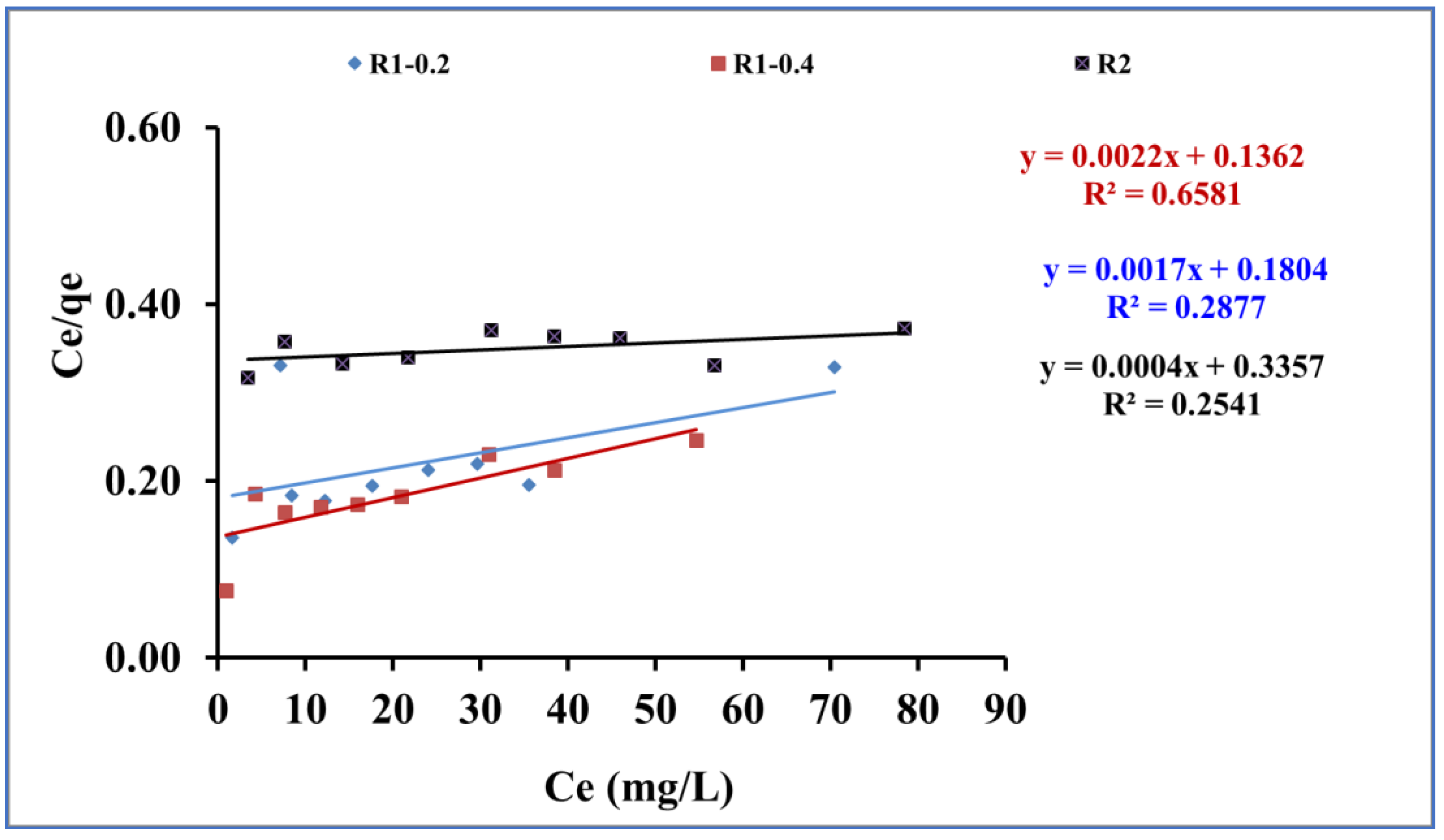
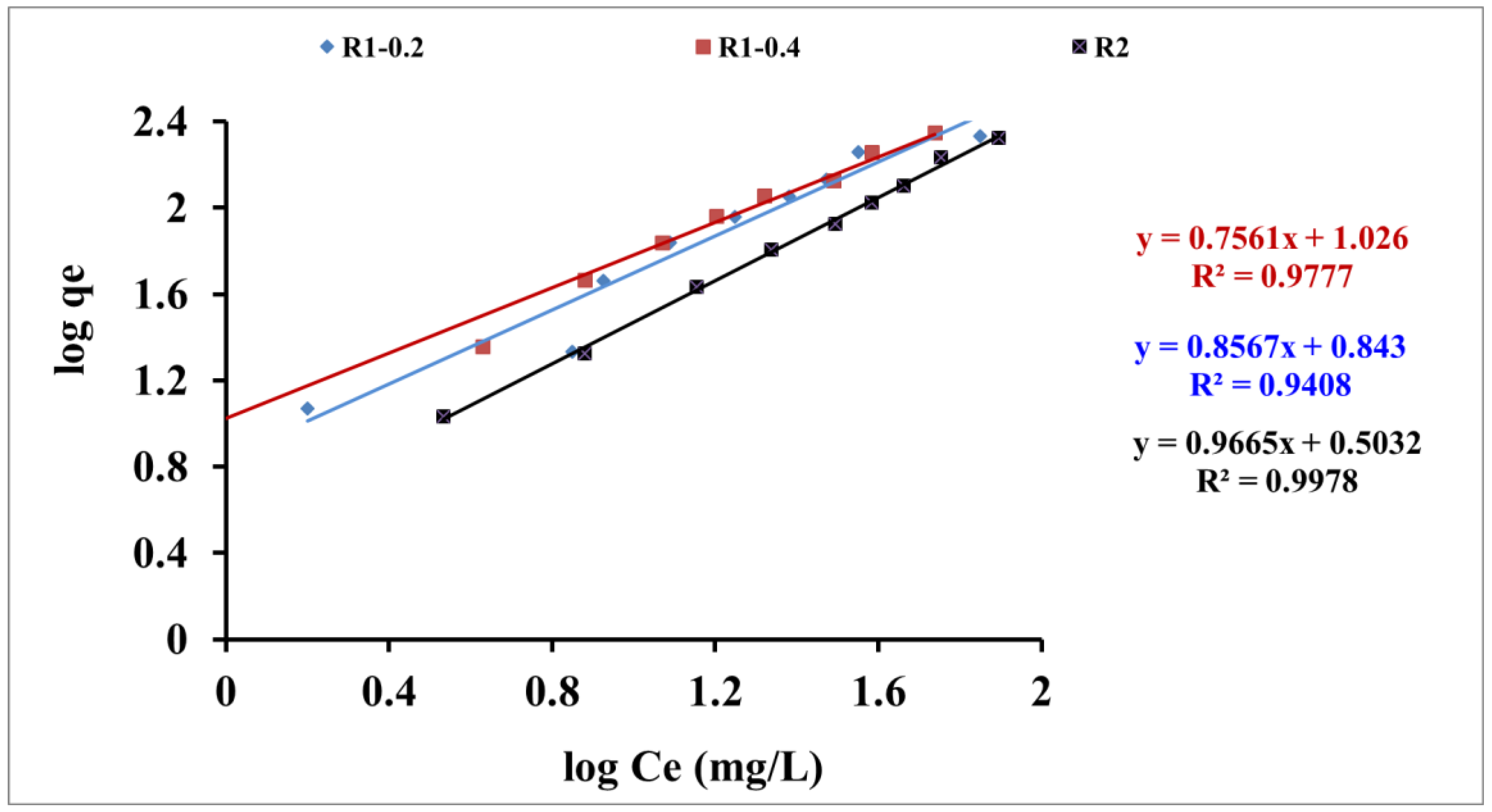
3.3. Isotherms Study
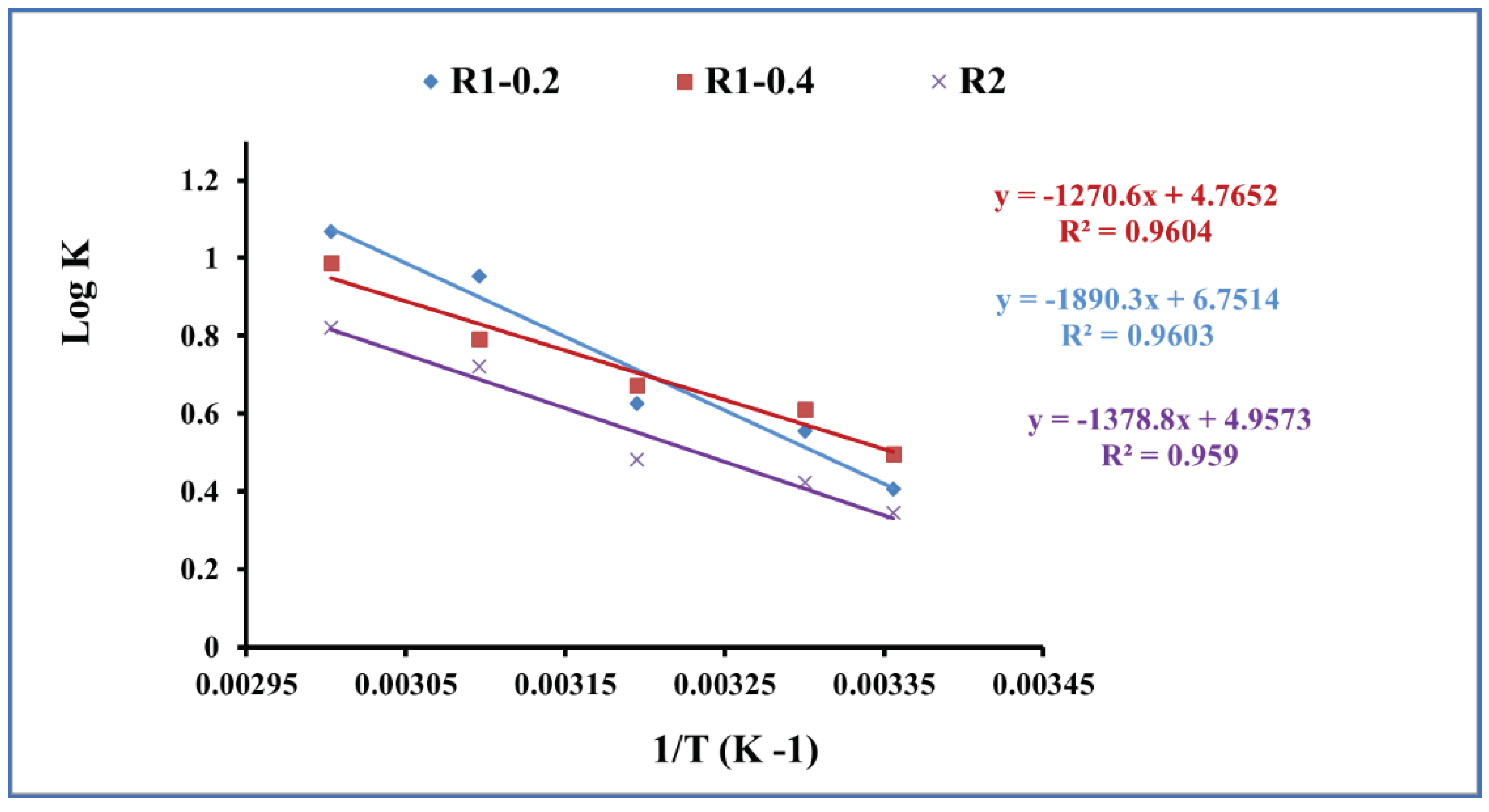
3.4. Thermodynamic Studies
4. Conclusions
Author Contributions
Acknowledgments
References
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water Treatment and Purification; Springer International Publishing, 2017; ISBN 9783319581361.
- Azmi, W.; Sani, R.K.; Banerjee, U.C. Biodegradation of Triphenylmethane Dyes;
- Pandey, N.; Shukla, S.K.; Singh, N.B. Water Purification by Polymer Nanocomposites: An Overview. Nanocomposites 2017, 3, 47–66. https://doi.org/10.1080/20550324.2017.1329983. [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. International Journal of Environmental Science and Technology 2019, 16, 1193–1226. [CrossRef]
- Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V. V; Ghaly, A. Ramakrishnan VV (2014) Production, Characterization and Treatment of Textile Effluents: A Critical Review. J Chem Eng Process Technol 2014, 5, 182. https://doi.org/10.4172/2157-7048.1000182. [CrossRef]
- Li, H. hong; Wang, Y. tao; Wang, Y.; Wang, H. xia; Sun, K. kai; Lu, Z. mei Bacterial Degradation of Anthraquinone Dyes. J Zhejiang Univ Sci B 2019, 20, 528–540. [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnology Research and Innovation 2019, 3, 275–290. https://doi.org/10.1016/j.biori.2019.09.001. [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini–Bandegharaei, A.; Dotto, G.L. Microwave Synthesis of Silica Nanoparticles and Its Application for Methylene Blue Adsorption. J Environ Chem Eng 2018, 6, 649–659. [CrossRef]
- Al-Wakeel, K.Z.; Abd El Monem, H.; Khalil, M.M.H. Removal of Divalent Manganese from Aqueous Solution Using Glycine Modified Chitosan Resin. J Environ Chem Eng 2015, 3, 179–186. [CrossRef]
- El-Moselhy, M.M.; Kamal, S.M. Selective Removal and Preconcentration of Methylene Blue from Polluted Water Using Cation Exchange Polymeric Material. Groundw Sustain Dev 2018, 6, 6–13. [CrossRef]
- Liu, X.; Chen, Z.-Q.; Han, B.; Su, C.-L.; Han, Q.; Chen, W.-Z. Biosorption of Copper Ions from Aqueous Solution Using Rape Straw Powders: Optimization, Equilibrium and Kinetic Studies. Ecotoxicol Environ Saf 2018, 150, 251–259. [CrossRef]
