1. Introduction
Chemical warfare agents (CWAs) are chemicals specially designed and synthesized to harm or kill living organisms, including humans. These agents can be classified into several categories based on their mechanism of action, such as nerve 1, 2 Certain hoarded U.S CWAs have been destroyed by incineration and neutralization process.3 However, sensitive and selective detection, quantification as well as decontamination of these agents are crucial for national security and public safety. Several analytical approaches for the detection of CWAs have been reported, which include gas chromatography coupled with mass spectrometry (GC-MS),4 colorimetric sensor arrays and biosensors,5 and surface acoustic wave sensors.6, 7 To further increase the selectivity, sensitivity, and reliability, especially portability for on-field applications of the detection,2 we present here an electrochemical technique using a miniaturized potentiostat for the analysis of CWAs mimics, which could be combined with drone technology for remote sensing of CWAs in the near future.
Electrochemical techniques render accurate, sensitive, selective, reproducible, and fast sample analysis of target analytes.8-10 Recent advancement in electrochemistry and computer technology has provided various high-end point of care devices which are often connected to smartphones or PCs to get the desired outcomes. Generally, sensors based on electrochemistry utilize several voltammetric techniques which include cyclic voltammetry (CV),11, 12 differential pulse voltammetry (DPV),13 and square wave voltammetry (SWV)14, 15 where the current response is recorded between a working electrode and a counter electrode when a potential is applied at the working electrode relative to a reference electrode. DPV holds high sensitivity because the applied pulse potential waveform can substantially deduct the background and the charging or capacitive current. Instead of using a benchtop electrochemical workstation, great efforts have been made over the years in developing compact, affordable, and effective miniaturized potentiostats for in-situ analysis.8, 16 Adams et al.21 developed miniaturized low-cost potentiostat that uses Microchip Technology ATxmega32E5 microcontroller. Such a mini device when installed in an unmanned aerial vehicle (UAV), also referred to as a micro-drone, could allow sensitive and selective detection of the analytes such as CWAs to be realized remotely. We expect that there will be a wide range of applications for this type of remote monitoring of unsupervised drones in various fields.
The rest of the paper is organized as follows: the next section describes the electrochemical and hardware system development. Next, the results section talks about pattern recognition, mainly the detection of signal signature with the addition of the CWA simulant. System validation and future directions are summarized in the discussion section.
