1. Introduction
Escherichia coli (E. coli) is a Gram-negative, rod-shaped bacterium that commonly resides in the gastrointestinal tracts of humans and animals, as well as the natural environment [
1]. While it typically maintains a symbiotic relationship with its host and rarely causes disease, certain pathogenic strains can infect multiple animal species, posing a significant health risk to both livestock and humans [
2]. E. coli can lead to a range of infections, including gastroenteritis, urinary tract infections, skin and soft tissue infections, and septicemia [
3]. It is expelled from the host via feces and can survive for extended periods in the environment, making it a potential source of water and soil pollution [
4,
5]. As such, E. coli testing is an essential part of routine epidemiological examinations for diagnosing various diseases. The emergence of antibiotic resistance is a growing global problem [
6]. Bacteria have developed resistance to antibiotics in response to the widespread and often inappropriate use of these drugs [
7]. Animal husbandry, where antibiotics are used for therapy and prophylaxis of infectious diseases, has contributed significantly to the development of antibiotic-resistant strains [
8]. E. coli, with its outer membrane barrier, has exhibited resistance to therapeutic levels of penicillin G, the first β-lactam antibiotic introduced into clinical practice. In this study, we report a massive mortality event in a donkey farm caused by an E. coli infection. The infection resulted in severe diarrhea, septicemia, and sudden death in young donkeys. The outbreak affected approximately 100 donkeys under four months of age within a herd of 340. The characteristics of the E. coli strain were determined through clinical observation, biochemical analysis, pathological examination, isolation and identification, and molecular sequencing. Mice experiments, conducted using the isolated bacteria, replicated the clinical and pathological symptoms observed in the donkeys. Molecular Evolutionary Genetic Analysis confirmed that the isolated bacteria, named CEG-GZL20, shared 99.98% sequence identity with E. coli. Antibiotic sensitivity testing revealed that CEG-GZL20 exhibited high resistance to most antibiotics, with sensitivity only observed to Tetracycline and Gentamicin. Due to the role of E. coli as a key component of the gut microbiota, promoting digestion, nutrient absorption, and immune health, diseases and mortality caused by this bacterium pose significant challenges for animal husbandry and human health. This study provides valuable insights into a comprehensive investigation of E. coli infection in donkeys, helping to identify etiological agents and evaluate donkeys as reservoir hosts for human pathogenic E. coli.
2. Materials and Methods
2.1. Ethical Statement
The samples used in this study were collected from deceased donkeys at a farm under the supervision of a local veterinarian and with the approval of the farm owner. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Jilin University (approval NO.20150226), ensuring compliance with ethical guidelines for animal research.
2.2. Source of Donkey Pathological Tissues
Pathological tissues were collected from deceased donkeys at a farm located in Qiqihar city, Heilongjiang province, China. All tissues were collected under aseptic conditions and transported in iceboxes to the Veterinary Medicine College of Jilin University for further analysis.
2.3. Blood Collection and Analysis
Blood samples were collected from jugular veins of both mother and cub donkeys using EDTA tubes (Hangzhou Microbiological Reagents Limited Company, Hangzhou China). Complete blood count (CBC) and blood biochemical analysis were performed using automated analyzers (Hangzhou China) to assess various blood parameters. The obtained results are presented in (S1, S2).
2.4. Mice and Treatment
Twenty-five Kunming strain mice were obtained from Changchun Biological Products Institute and housed in the Laboratory Animal Facility of Jilin University. The mice were provided with free access to food and water and were maintained at a temperature-controlled room (22 ± 0.5 °C). To study the pathogenicity of the isolated bacteria, the mice were divided into five groups, including one control group, one group inoculated with standard E. coli, and three groups inoculated with different concentrations of the CEG-GZL20 bacteria strain. After inoculation, the mice were closely monitored every 4 hours for the development of clinical signs. Mice displaying clinical symptoms were euthanized and subjected to necropsy following standard protocols.