- Polat, H.; Erdogan, D. Heavy Metal Removal from Waste Waters by Ion Flotation. J Hazard Mater 2007, 148, 267–273. https://doi.org/10.1016/j.jhazmat.2007.02.013. [CrossRef]
- Akar, S.T.; Akar, T.; Çabuk, A. Decolorization of a Textile Dye, Reactive Red 198 (RR198), by Aspergillus Parasiticus Fungal Biosorbent. Brazilian Journal of Chemical Engineering 2009, 26, 399–405. https://doi.org/10.1590/S0104-66322009000200018. [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste Materials for Activated Carbon Preparation and Its Use in Aqueous-Phase Treatment: A Review. J Environ Manage 2007, 85, 833–846. [CrossRef]
- Hajeb, P.; Sloth, J.J.; Shakibazadeh, Sh.; Mahyudin, N.A.; Afsah-Hejri, L. Toxic Elements in Food: Occurrence, Binding, and Reduction Approaches. Compr Rev Food Sci Food Saf 2014, 13, 457–472. https://doi.org/10.1111/1541-4337.12068. [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J Mol Liq 2019, 290, 111197. https://doi.org/10.1016/j.molliq.2019.111197. [CrossRef]
- Qiao, W.; Zhang, P.; Sun, L.; Ma, S.; Xu, W.; Xu, S.; Niu, Y. Adsorption Performance and Mechanism of Schiff Base Functionalized Polyamidoamine Dendrimer/Silica for Aqueous Mn(II) and Co(II). Chinese Chemical Letters 2020, 31, 2742–2746. https://doi.org/10.1016/j.cclet.2020.04.036. [CrossRef]
- Zhan, H.; Bian, Y.; Yuan, Q.; Ren, B.; Hursthouse, A.; Zhu, G. Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4. Processes 2018, 6, 33. https://doi.org/10.3390/pr6040033. [CrossRef]
- López, Y.C.; Ortega, G.A.; Martínez, M.A.; Reguera, E. Magnetic Prussian Blue Derivative like Absorbent Cages for an Efficient Thallium Removal. J Clean Prod 2020, doi:10.1016/j.jclepro.2020.124587. [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9. [CrossRef]
- Yazdimamaghani, M.; Pourvala, T.; Motamedi, E.; Fathi, B.; Vashaee, D.; Tayebi, L. Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by in Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant. Materials 2013, 6, 3727–3741. https://doi.org/10.3390/ma6093727. [CrossRef]