2. Materials and Methods
2.1. Miniature Drone-Based Design
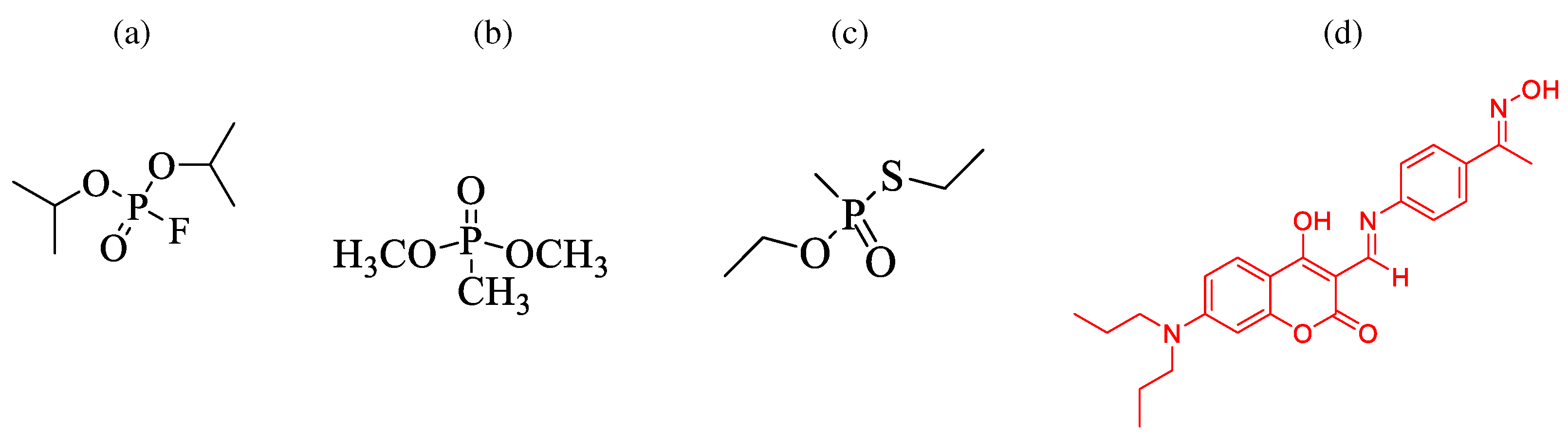
Our proposed design uses a commercially available PalmSens Emstat4 HR miniaturized USB-powered potentiostat to obtain electrochemical results. Emstat4S could be easily mounted on drones for remote sensing because of its compact design. To begin with, the redox properties of a coumarin-enamine derivative (CE2) was obtained using DPV technique which are validated using the laboratory standard CHI 660A potentiostat for reproducibility. The oxime moiety containing coumarin-enamine molecular probe is specifically designed in a way to sensitively capture some of the nerve agents mimic that include diisopropyl fluorophosphate (DFP), , O,S-diethyl methyl phosphonothioate (DEMPT), diisopropyl chlorophosphate (DICP) )
17-19 (ref. ) (
Figure 1). Then, the current response before and after addition of stimulant is collected from the Emstat4S potentiostat. Further, the sensor’s linear range of detection, sensitivity, cross-selectivity, stability, and reproducibility are studied. The collected current responses (output signals) against potential sweep with added stimulant (input signals) could be used to build the logic gate. Also, this method will assist in the quick study of the impacted site by converting the given output signals to electronic signals with a simple on/off programmed logic gate system. This enables us to build a networked device that completes the task without prior supervision.
2.2. Electrochemistry
A three-electrode cell system was employed for the electrochemical studies with a glassy carbon electrode (GCE, 3 mm in diameter) as the working electrode, an Ag/Ag+ [10 mM AgNO3 with 0.10 M tetrabutylammonium perchlorate (TBAP) in acetonitrile (MeCN)] as the reference electrode, and a platinum (Pt) wire as the counter electrode. The molecular probe CE2 was dissolved in MeCN with 0.10 M TBAP as electrolyte. DPV studies of the compound (CE2) were carried out to find the redox properties of underlying different functional moiety present in the CE2. Further subsequent additions of nerve agent mimics to the CE2 were tested and their effects in redox behavior were observed. Our earlier work was centered on the synthesis, photophysical property,19 and proposed configuration of miniatured electrochemical device setup.20 The current work is an extension of electrochemical studies with the proposed device setup.
3. Results
3.1. Emstate4 HR Postentiostat Validation
Validation of Emstate4 HR potentiostat was carried out by comparing the obtained results with ones measured with the CHI 660A electrochemical workstation. DPV studies were carried out in both instruments and signals were analyzed before and after the addition of nerve agent stimulants.
