Submitted:
08 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
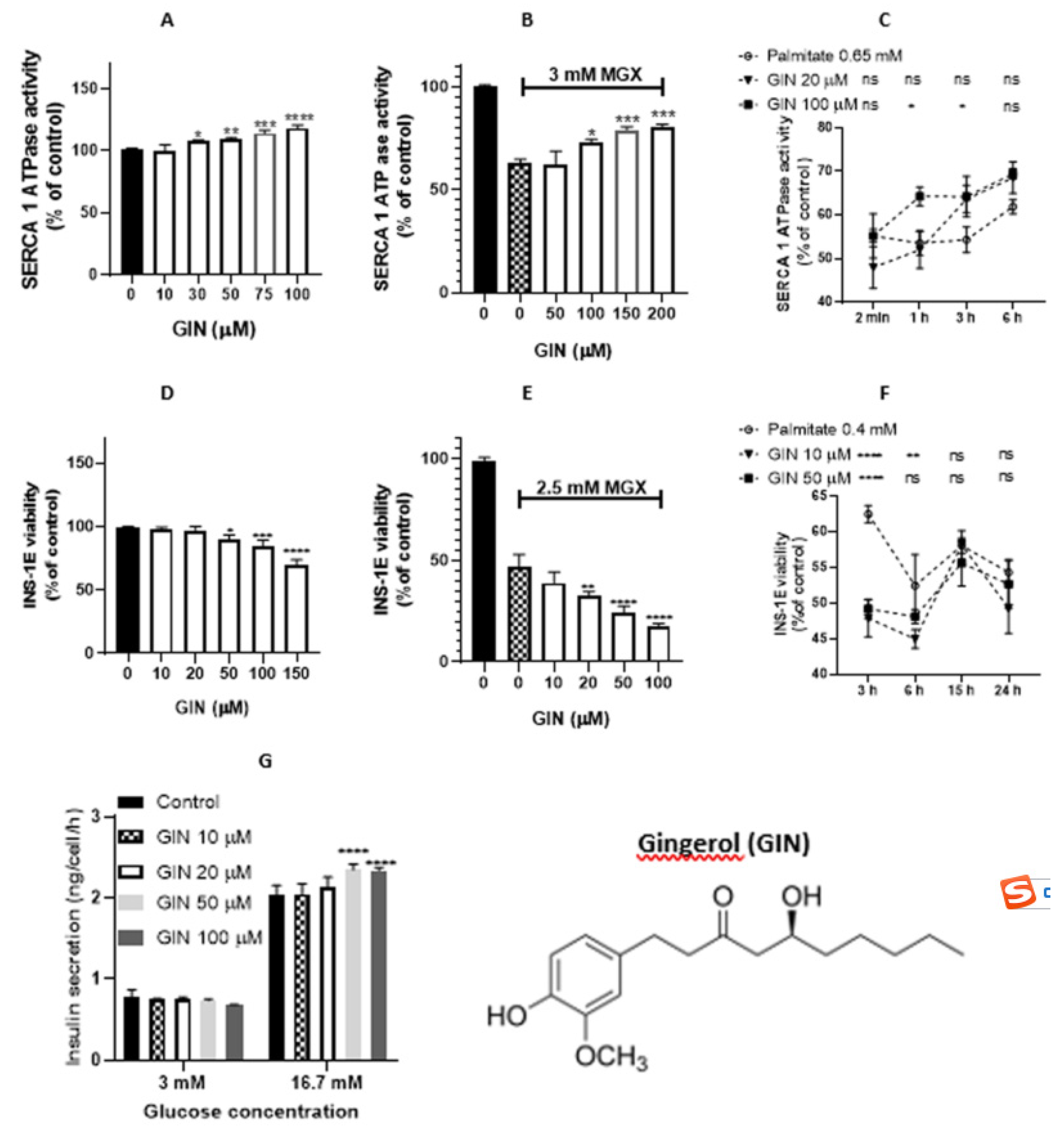
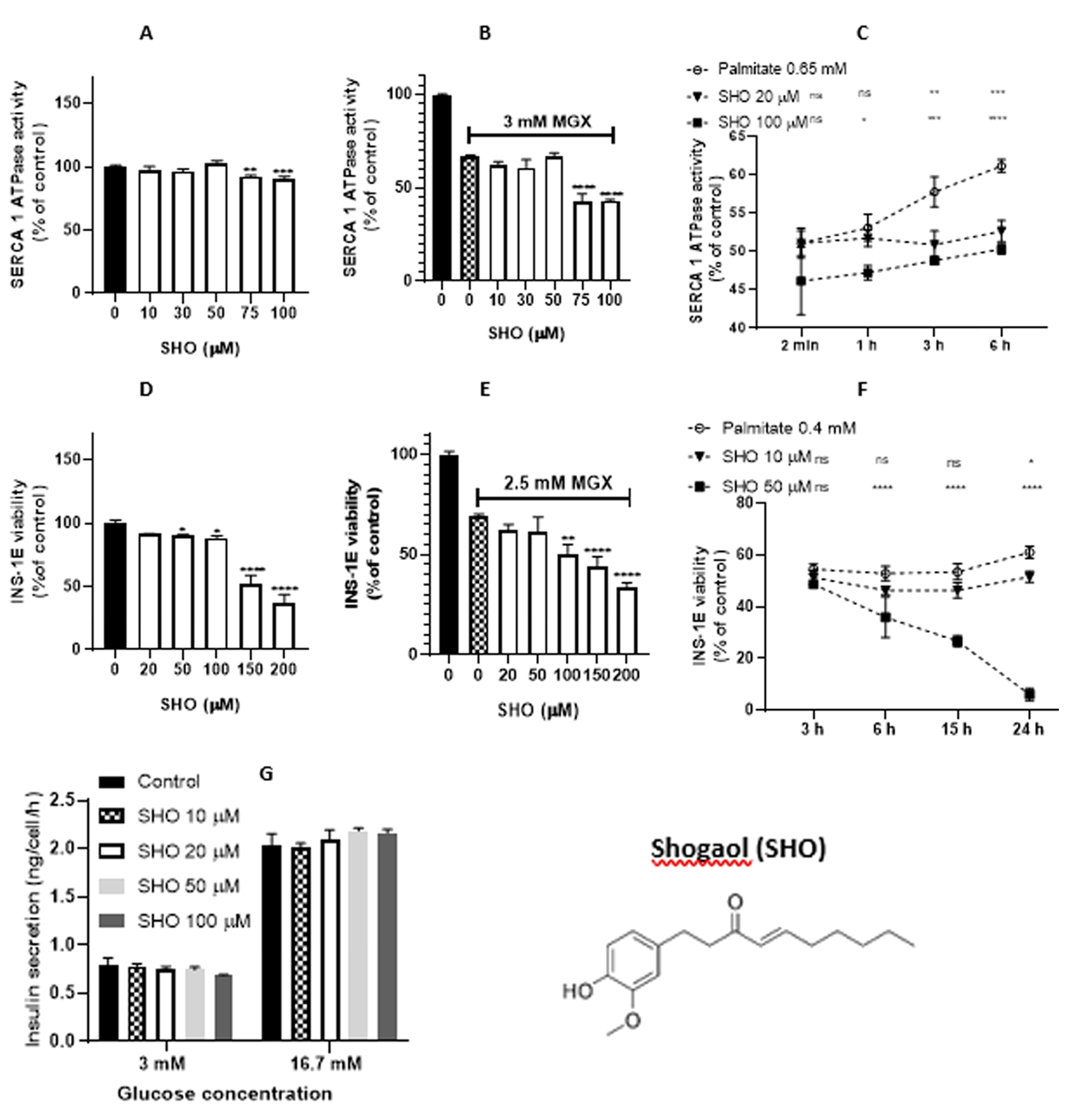
2.1. [6]-Gingerol and [6]-shogaol
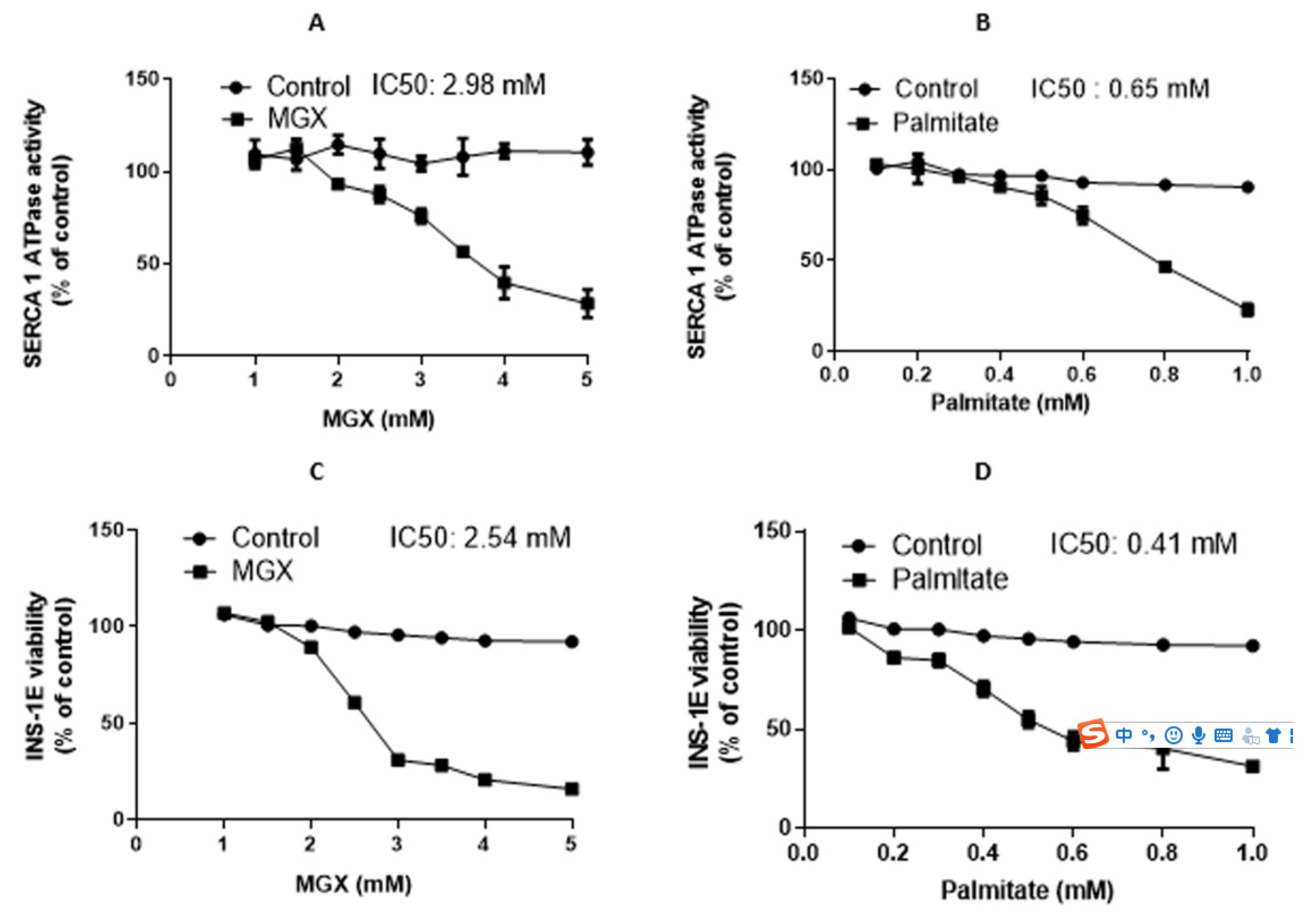
2.1.1. [6]-Gingerol and [6]-shogaol in the noncellular system
2.1.2. [6]-Gingerol and [6]-shogaol in the cellular system
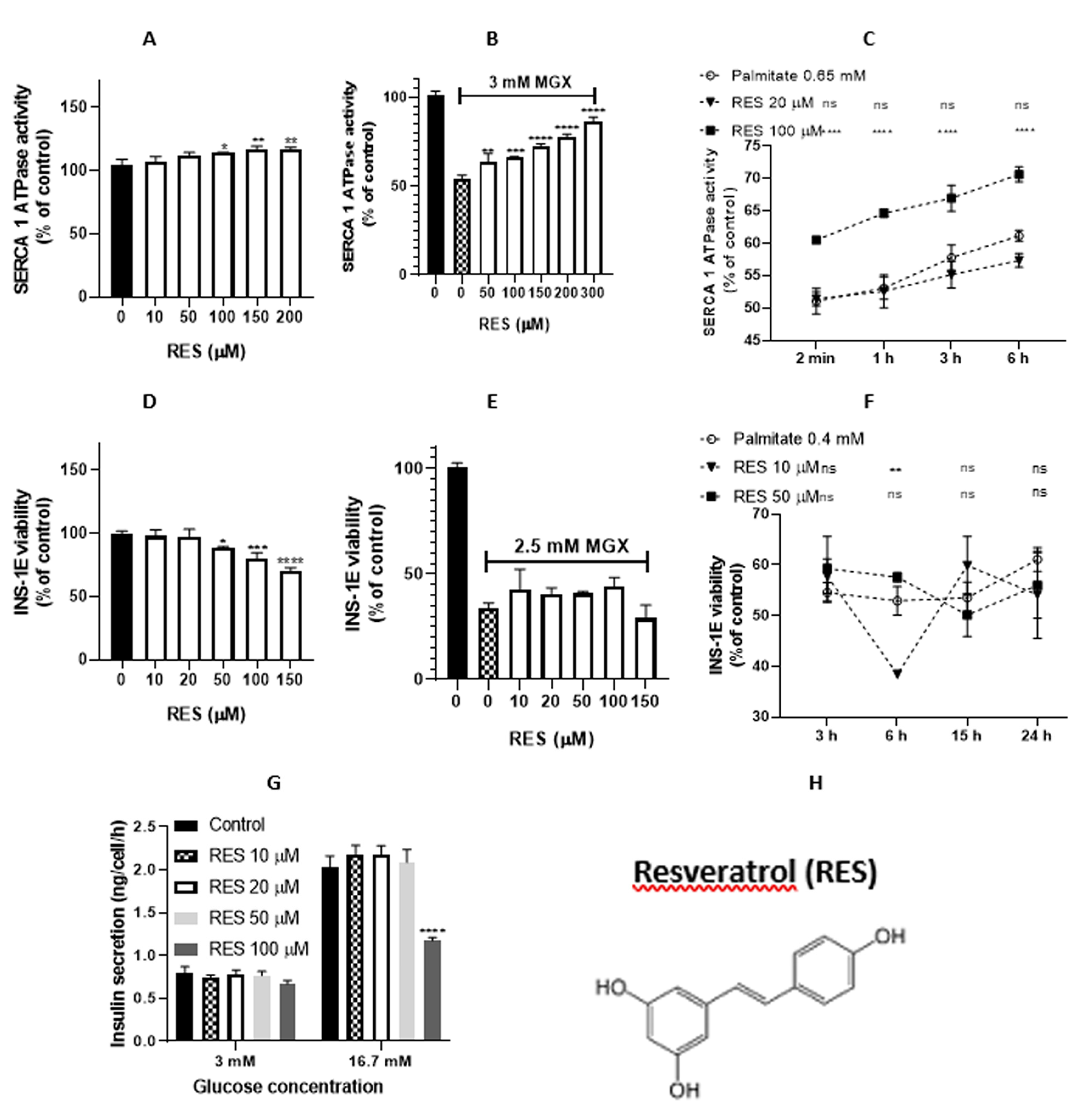
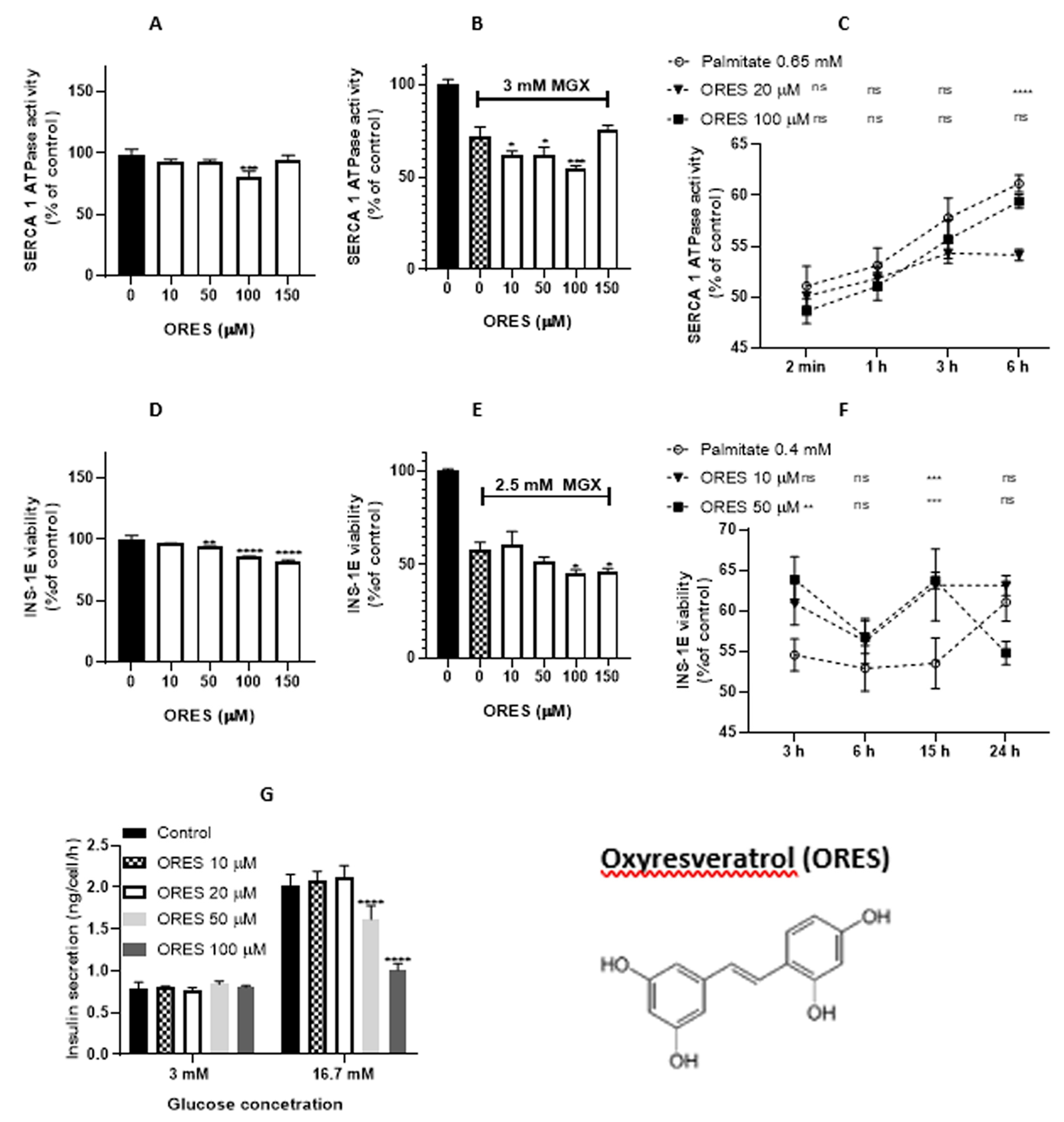
2.2. Resveratrol and oxyresveratrol
2.2.1. Resveratrol and oxyresveratrol in the noncellular system
2.2.2. Resveratrol and oxyresveratrol in the cellular system
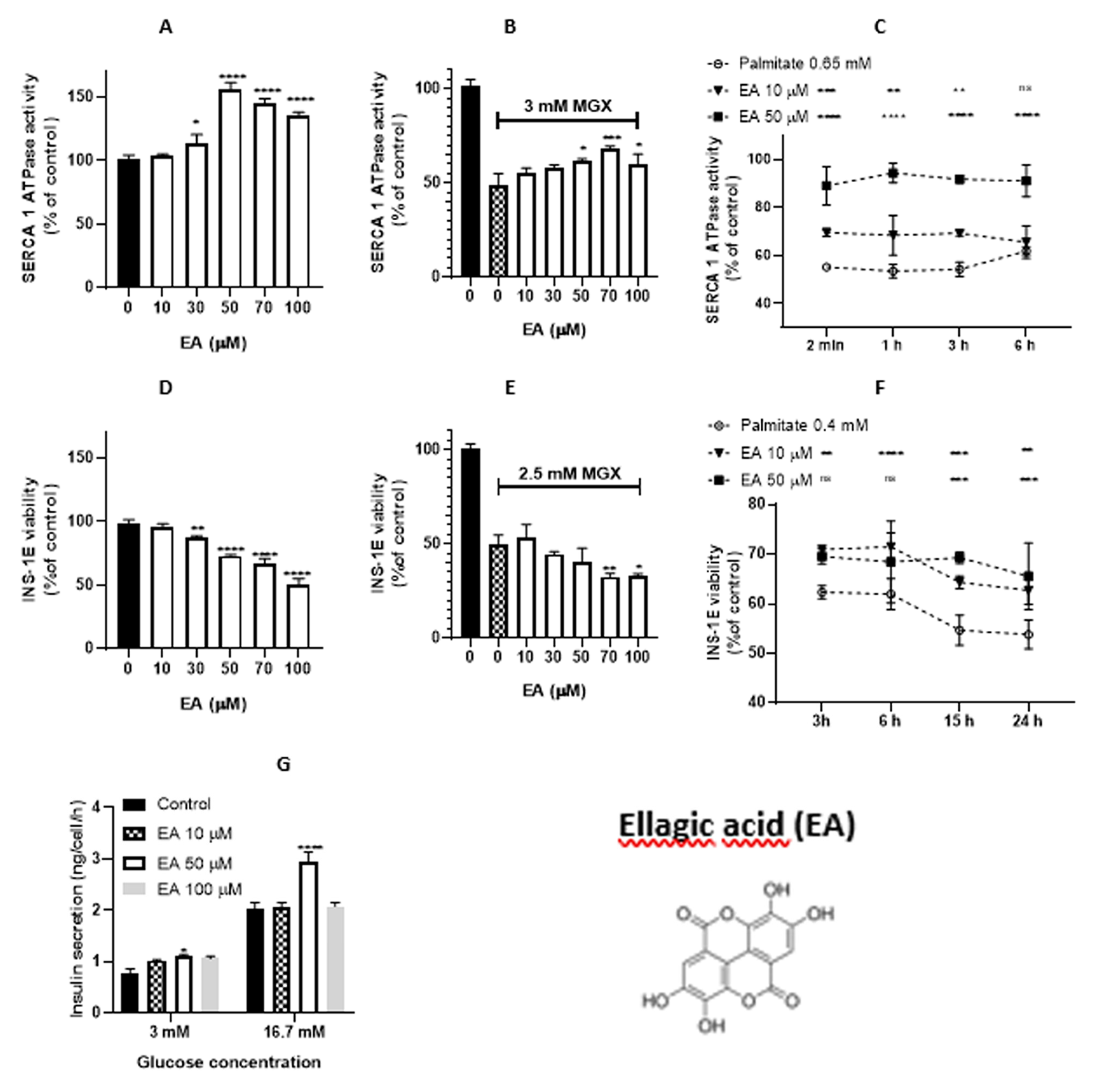
2.3. Ellagic acid
2.3.1. Ellagic acid in the noncellular system
2.3.2. Ellagic acid in the cellular system
2.4. In silico study
3. Discussion
3.2. Resveratrol (RES) and oxyresveratrol (ORES)
3.4. Ellagic acid (EA)
4. Materials and methods
4.1. Compounds evaluated
4.2. SERCA1 activity measurement
4.2.1. NADH-coupled enzyme assay
4.3. Cell culture
4.4. Cytotoxicity assay (MTT)
4.5. Insulin release
4.6. In silico study
4.7. Statistical analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol 2018, 28, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA Control of Cell Death and Survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Urano, F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr Diab Rep 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Remmen, H. Van The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) Pump: A Potential Target for Intervention in Aging and Skeletal Muscle Pathologies. Skelet Muscle 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.X.; Raghavan, M.; Satin, L.S. The Endoplasmic Reticulum and Calcium Homeostasis in Pancreatic Beta Cells. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Ogunbayo, O.A.; Harris, R.M.; Waring, R.H.; Kirk, C.J.; Michelangeli, F. Inhibition of the Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase by Flavonoids: A Quantitative Structure-Activity Relationship Study. IUBMB Life 2008, 60, 853–858. [Google Scholar] [CrossRef]
- Kranias, E.G.; Hajjar, R.J. Modulation of Cardiac Contractility by the Phopholamban/SERCA2a Regulatome. Circ Res 2012, 110, 1646–1660. [Google Scholar] [CrossRef]