- De Los Santos Valladares, L.; Bustamante Domínguez, A.; León Félix, L.; Kargin, J.B.; Mukhambetov, D.G.; Kozlovskiy, A.L.; Moreno, N.O.; Flores Santibañez, J.; Castellanos Cabrera, R.; Barnes, C.H.W. Characterization and Magnetic Properties of Hollow α-Fe2O3 Microspheres Obtained by Sol Gel and Spray Roasting Methods. Journal of Science: Advanced Materials and Devices 2019, 4, 483–491. https://doi.org/10.1016/j.jsamd.2019.07.004. [CrossRef]
- S., A.J.; T., R.; Yimam, A. Magnetic Hetero-Structures as Prospective Sorbents to Aid Arsenic Elimination from Life Water Streams. Water Science 2018, 32, 151–170. https://doi.org/10.1016/j.wsj.2017.05.001. [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A Critical Review on the Electrospun Nanofibrous Membranes for the Adsorption of Heavy Metals in Water Treatment. J Hazard Mater 2021, 401. [CrossRef]
- Habila, M.A.; Alothman, Z.A.; Mohamed El-Toni, A.; Labis, J.P.; Khan, A.; Al-Marghany, A.; Elafifi, H.E. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules 2017, 22. https://doi.org/10.3390/molecules22122074. [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; Alothman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, Synthesis and Applications of Core-Shell, Hollow Core, and Nanorattle Multifunctional Nanostructures. Nanoscale 2016, 8. https://doi.org/10.1039/c5nr07004j. [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Li, X.; Zhang, F.; Soylak, M. Mercaptobenzothiazole-Functionalized Magnetic Carbon Nanospheres of Type Fe3O4@SiO2@C for the Preconcentration of Nickel, Copper and Lead Prior to Their Determination by ICP-MS. Microchimica Acta 2016, 183, 2377–2384. https://doi.org/10.1007/s00604-016-1880-x. [CrossRef]
- Salamat, S.; Younesi, H.; Bahramifar, N. Synthesis of Magnetic Core–Shell Fe3O4@TiO2 Nanoparticles from Electric Arc Furnace Dust for Photocatalytic Degradation of Steel Mill Wastewater. RSC Adv 2017, 7, 19391–19405. https://doi.org/10.1039/C7RA01238A. [CrossRef]
- Shi, L.; Dong, B.; Gao, R.; Su, G.; Liu, W.; Xia, C.; Zhao, F.; Cao, L. Hierarchical Fe3O4@titanate Microspheres with Superior Removal Capability for Water Treatment: In Situ Growth and Structure Tailoring via Hydrothermal Assisted Etching. RSC Adv 2015, 5, 73126–73132. https://doi.org/10.1039/C5RA06362K. [CrossRef]
- Zheng, J.; Cheng, C.; Fang, W.J.; Chen, C.; Yan, R.W.; Huai, H.X.; Wang, C.C. Surfactant-Free Synthesis of a Fe3O4@ZIF-8 Core–Shell Heterostructure for Adsorption of Methylene Blue. CrystEngComm 2014, 16, 3960–3964. https://doi.org/10.1039/C3CE42648C. [CrossRef]