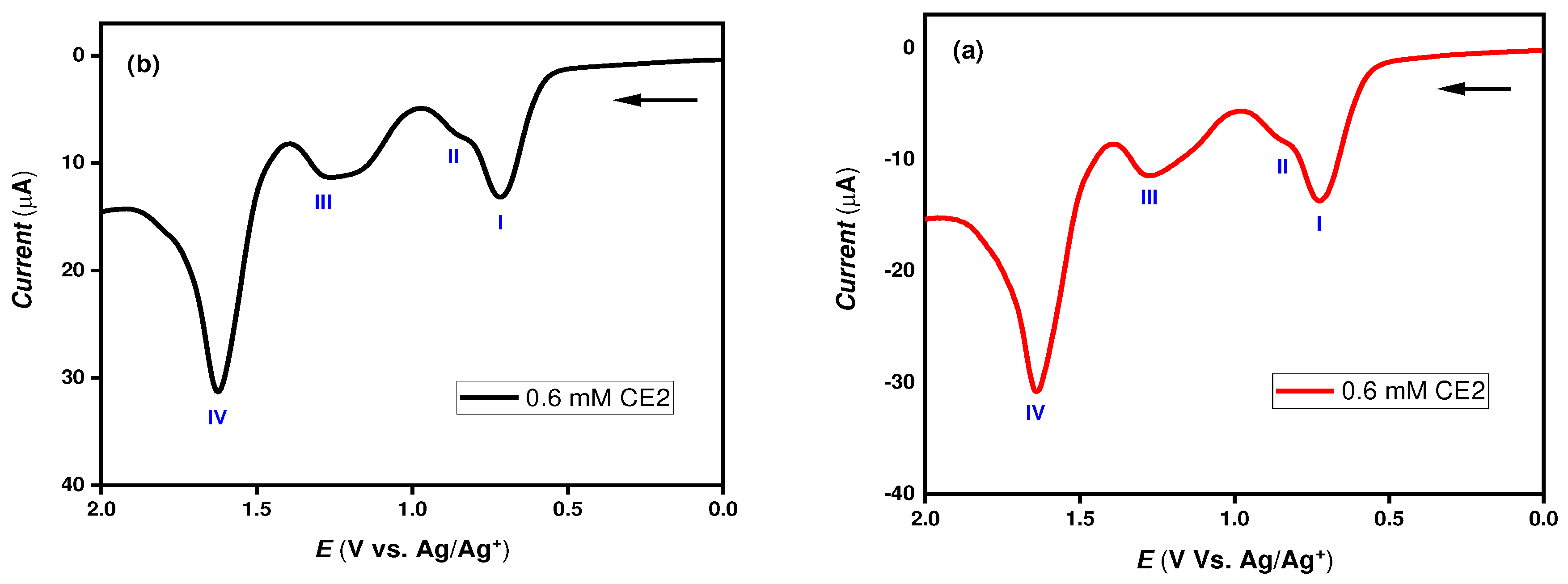
Figure 2 shows the DPV voltammograms of compound CE2 from the two potentiostats, and
Table 1 lists its oxidation peak potentials with and without addition of the stimulants DFP and O,S-DEMPT. Four oxidation peaks exemplified as (I, II, III, IV) were obtained. The oxime functional group in compound CE2 is attributed to oxidation at 1.29 V represented as (III). Initial DPV experiments proved that CHI 660A and Emstate4 HR voltammograms were reproducible generating identical current responses. The results indicate that the miniature Emstat4 HR has produced very similar results to CHI 660A in terms of peak voltages and signal waveforms shape. This means that Emstat4 HR is reliable and the expectation to produce accurate measurements is validated.
3.2. Electrochemical Detection of CWAs
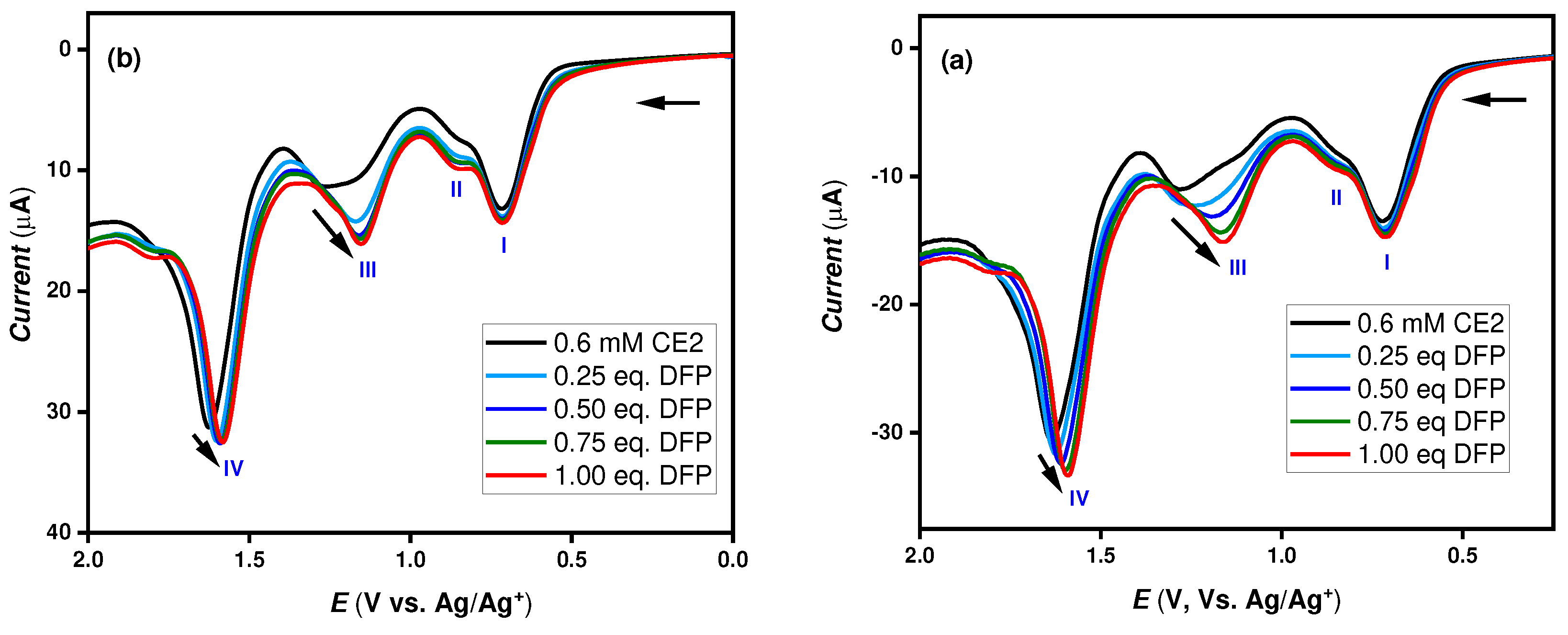
In both CHI 660A and Emstate4 HR potentiostat, the oxidation peak (III) exhibited a slight change by about ~ 0.13 V from 1.29 V to 1.16 V with the addition of the nerve agent mimic DFP which can be ascribed due to covalent bond formation between oxime of CE2 and phosphorous center of DFP as shown in
Figure 3 (a) and (b). Besides, the oxidation peak (IV) showed a marginal shift of ~ 0.05 V from 1.64 V to 1.59 V, which is due to disruption of the enamine moiety in the CE2 structure by free F