2.5. Isolation and Characterization of Bacteria
To identify the bacteria responsible for the infection, the tissues from liver, spleen, kidney, and lung were collected and subjected to bacterial isolation. Bacteria were isolated using Luria broth (LB) agar plates containing 2% agar. After incubation at 37°C for 18 hours, large, white, and moist colonies were observed. Gram staining and biochemical testing were performed to characterize the isolated bacteria according to standard protocols.
2.6. Antibiotic Sensitivity Test
To determine the antibiotic sensitivity of the isolated bacteria, a disk diffusion test was performed according to the Bauer-Kirby method [
9]. The test involved placing antibiotic disks on inoculated agar plates and measuring the zone of inhibition after incubation. The results were interpreted according to the guidelines provided by the American Society for Microbiology.
2.7. H&E Staining
Tissue samples were collected from the mice and processed for histopathological analysis. The samples were dehydrated in increasing concentrations of ethanol, infiltrated with paraffin, blocked on polylysine-coated glass slides, and dried. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. The slides were examined under a Nikon epifluorescence microscope, and images were captured using a CCD camera.
2.8. Statistical Analysis
All experiments were performed at least three times, and the results were expressed as mean ± standard deviation (SD). Differences between mean values were assessed using the two-tailed Student's t-test, with p ≤ 0.05 considered statistically significant.
3. Results
3.1. Epidemiological Investigation and Clinical Observation