- Saini, J.; Garg, V.K.; Gupta, R.K. Removal of Methylene Blue from Aqueous Solution by Fe3O4@Ag/SiO2 Nanospheres: Synthesis, Characterization and Adsorption Performance. J Mol Liq 2018, 250, 413–422. https://doi.org/10.1016/j.molliq.2017.11.180. [CrossRef]
- Jaseela, P.K.; Garvasis, J.; Joseph, A. Selective Adsorption of Methylene Blue (MB) Dye from Aqueous Mixture of MB and Methyl Orange (MO) Using Mesoporous Titania (TiO2) – Poly Vinyl Alcohol (PVA) Nanocomposite. J Mol Liq 2019, 286, 110908. https://doi.org/10.1016/J.MOLLIQ.2019.110908. [CrossRef]
- Zhan, Y.; Zhao, S.; Wan, X.; He, S. Hierarchical Fe3O4-Derived Organic/Inorganic Hybrids Constructed by Modified Bio-Inspired Functionalization: Efficient Adsorbents for Water-Soluble Methylene Blue and Mechanism. Journal of Chemical Technology & Biotechnology 2019, 94, 1638–1650. https://doi.org/10.1002/JCTB.5933. [CrossRef]
- Schneider, M.; Ballweg, T.; Groß, L.; Gellermann, C.; Sanchez-Sanchez, A.; Fierro, V.; Celzard, A.; Mandel, K.; Schneider, M.; Ballweg, T.; et al. Magnetic Carbon Composite Particles for Dye Adsorption from Water and Their Electrochemical Regeneration. Particle & Particle Systems Characterization 2019, 36, 1800537. https://doi.org/10.1002/PPSC.201800537. [CrossRef]
- Akbarbandari, F.; Zabihi, M.; Faghihi, M. Synthesis of the Magnetic Core–Shell Bi-Metallic and Tri-Metallic Metal–Organic Framework Nanocomposites for Dye Adsorption. Water Environment Research 2021, 93, 906–920. https://doi.org/10.1002/WER.1481. [CrossRef]
- El-Toni, A.M.; Ibrahim, M.A.; Labis, J.P.; Khan, A.; Alhoshan, M. Optimization of Synthesis Parameters for Mesoporous Shell Formation on Magnetic Nanocores and Their Application as Nanocarriers for Docetaxel Cancer Drug. International Journal of Molecular Sciences 2013, Vol. 14, Pages 11496-11509 2013, 14, 11496–11509. https://doi.org/10.3390/IJMS140611496. [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe 3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J Am Chem Soc 2008, 130, 28–29. https://doi.org/10.1021/JA0777584/SUPPL_FILE/JA0777584-FILE002.PDF. [CrossRef]