- ions from the DFP simulant.
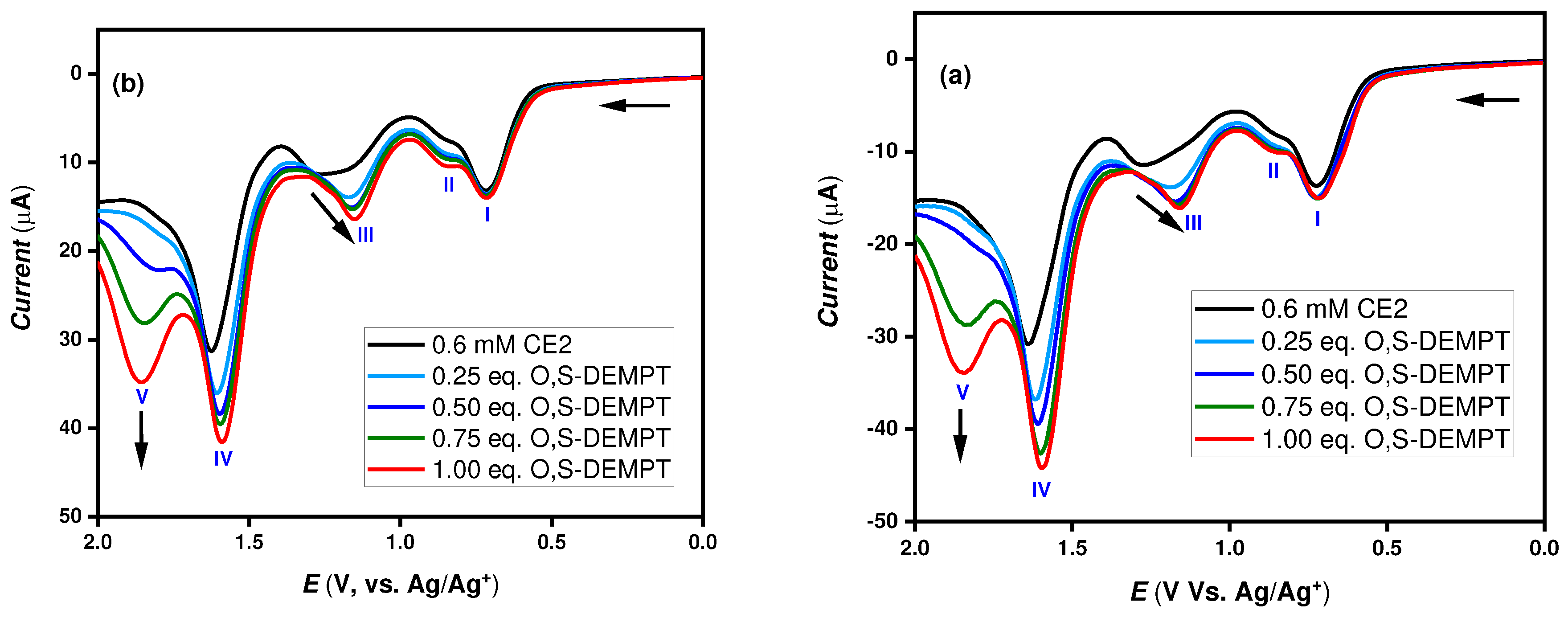
19 When the thiol-containing nerve agent mimic O,S-DEMPT was added, it exhibited a new oxidation peak (V) at 1.86 V. Given that the current increased, with the increasing concentration of the stimulant. It was presumed that this originated from O,S-DEMPT. The oxidation peak (III) also showed a similar shift ~ 0.13 V,
Figure 4 (a) and (b) as the pervious stimulant DFP. However, we have observed that other oxidation peaks (I and II) remain unchanged with the added nerve agent stimulant.
In addition,
Figure 3 and
Figure 4 show the DPV results with increasing concentration of DFP and O,S-DEMPT simulants with the molecular probe CE2 respectively. As seen by the arrow indicated in
Figure 3 and
Figure 4, there is a slight shift in peak current (III) with increasing addition of simulants from 0.25 to 1.00 eq. The new peak (V) appeared only when O,S-DEMPT concentration exceeded 0.75 eq.
4. Discussion
Figure 4 (a) DPV studies of 0.6 mM CE2 with varying addition of O, S-DEMPT simulant to CHI 660A instrument (left) and (b) Emstat4 HR potentiostat (right).5. Conclusions
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
The Future work will focus on optimizing sensitivity, cross selectivity, reproducibility, logic gate construction of the designed probe with the nerve agent mimics. This approach could bring in new promising innovative technologies for on-field detection of CWAs. Future direction also involves incorporating different modalities including electrogenerated chemiluminescence (ECL) signatures, which is expected to improve robustness of the detection system. Also, we are investigating how optical, electrochemical and ECL techniques can be incorporated into films (i.e., Molecular imprinted polymers–MIPs and conductive polymers–CPs) to prepare visual materials designed to indicate the presence (and potential concentration) of specific CWAs.
Funding
Financial support from the US Department of Defense, the US Army Engineer Research and Development Center ERDC (Contract W912HZ-19-2-0044) is greatly acknowledged.
References
- Picard, B.; Chataigner, I.; Maddaluno, J.; Legros, J. Introduction to chemical warfare agents, relevant simulants and modern neutralisation methods. Organic & Biomolecular Chemistry 2019, 17, 6528–6537. [Google Scholar]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and Detection of Chemical Warfare Agents. Chemical Reviews 2011, 111, 5345–5403. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Y. U.S. Meets Milestone in Chemical Weapons Stockpile Destruction. U.S. Department of Defense 2022.