- Bhupathy, P.; Babu, G.J.; Periasamy, M. Sarcolipin and Phospholamban as Regulators of Cardiac Sarcoplasmic Reticulum Ca2+ ATPase. J Mol Cell Cardiol 2007, 42, 903–911. [Google Scholar] [CrossRef]
- Anderson, D.M.; Anderson, K.M.; Chang, C.-L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A Micropeptide Encoded by a Putative Long Noncoding RNA Regulates Muscle Performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef]
- Kho, C.; Lee, A.; Jeong, D.; Oh, J.G.; Chaanine, A.H.; Kizana, E.; Park, W.J.; Hajjar, R.J. SUMO1-Dependent Modulation of SERCA2a in Heart Failure. Nature 2011, 477, 601–605. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R. Diabetes Increases Formation of Advanced Glycation End Products on Sarco(Endo)Plasmic Reticulum Ca2+-ATPase. Diabetes 2004, 53, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, J.; Weinstein, L.; Poksay, K.S.; Nishinaga, B.; Bredesen, D.E.; Rao, R. V Coupling Endoplasmic Reticulum Stress to the Cell Death Program in Mouse Melanoma Cells: Effect of Curcumin. Apoptosis 2008, 13, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.D.; McIntosh, D.B.; Woolley, D.G.; Andersen, J.P. Modulatory ATP Binding Affinity in Intermediate States of E2P Dephosphorylation of Sarcoplasmic Reticulum Ca2+-ATPase. Journal of Biological Chemistry 2011, 286, 11792–11802. [Google Scholar] [CrossRef]
- Clausen, J.D.; Andersen, J.P. Glutamate 90 at the Luminal Ion Gate of Sarcoplasmic Reticulum Ca2+-ATPase Is Critical for Ca2+ Binding on Both Sides of the Membrane. Journal of Biological Chemistry 2010, 285, 20780–20792. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, U.; Elumalai, S.; Moon, J.S.; Jeon, J.H.; Kim, N.D.; Park, K.G.; Won, K.C.; Leem, J.; Lee, I.K. Myricetin Protects against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5. Diabetes Metab J 2019, 43, 192–205. [Google Scholar] [CrossRef]
- Kono, T.; Ahn, G.; Moss, D.R.; Gann, L.; Zarain-Herzberg, A.; Nishiki, Y.; Fueger, P.T.; Ogihara, T.; Evans-Molina, C. PPAR-γ Activation Restores Pancreatic Islet SERCA2 Levels and Prevents β-Cell Dysfunction under Conditions of Hyperglycemic and Cytokine Stress. Molecular Endocrinology 2012, 26, 257–271. [Google Scholar] [CrossRef]
- Yamamoto, W.R.; Bone, R.N.; Sohn, P.; Syed, F.; Reissaus, C.A.; Mosley, A.L.; Wijeratne, A.B.; True, J.D.; Tong, X.; Kono, T.; et al. Endoplasmic Reticulum Stress Alters Ryanodine Receptor Function in the Murine Pancreatic β Cell. Journal of Biological Chemistry 2019, 294, 168–181. [Google Scholar] [CrossRef]