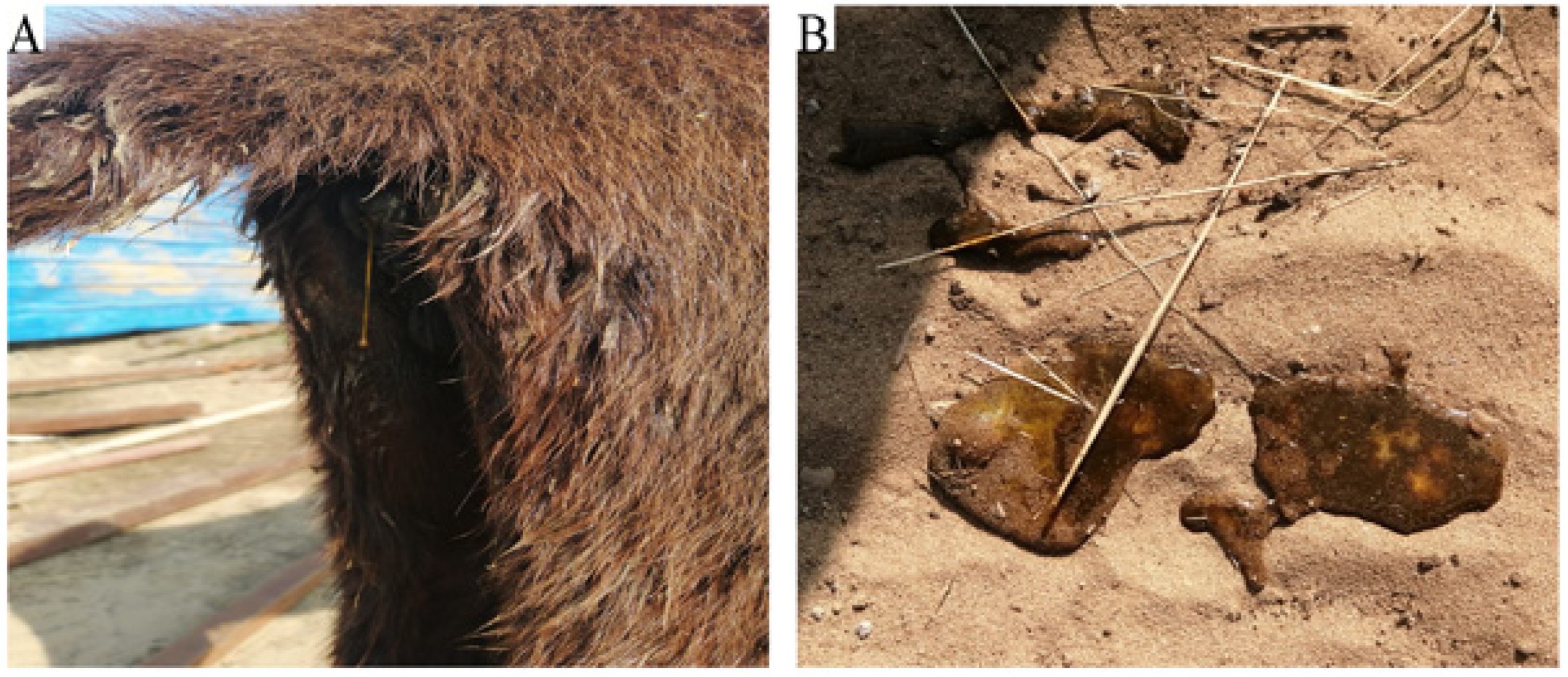
The farm had a total of 340 donkeys, with over 100 under four months old. An outbreak resulted in sudden death, severe diarrhea, and depression in 20 young donkeys (
Table 1). Clinical examination revealed elevated body temperatures (39.5℃), arrhythmic heart rates (45 bpm), and watery feces in affected donkeys (
Figure 1A,B). An investigation traced the outbreak to recent changes in feeding material.
3.2. Gross Lesions
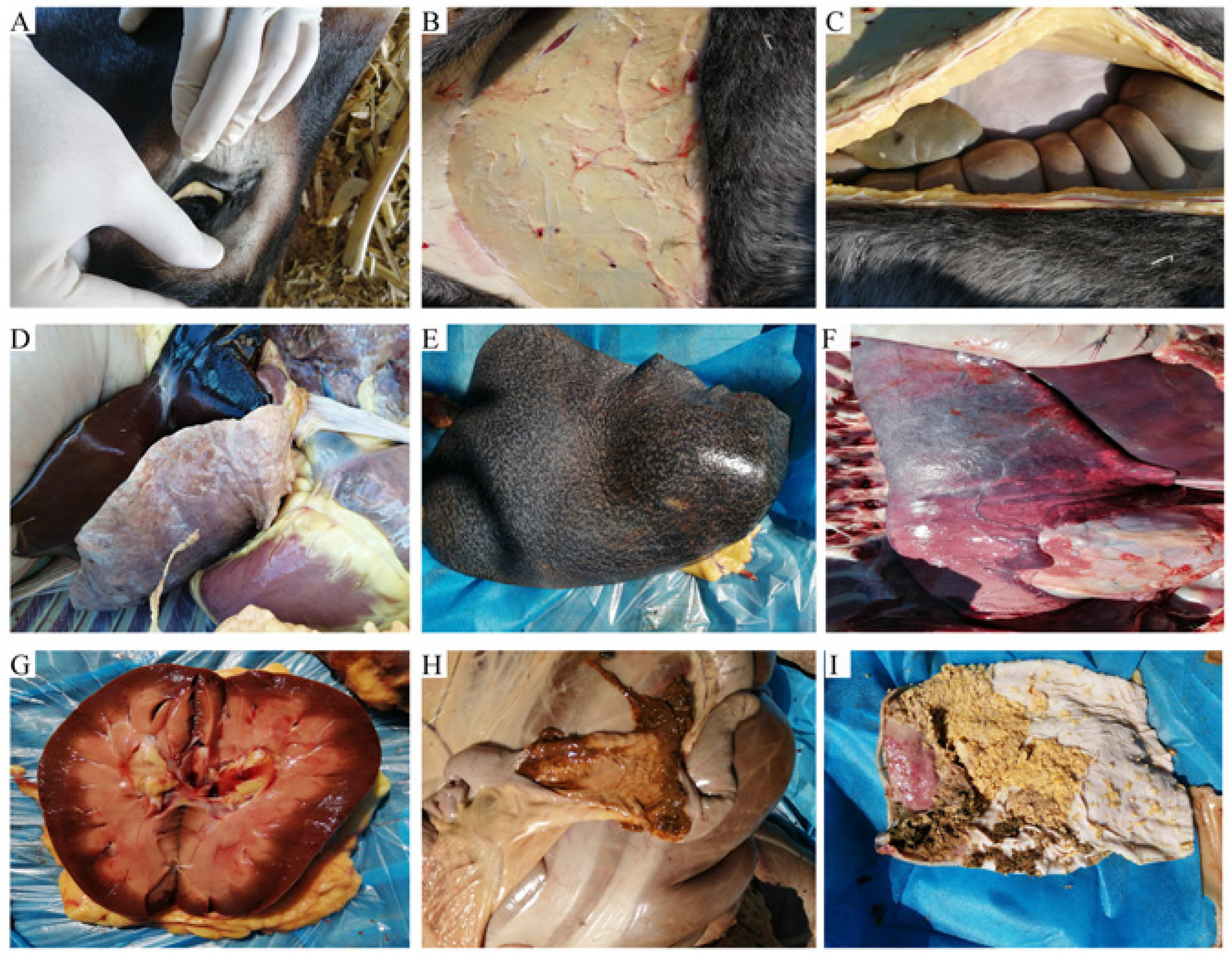
Autopsies were performed on deceased donkeys, revealing various gross lesions. The conjunctiva mucosa appeared pale, the tissue under the skin was pale and enlarged, and the abdomen was filled with red-colored fluid. The heart, liver, spleen, lungs, and kidneys exhibited swelling, bleeding points, and