- Rozsypal, T.; Kobliha, Z.J.A.L. Identification of Nitrogen Mustard Chemical Warfare Agents in Sand by Gas Chromatography–Mass Spectrometry (GC-MS) in a Military Deployable Laboratory. 2022, 56, 1–13. [Google Scholar] [CrossRef]
- Davidson, C.E.; Dixon, M.M.; Williams, B.R.; Kilper, G.K.; Lim, S.H.; Martino, R.A.; Rhodes, P.; Hulet, M.S.; Miles, R.W.; Samuels, A.C.; Emanuel, P.A.; Miklos, A.E. Detection of Chemical Warfare Agents by Colorimetric Sensor Arrays. ACS Sensors 2020, 5, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Sharma, M.U.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sensors and Actuators B: Chemical 2013, 178, 636–647. [Google Scholar] [CrossRef]
- Mohan, J.M.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Current Opinion in Electrochemistry 2022, 33, 100930. [Google Scholar] [CrossRef]
- Adams, S.D.; Doeven, E.H.; Quayle, K.; Kouzani, A.Z. MiniStat: Development and Evaluation of a Mini-Potentiostat for Electrochemical Measurements. IEEE Access 2019, 7, 31903–31912. [Google Scholar] [CrossRef]
- Lee, S.C.-H.; Burke, P.J. NanoStat: An open source, fully wireless potentiostat. Electrochimica Acta 2022, 422, 140481. [Google Scholar] [CrossRef]
- Prinith, N.S.; Manjunatha, J.G. Surfactant modified electrochemical sensor for determination of Anthrone – A cyclic voltammetry. Materials Science for Energy Technologies 2019, 2, 408–416. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.; Banks, C.E.J.B. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Meloni, F.; Halicka, K.; Szyszka, A.; Zucca, A.; Pilo, M.I.; Cabaj, J. Differential pulse voltammetry and chronoamperometry as analytical tools for epinephrine detection using a tyrosinase-based electrochemical biosensor. RSC Advances 2022, 12, 25342–25353. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Analytical Methods 2013, 5, 2158–2173. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Li, M.; Qu, L.; Liu, Z. Acetylcholinesterase–Cu3(PO4)2 hybrid nanoflowers for electrochemical detection of dichlorvos using square-wave voltammetry. Analytical Methods 2022, 14, 3911–3920. [Google Scholar] [CrossRef] [PubMed]
- Hoilett, O.S.; Walker, J.F.; Balash, B.M.; Jaras, N.J.; Boppana, S.; Linnes, J.C. KickStat: A Coin-Sized Potentiostat for High-Resolution Electrochemical Analysis Sensors [Online], 2020.
- Diauudin, F.N.; Rashid, J.I.A.; Knight, V.F.; Wan Yunus, W.M.Z.; Ong, K.K.; Kasim, N.A.M.; Abdul Halim, N.; Noor, S.A.M. A review of current advances in the detection of organophosphorus chemical warfare agents based biosensor approaches. Sensing and Bio-Sensing Research 2019, 26, 100305. [Google Scholar] [CrossRef]
- Wahl, J.H.; Colburn, H.A. Extraction of chemical impurities for forensic investigations: A case study for indoor releases of a sarin surrogate. Building and Environment 2010, 45, 1339–1345. [Google Scholar] [CrossRef]
- Mia, R.; Cragg, P.J.; Wallace, K.J. Low Molecular Weight Fluorescent probes for the detection of organophosphates. Journal of Luminescence 2021, 235, 118053. [Google Scholar] [CrossRef]
- Dawoud, A.; Mia, R.; Biswakarma, A.; Motchaalangaram, J.; Miao, W.; Wallace, K.J.I.J.o.A.; Engineering, M. Embedded Electrochemistry with a Miniaturized, Drone-Based, Potentiostat System for Remote Detection Chemical Warfare Agents. 2022, 16, 112–115. [Google Scholar]
- Adams, S.D.; Doeven, E.H.; Quayle, K.; Kouzani, A.Z. IEEE Access MiniStat: Development and Evaluation of a Mini-Potentiostat for Electrochemical Measurements. 2019, 7, 31903–31912. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).