- Lockridge, A.; Jo, S.; Gustafson, E.; Damberg, N.; Mohan, R.; Olson, M.; Abrahante, J.E.; Alejandro, E.U. Islet O-GlcNAcylation Is Required for Lipid Potentiation of Insulin Secretion through SERCA2. Cell Rep 2020, 31, 107609. [Google Scholar] [CrossRef]
- Ahmad, F.; Shen, W.; Vandeput, F.; Szabo-Fresnais, N.; Krall, J.; Degerman, E.; Goetz, F.; Klussmann, E.; Movsesian, M.; Manganiello, V. Regulation of Sarcoplasmic Reticulum Ca2+ ATPase 2 (SERCA2) Activity by Phosphodiesterase 3A (PDE3A) in Human Myocardium. Journal of Biological Chemistry 2015, 290, 6763–6776. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Pitt, S.J.; Smith, T.K.; Ajjan, R.A.; Stewart, A.J. Lipidomic Profiling of Plasma Free Fatty Acids in Type-1 Diabetes Highlights Specific Changes in Lipid Metabolism. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2021, 1866, 158823. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Blindauer, C.A.; Stewart, A.J. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Bagherieh, M.; Kheirollahi, A.; Zamani-Garmsiri, F.; Emamgholipour, S.; Meshkani, R. Folic Acid Ameliorates Palmitate-Induced Inflammation through Decreasing Homocysteine and Inhibiting NF-ΚB Pathway in HepG2 Cells. Arch Physiol Biochem 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Villanueva, A.H.; Oun, A.; Buist-Homan, M.; Blokzijl, H.; Faber, K.N.; Dolga, A.; Moshage, H. Protective Effect of Metformin against Palmitate-Induced Hepatic Cell Death. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2020, 1866, 165621. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Regulating JNK/NF-KB Pathway and ROS Production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.-K.; Ha, C.-M.; Thoudam, T.; Cha, S.-K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.-K.; Park, K.-S. Oxidative Stress and Calcium Dysregulation by Palmitate in Type 2 Diabetes. Exp Mol Med 2017, 49, e291–e291. [Google Scholar] [CrossRef]
- Ciregia, F.; Bugliani, M.; Ronci, M.; Giusti, L.; Boldrini, C.; Mazzoni, M.R.; Mossuto, S.; Grano, F.; Cnop, M.; Marselli, L.; et al. Palmitate-Induced Lipotoxicity Alters Acetylation of Multiple Proteins in Clonal β Cells and Human Pancreatic Islets. Sci Rep 2017, 7, 13445. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol Rev 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, Obesity, and Diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef]