- Li, W.; Yang, J.; Wu, Z.; Wang, J.; Li, B.; Feng, S.; Deng, Y.; Zhang, F.; Zhao, D. A Versatile Kinetics-Controlled Coating Method to Construct Uniform Porous TiO 2 Shells for Multifunctional Core-Shell Structures. J Am Chem Soc 2012, 134, 11864–11867. https://doi.org/10.1021/JA3037146/SUPPL_FILE/JA3037146_SI_001.PDF. [CrossRef]
- El-Ashtoukhy, E.S.Z.; Fouad, Y.O. Liquid-Liquid Extraction of Methylene Blue Dye from Aqueous Solutions Using Sodium Dodecylbenzenesulfonate as an Extractant. Alexandria Engineering Journal 2015, 54, 77–81. https://doi.org/10.1016/j.aej.2014.11.007. [CrossRef]
- Eslami, H.; Sedighi Khavidak, S.; Salehi, F.; Khosravi, R.; Fallahzadeh, R.A.; Peirovi, R.; Sadeghi, S. Biodegradation of Methylene Blue from Aqueous Solution by Bacteria Isolated from Contaminated Soil; Kurdistan University of Medical Sciences, 2016; Vol. 5;
- Rahman, R.; K2, N.M. DEGRADATION OF METHYLENE BLUE IN TEXTILE WASTE WATER USING ACTIVATED SAWDUST AND EGGSHELL BIOSORBENT; Vol. 2;
- Yan, Y.; Zhang, M.; Gong, K.; Su, L.; Guo, Z.; Mao, L. Adsorption of Methylene Blue Dye onto Carbon Nanotubes: A Route to an Electrochemically Functional Nanostructure and Its Layer-by-Layer Assembled Nanocomposite. Chemistry of Materials 2005, 17, 3457–3463. https://doi.org/10.1021/cm0504182. [CrossRef]
- Karthik, R.; Muthezhilan, R.; Jaffar Hussain, A.; Ramalingam, K.; Rekha, V. Effective Removal of Methylene Blue Dye from Water Using Three Different Low-Cost Adsorbents. Desalination Water Treat 2016, 57, 10626–10631. https://doi.org/10.1080/19443994.2015.1039598. [CrossRef]
- Vijayalaks, G.; Ramkumar, B.; Mohan, S.C. Isotherm and Kinetic Studies of Methylene Blue Adsorption Using Activated Carbon Prepared from Teak Wood Waste Biomass. Journal of Applied Sciences 2019, 19, 827–836. https://doi.org/10.3923/jas.2019.827.836. [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of Activated Carbon as Adsorbents for Efficient Removal of Methylene Blue: Kinetics and Equilibrium Study. Journal of Industrial and Engineering Chemistry 2014, 20, 2317–2324. https://doi.org/10.1016/j.jiec.2013.10.007. [CrossRef]