abnormal tissue consistency. The gastrointestinal tract showed mucous hemorrhage, ulcers, and disrupted tissue structure (
Figure 2).
3.3. Pathological Changes in Donkeys
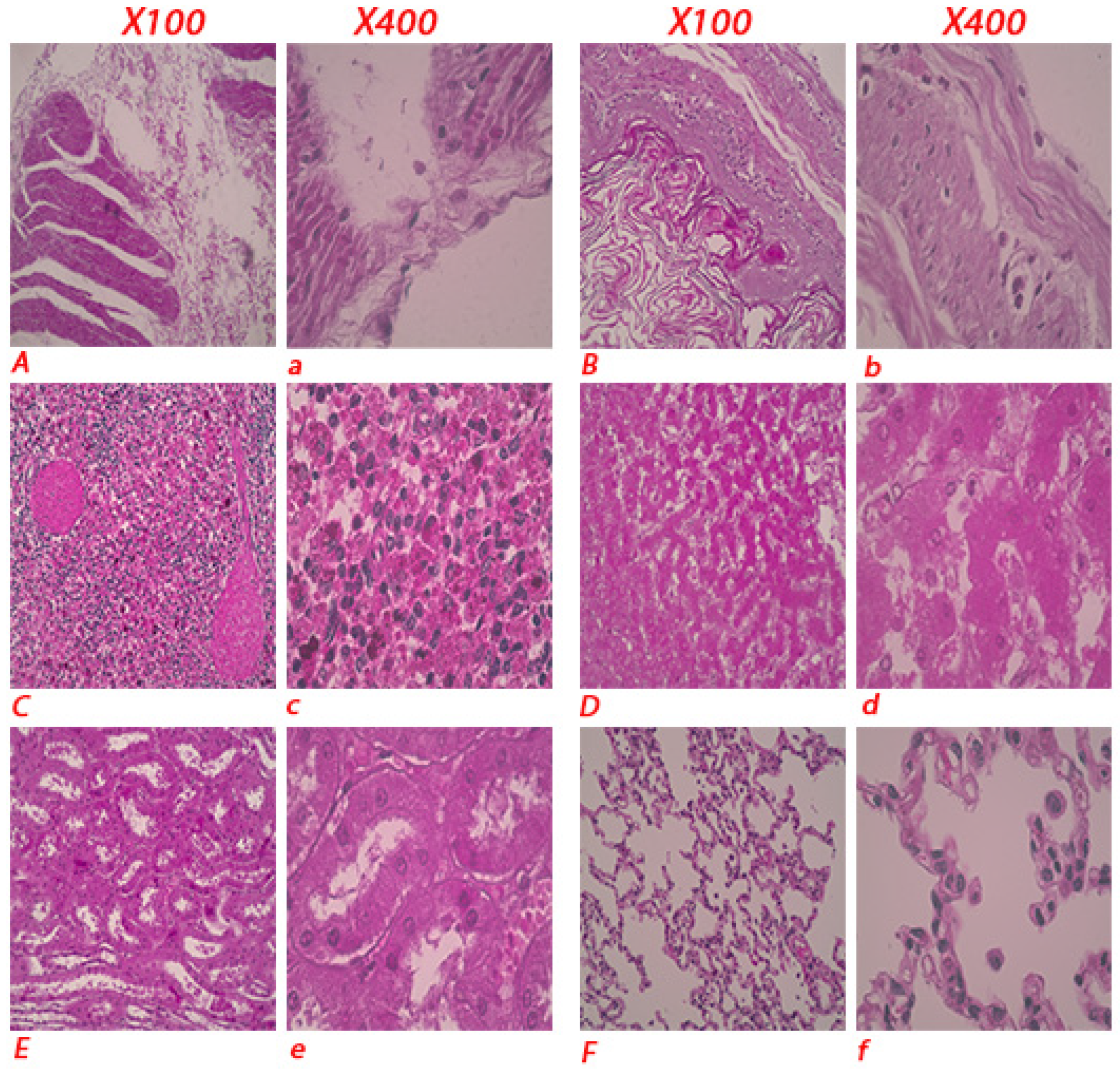
Histopathological examination of tissues revealed disrupted tissue structures in the intestines and stomach, necrosis and congestion in the spleen and liver, lung inflammation, and swollen and fragile kidneys (
Figure 3).
3.4. Detection of Potential Pathogens
Smear slides were prepared from liver, spleen, kidney, and lung samples for gram staining, and large, white, and moist colonies were observed. Biochemical analysis revealed characteristics indicating bacterial presence (
Table 2).
3.5. Bacterial Isolation
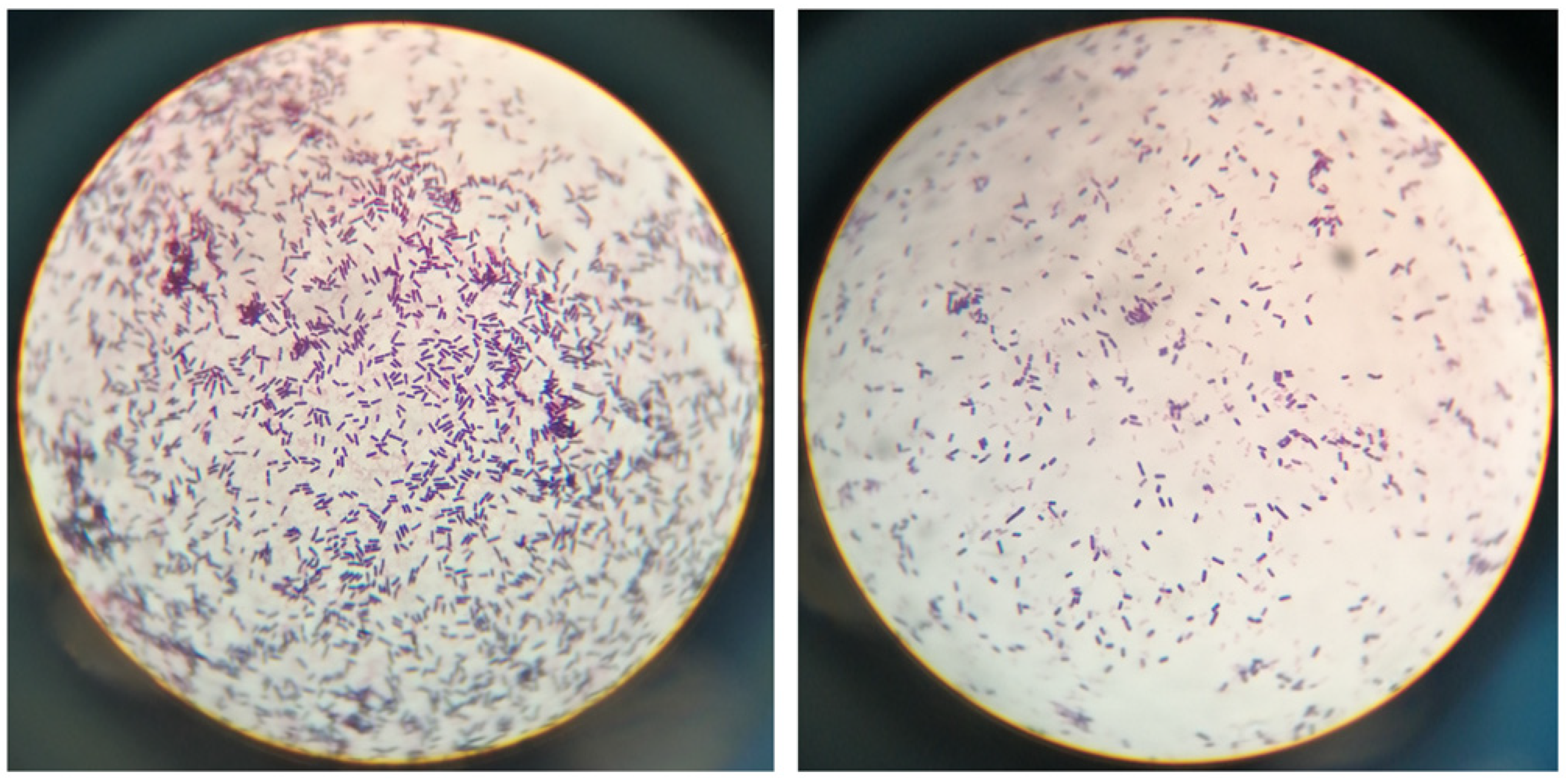
To identify the bacteria, we performed an isolation procedure on the aforementioned samples. Bacteria were isolated from aseptically collected liver, lung, spleen, and kidney samples using Luria broth (LB) plates containing 2% agar powder. Following streaking and incubation at 37℃ for 18 hours, we observed numerous large, white, and moist colonies. The bacteria colonies were then examined under light microscopy after H&E staining, and their morphology was found to be identical to the bacteria observed in the tissue samples (
Figure 4).
3.6. Biochemical Properties
To characterize the isolated bacteria, we conducted biochemical property tests according to the manufacturer's instructions. The bacteria showed negative results for the decomposition of indole, methyl red, sorbitol, xylose, lysine, ornithine, citrate, VP, urea, phenylalanine, and hydrogen sulfide (
Table 3).