- Desai, K.M.; Wu, L. FREE RADICAL GENERATION BY METHYLGLYOXAL IN TISSUES. Drug Metabol Drug Interact 2008, 23. [Google Scholar] [CrossRef]
- Dhar, A.; Desai, K.; Kazachmov, M.; Yu, P.; Wu, L. Methylglyoxal Production in Vascular Smooth Muscle Cells from Different Metabolic Precursors. Metabolism 2008, 57, 1211–1220. [Google Scholar] [CrossRef]
- Chang, T.; Wu, L. Methylglyoxal, Oxidative Stress, and Hypertension. Can J Physiol Pharmacol 2006, 84, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Zizkova, P.; Viskupicova, J.; Heger, V.; Rackova, L.; Majekova, M.; Horakova, L. Dysfunction of SERCA Pumps as Novel Mechanism of Methylglyoxal Cytotoxicity. Cell Calcium 2018, 74. [Google Scholar] [CrossRef] [PubMed]
- Namekata, I.; Hamaguchi, S.; Wakasugi, Y.; Ohhara, M.; Hirota, Y.; Tanaka, H. Ellagic Acid and Gingerol, Activators of the Sarco-Endoplasmic Reticulum Ca2+-ATPase, Ameliorate Diabetes Mellitus-Induced Diastolic Dysfunction in Isolated Murine Ventricular Myocardia. Eur J Pharmacol 2013, 706, 48–55. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and Shogaols: Important Nutraceutical Principles from Ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.K.; Ryoo, Z.Y.; Ha, J.J.; Oh, D.Y.; Kim, M.O.; Kim, S.H. Beneficial Effects of 6-Shogaol on Hyperglycemia, Islet Morphology and Apoptosis in Some Tissues of Streptozotocin-Induced Diabetic Mice. Diabetol Metab Syndr 2019, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.H.; Gonda, T.; Hunyadi, A. Medicinal Chemistry Inspired by Ginger: Exploring the Chemical Space around 6-Gingerol. RSC Adv 2021, 11, 26687–26699. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J Ethnopharmacol 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Sikdar, S.; Paul, A.; Ghosh, S.; Khuda-Bukhsh, A.R. [6]-Gingerol Isolated from Ginger Attenuates Sodium Arsenite Induced Oxidative Stress and Plays a Corrective Role in Improving Insulin Signaling in Mice. Toxicol Lett 2012, 210, 34–43. [Google Scholar] [CrossRef]