- AlOthman, Z.A.; Habila, M.A.; Ali, R.; Abdel Ghafar, A.; El-din Hassouna, M.S. Valorization of Two Waste Streams into Activated Carbon and Studying Its Adsorption Kinetics, Equilibrium Isotherms and Thermodynamics for Methylene Blue Removal. Arabian Journal of Chemistry 2014, 7. https://doi.org/10.1016/j.arabjc.2013.05.007. [CrossRef]
- Al-Othman, Z.A.; Habila, M.A.; Ali, R.; Hassouna, M.S.E.-D. Kinetic and Thermodynamic Studies for Methylene Blue Adsorption Using Activated Carbon Prepared from Agricultural and Municipal Solid Wastes. Asian Journal of Chemistry 2013, 25. https://doi.org/10.14233/ajchem.2013.14723. [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Ibrahim, M.A.; Labis, J.P.; ALOthman, Z.A. Simple and Facile Synthesis of Amino Functionalized Hollow Core-Mesoporous Shell Silica Spheres Using Anionic Surfactant for Pb(II), Cd(II), and Zn(II) Adsorption and Recovery. Chemical Engineering Journal 2014, 251, 441–451. https://doi.org/10.1016/j.cej.2014.04.072. [CrossRef]
- Habila, M.; Sheikh Moshab, M.; Mohamed El-Toni, A.; S. Al-Awadi, A.; A. ALOthman, Z. Facile Strategy for Fabricating an Organosilica-Modified Fe3O4 (OS/Fe3O4) Hetero-Nanocore and OS/Fe3O4@SiO2 Core–Shell Structure for Wastewater Treatment with Promising Recyclable Efficiency. ACS Omega 2023, 8, 7626–7638. https://doi.org/10.1021/acsomega.2c07214. [CrossRef]
- Mondal, K.; Lalvani, S.B. Modeling of Mass Transfer Controlled Adsorption Rate Based on the Langmuir Adsorption Isotherm. Sep Sci Technol 2000. https://doi.org/10.1081/SS-100102357. [CrossRef]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis Tef) Agricultural Waste. Journal of Thermodynamics 2013. https://doi.org/10.1155/2013/375830. [CrossRef]
- Moganavally, P.; Deepa, M.; Sudha, P.N.; Suresh, R. Adsorptive Removal of Lead and Cadmium Ions Using Cross-Linked CMC Schiff Base: Isotherm, Kinetics and Catalytic Activity. Oriental Journal of Chemistry 2016, 32, 441–453. https://doi.org/10.13005/ojc/320150. [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J Chem 2017, 2017, 1–11. https://doi.org/10.1155/2017/3039817. [CrossRef]
- Langmuir, I. THE CONSTITUTION AND FUNDAMENTAL PROPERTIES OF SOLIDS AND LIQUIDS. PART I. SOLIDS. J Am Chem Soc 1916, 38, 2221–2295. https://doi.org/10.1021/ja02268a002. [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. Journal of Physical Chemistry A 1906, 57, 385–470.