- Annamalai, G.; Kathiresan, S.; Kannappan, N. [6]-Shogaol, a Dietary Phenolic Compound, Induces Oxidative Stress Mediated Mitochondrial Dependant Apoptosis through Activation of Proapoptotic Factors in Hep-2 Cells. Biomedicine & Pharmacotherapy 2016, 82, 226–236. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Sang, S. Bioactive Ginger Constituents Alleviate Protein Glycation by Trapping Methylglyoxal. Chem Res Toxicol 2015, 28, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bose, D.D.; Thomas, D.W. Paradoxical Effects of Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Activator Gingerol on NG115-401L Neuronal Cells: Failure to Augment ER Ca2+ Uptake and Protect against ER Stress-Induced Cell Death. Eur J Pharmacol 2015, 762, 165–173. [Google Scholar] [CrossRef]
- Isa, Y.; Miyakawa, Y.; Yanagisawa, M.; Goto, T.; Kang, M.-S.; Kawada, T.; Morimitsu, Y.; Kubota, K.; Tsuda, T. 6-Shogaol and 6-Gingerol, the Pungent of Ginger, Inhibit TNF-α Mediated Downregulation of Adiponectin Expression via Different Mechanisms in 3T3-L1 Adipocytes. Biochem Biophys Res Commun 2008, 373, 429–434. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Liu, I.-M. 6-Gingerol Prevents Adipogenesis and the Accumulation of Cytoplasmic Lipid Droplets in 3T3-L1 Cells. Phytomedicine 2013, 20, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Chemical and Pharmacological Basis of Ginger (Zingiber Officinale Roscoe) as Potential Therapy for Diabetes and Metabolic Syndrome. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; 2019; pp. 639–687. [Google Scholar]
- Pavlović, N.; Đanić, M.; Stanimirov, B.; Goločorbin-Kon, S.; Stankov, K.; Lalić-Popović, M.; Mikov, M. In Silico Discovery of Resveratrol Analogues as Potential Agents in Treatment of Metabolic Disorders. Curr Pharm Des 2019, 25, 3776–3783. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of Resveratrol to Inhibit Advanced Glycation End Product Formation and Carbohydrate-Hydrolyzing Enzyme Activity, and to Conjugate Methylglyoxal. Food Chem 2017, 216, 153–160. [Google Scholar] [CrossRef]
- Wang, W.; Yang, R.; Yao, H.; Wu, Y.; Pan, W.; Jia, A.-Q. Inhibiting the Formation of Advanced Glycation End-Products by Three Stilbenes and the Identification of Their Adducts. Food Chem 2019, 295, 10–15. [Google Scholar] [CrossRef]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Horn, T.F.W. Oxyresveratrol and Resveratrol Are Potent Antioxidants and Free Radical Scavengers: Effect on Nitrosative and Oxidative Stress Derived from Microglial Cells. Nitric Oxide 2003, 9, 64–76. [Google Scholar] [CrossRef]