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Badjah, A.Y. Kinetics, Thermodynamics, and Modeling of Amido Black Dye Photodegradation in Water Using Co/TiO2 Nanoparticles. Photochem Photobiol 2018, 94, 935–941. https://doi.org/10.1111/PHP.12937. [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Badjah, A.Y. Kinetics, Thermodynamics, and Modeling of Amido Black Dye Photodegradation in Water Using Co/TiO2 Nanoparticles. Photochem Photobiol 2018, 94, 935–941. https://doi.org/10.1111/PHP.12937. [CrossRef]
- Ghate, E.; Ganjidoust, H.; Ayati, B. The Thermodynamics, Kinetics, and Isotherms of Sulfamethoxazole Adsorption Using Magnetic Activated Carbon Nanocomposite and Its Reusability Potential. Nanotechnology for Environmental Engineering 2021, 6. https://doi.org/10.1007/S41204-021-00127-Y. [CrossRef]
- Othman, Z.A.A.; Hashem, A.; Habila, M.A. Kinetic, Equilibrium and Thermodynamic Studies of Cadmium (II) Adsorption by Modified Agricultural Wastes. Molecules 2011, 16. https://doi.org/10.3390/molecules161210443. [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Ali, R.; Ghafar, A.A.; Hassouna, M.S.E.-D. Removal of Tartrazine Dye onto Mixed-Waste Activated Carbon: Kinetic and Thermodynamic Studies. Clean (Weinh) 2014, 42, 1824–1831. https://doi.org/10.1002/clen.201300191. [CrossRef]
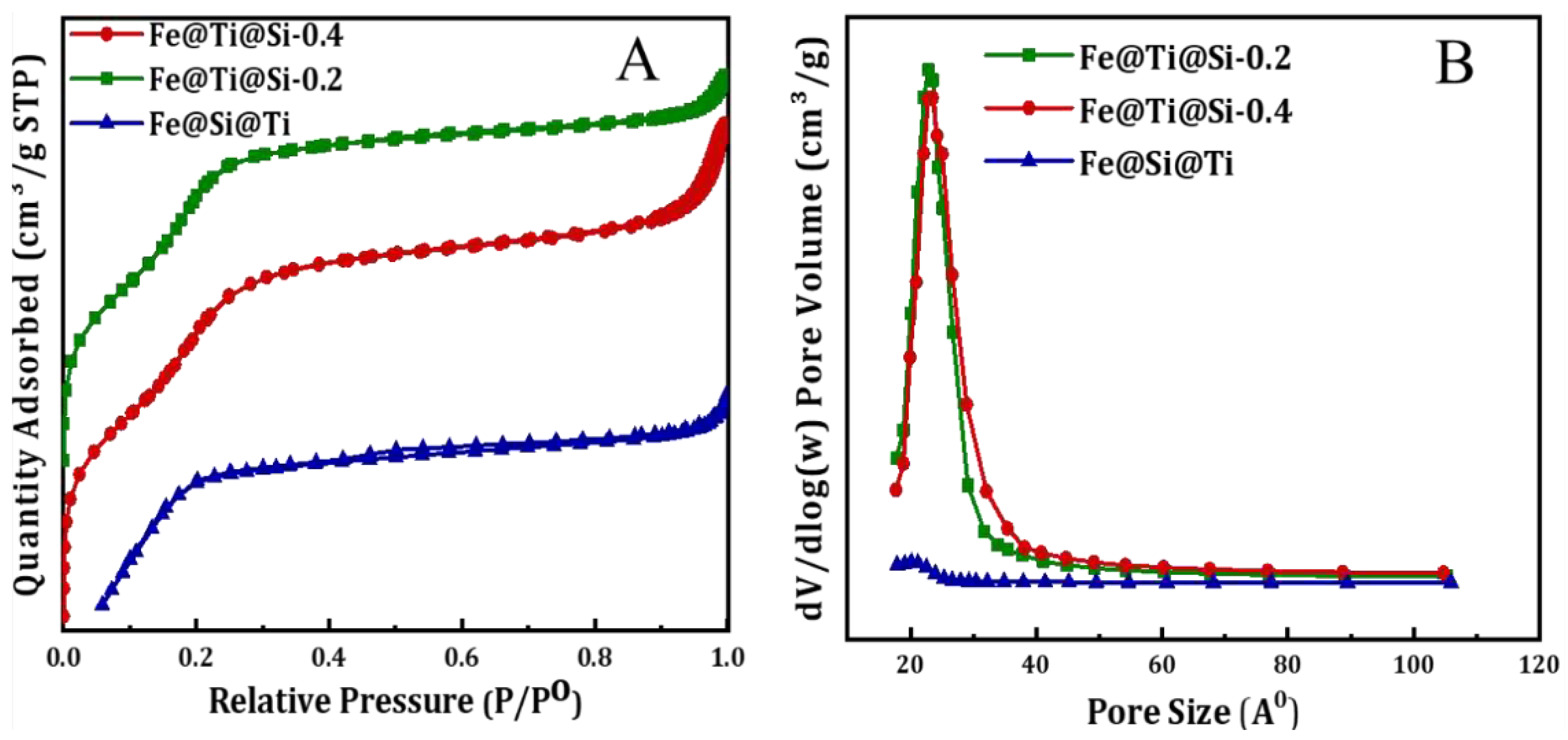