- Sonnett, T.E.; Levien, T.L.; Gates, B.J.; Robinson, J.D.; Campbell, R.K. Diabetes Mellitus, Inflammation, Obesity: Proposed Treatment Pathways for Current and Future Therapies. Annals of Pharmacotherapy 2010, 44, 701–711. [Google Scholar] [CrossRef]
- Pereira, L.; Matthes, J.; Schuster, I.; Valdivia, H.H.; Herzig, S.; Richard, S.; Gómez, A.M. Mechanisms of [Ca2+]i Transient Decrease in Cardiomyopathy of Db / Db Type 2 Diabetic Mice. Diabetes 2006, 55, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ahmed, S.; Grupp, I.L.; Matlib, M.A. Altered SR Protein Expression Associated with Contractile Dysfunction in Diabetic Rat Hearts. American Journal of Physiology-Heart and Circulatory Physiology 2001, 281, H1137–H1147. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Zhong, Y.; Hoit, B.D.; Grupp, I.L.; Hahn, H.; Dilly, K.W.; Guatimosim, S.; Lederer, W.J.; Matlib, M.A. Defective Intracellular Ca 2+ Signaling Contributes to Cardiomyopathy in Type 1 Diabetic Rats. American Journal of Physiology-Heart and Circulatory Physiology 2002, 283, H1398–H1408. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M.; Gupta, M. Resveratrol, an Activator of SIRT1, Upregulates Sarcoplasmic Calcium ATPase and Improves Cardiac Function in Diabetic Cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology 2010, 298, H833–H843. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kono, T.; Evans-Molina, C. The Role of Peroxisome Proliferator-Activated Receptor γ in Pancreatic β Cell Function and Survival: Therapeutic Implications for the Treatment of Type 2 Diabetes Mellitus. Diabetes Obes Metab 2010, 12, 1036–1047. [Google Scholar] [CrossRef]
- Dubois, M.; Pattou, F.; Kerr-Conte, J.; Gmyr, V.; Vandewalle, B.; Desreumaux, P.; Auwerx, J.; Schoonjans, K.; Lefebvre, J. Expression of Peroxisome Proliferator-Activated Receptor γ (PPARγ) in Normal Human Pancreatic Islet Cells. Diabetologia 2000, 43, 1165–1169. [Google Scholar] [CrossRef]
- Rosen, E.D.; Kulkarni, R.N.; Sarraf, P.; Ozcan, U.; Okada, T.; Hsu, C.-H.; Eisenman, D.; Magnuson, M.A.; Gonzalez, F.J.; Kahn, C.R.; et al. Targeted Elimination of Peroxisome Proliferator-Activated Receptor γ in β Cells Leads to Abnormalities in Islet Mass without Compromising Glucose Homeostasis. Mol Cell Biol 2003, 23, 7222–7229. [Google Scholar] [CrossRef]