| Sample | BET S. A. m2/g | Pore volume cm3/g | Pore size Ao |
|---|---|---|---|
| Fe@Ti@m-Si-0.2(R1-0.2) | 1133.28 | 0.74 | 25.79 |
| Fe@Ti@m-Si-0.4(R1-0.4) | 1207.49 | 0.88 | 29.86 |
| Fe@ m-Si@Ti (R2) | 52.27 | 0.03 | 24.02 |
| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| qe,exp (mg/g) | K1(min−1) | qe,cal(mg/g) | R2 | k2(g/mg.min) | qe,cal(mg/g) | R2 | |
| R1 -0.2 | 128 | 0.031 | 176.27 | 0.91 | 1.71*10-4 | 222.22 | 0.98 |
| R1-0.4 | 118 | 0.03 | 130.16 | 0.93 | 4.16*10-4 | 156.25 | 0.99 |
| R2 | 133 | 0.032 | 116.35 | 0.92 | 4.12*10-4 | 172.41 | 0.99 |
| Langmuir Constants | Freundlich Constants | ||||||
|---|---|---|---|---|---|---|---|
| KL | b | Qmax. | R2 | KF | n | R2 | |
| R1 -0.2 | 5.54 | 9.4*10-3 | 588.2 | 0.28 | 6.96 | 1.16 | 0.94 |
| R1-0.4 | 7.34 | 0.016 | 454.5 | 0.65 | 10.61 | 1.32 | 0.97 |
| R2 | 2.97 | 1.19*10-3 | 2500 | 0.25 | 3.18 | 1.03 | 0.99 |
| Temperature T(K) | Thermodynamic Parameters | |||
|---|---|---|---|---|
| ΔG° (kJ/mol) | ΔS° (J/mol/K) | ΔH° (kJ/mol) | ||
| R1-0.2 | 273 | -2.3 | 129.3 | 36.19 |
| 278 | -3.2 | |||
| 288 | -3.7 | |||
| 298 | -5.9 | |||
| 308 | -6.8 | |||
| R1-0.4 | 273 | -2.8 | 91.2 | 24.33 |
| 278 | -3.5 | |||
| 288 | -4.0 | |||
| 298 | -4.9 | |||
| 308 | -6.3 | |||
| R2 | 273 | -2.0 | 94.9 | 26.40 |
| 278 | -2.5 | |||
| 288 | -2.9 | |||
| 298 | -4.5 | |||
| 308 | -5.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