- Zarain-Herzberg, A.; Alvarez-Fernandez, G. Sarco(Endo)Plasmic Reticulum Ca 2+ -ATPase-2 Gene: Structure and Transcriptional Regulation of the Human Gene. The Scientific World JOURNAL 2002, 2, 1469–1483. [Google Scholar] [CrossRef]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and Curcumin Enhance Pancreatic β-Cell Function by Inhibiting Phosphodiesterase Activity. Journal of Endocrinology 2014, 223, 107–117. [Google Scholar] [CrossRef]
- Mohammed, E.T.; Hashem, K.S.; Abdelazem, A.Z.; Foda, F.A.M.A. Prospective Protective Effect of Ellagic Acid as a SIRT1 Activator in Iron Oxide Nanoparticle-Induced Renal Damage in Rats. Biol Trace Elem Res 2020, 198, 177–188. [Google Scholar] [CrossRef]
- Dominy, J.E.; Lee, Y.; Jedrychowski, M.P.; Chim, H.; Jurczak, M.J.; Camporez, J.P.; Ruan, H.-B.; Feldman, J.; Pierce, K.; Mostoslavsky, R.; et al. The Deacetylase Sirt6 Activates the Acetyltransferase GCN5 and Suppresses Hepatic Gluconeogenesis. Mol Cell 2012, 48, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Järvenpää, J.; Huovinen, M.; Schroderus, A.-M.; Ihantola, E.-L.; Küblbeck, J.; Khadeer, M.; Moaddel, R.; Lahtela-Kakkonen, M. Effects of Galloflavin and Ellagic Acid on Sirtuin 6 and Its Anti-Tumorigenic Activities. Biomedicine & Pharmacotherapy 2020, 131, 110701. [Google Scholar] [CrossRef]
- Prestle, J.; Quinn, F.; Smith, G. Ca2+-Handling Proteins and Heart Failure: Novel Molecular Targets? Curr Med Chem 2003, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Coll, K.E.; Johnson, R.G.; McKenna, E. Relationship between Phospholamban and Nucleotide Activation of Cardiac Sarcoplasmic Reticulum Ca 2+ Adenosinetriphosphatase. Biochemistry 1999, 38, 2444–2451. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Sheng, Z. Ellagic Acid Activated PPAR Signaling Pathway to Protect Ileums Against Castor Oil-Induced Diarrhea in Mice: Application of Transcriptome Analysis in Drug Screening. Front Pharmacol 2020, 10. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhou, B.; Jin, L.; Yu, H.; Liu, L.; Liu, Y.; Qin, C.; Xie, S.; Zhu, F. In Vitro Antioxidant and Antiproliferative Effects of Ellagic Acid and Its Colonic Metabolite, Urolithins, on Human Bladder Cancer T24 Cells. Food and Chemical Toxicology 2013, 59, 428–437. [Google Scholar] [CrossRef]
- Harakeh, S.; Almuhayawi, M.; Jaouni, S. Al; Almasaudi, S.; Hassan, S.; Amri, T. Al; Azhar, N.; Abd-Allah, E.; Ali, S.; El-Shitany, N.; et al. Antidiabetic Effects of Novel Ellagic Acid Nanoformulation: Insulin-Secreting and Anti-Apoptosis Effects. Saudi J Biol Sci 2020, 27, 3474–3480. [Google Scholar] [CrossRef]
- Supervisor, Z.Z. Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus Nigra. Molecules 2019, 24, 2497. [Google Scholar]
- Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int J Mol Sci 2017, 18, 168. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Creech, J.; Jiménez-Vázquez, E.N.; Guerrero-Serna, G.; Wang, N.; da Rocha, A.M.; Herron, T.J.; Espinoza-Fonseca, L.M. A Multiscale Approach for Bridging the Gap between Potency, Efficacy, and Safety of Small Molecules Directed at Membrane Proteins. Sci Rep 2021, 11, 16580. [Google Scholar] [CrossRef]
- Rossi, J.P.F.C.; Garrahan, P.J.; Rega, A.F. The Activation of Phosphatase Activity of the Ca2+-ATPase from Human Red Cell Membranes by Calmodulin, ATP and Partial Proteolysis. Biochimica et Biophysica Acta (BBA) - Biomembranes 1986, 858, 21–30. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L. V; Levchenko, S. V; O’Neill, D.P.; et al. Advances in Methods and Algorithms in a Modern Quantum Chemistry Program Package. Phys Chem Chem Phys 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr Top Med Chem 2008, 8, 1555–1572. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Terauchi, M.; Kikuchi, Y.; Nakao, A.; Okubo, J.; Yoshinaga, T.; Hiratsuka, H.; Kobayashi, M.; Hoshi, T. Deprotonation Processes of Ellagic Acid in Solution and Solid States. Monatshefte für Chemie /Chemical Monthly 2003, 134, 811–821. [Google Scholar] [CrossRef]
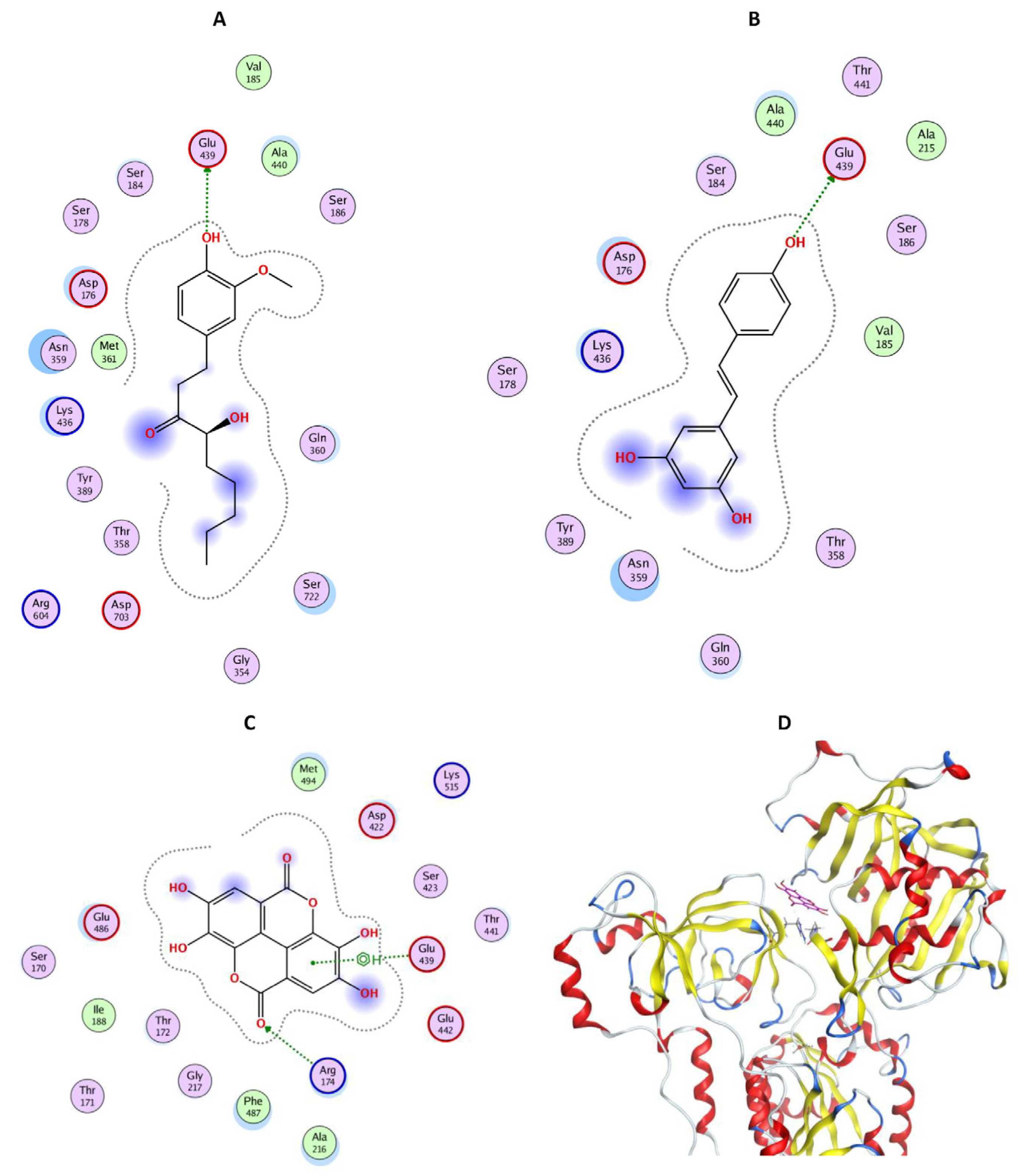
| compound | EHOMO | ELUMO | h_logD | h_logP |
|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | |||
| gingerol | -542.2 | -78.4 | 3.14 | 3.14 |
| shogaol | -530.4 | -38.1 | 4.66 | 4.66 |
| resveratrol | -507.4 | -115.2 | 2.76 | 2.75 |
| oxyresveratrol | -516.9 | -119.1 | 2.31 | 2.28 |
| ellagic acid | -581.3 | -196.4 | 1.95 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