3.7. Identification of the Isolated Bacteria as E. Coli
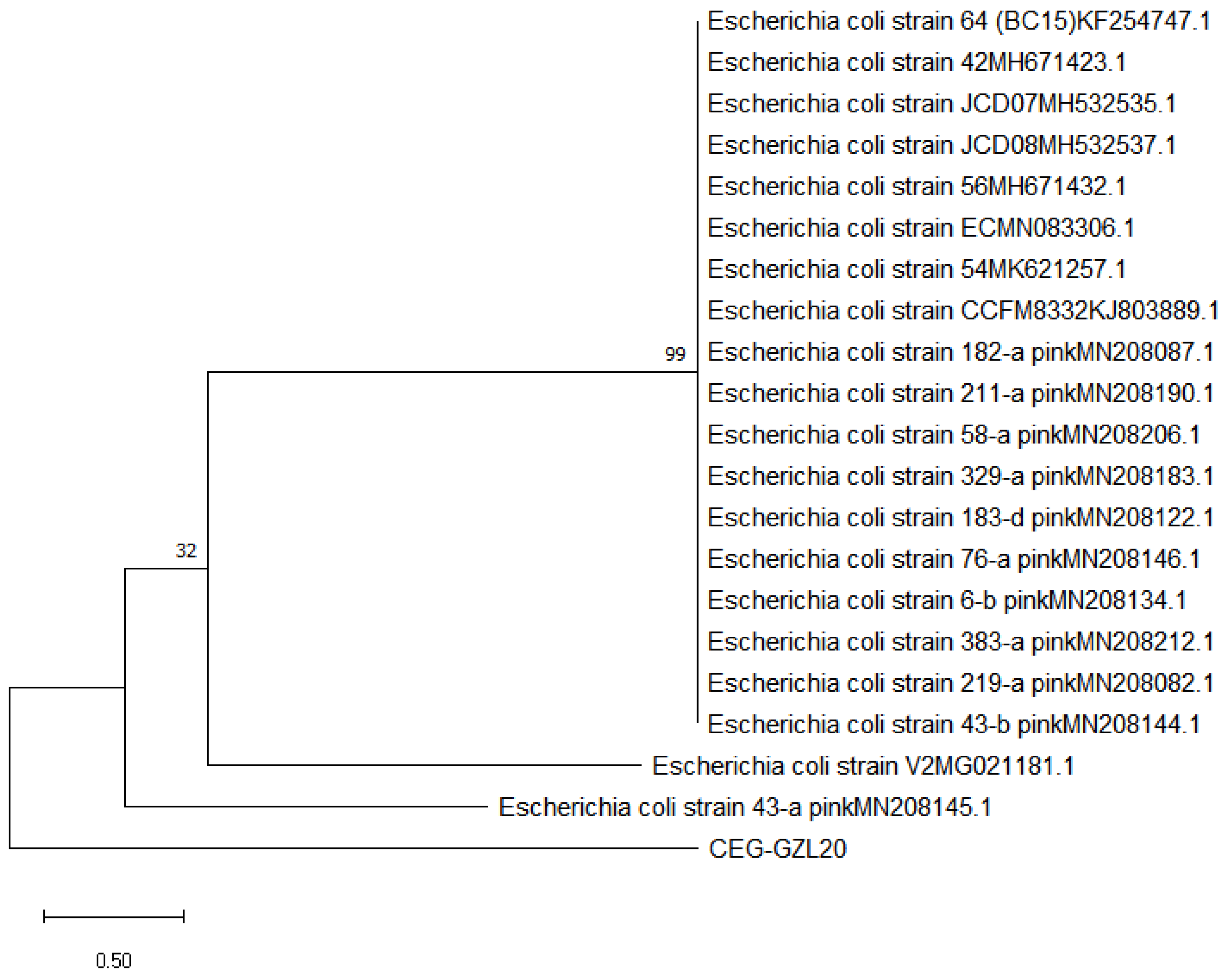
To molecularly determine the identity of the bacteria CEG-GZL20, we amplified the bacterial 16S rRNA genome. The amplified fragment had a size of 1391 bp, and sequencing analysis revealed a 99% sequence identity with E. coli, indicating that the isolated bacteria were indeed E. coli (
Figure 5).
3.8. Antibiotic-Sensitivity Test
To provide recommendations for more effective clinical therapy, we performed an antibiotic-sensitivity test using the disk diffusion method. After incubation at 37℃ for 36 hours, we identified two antibiotics (Tetracycline and Gentamicin) out of a total of 20 as sensitive. The results of the test are presented in
Table 4.
3.9. Effect of E. Coli on the Survival Rate of Mice
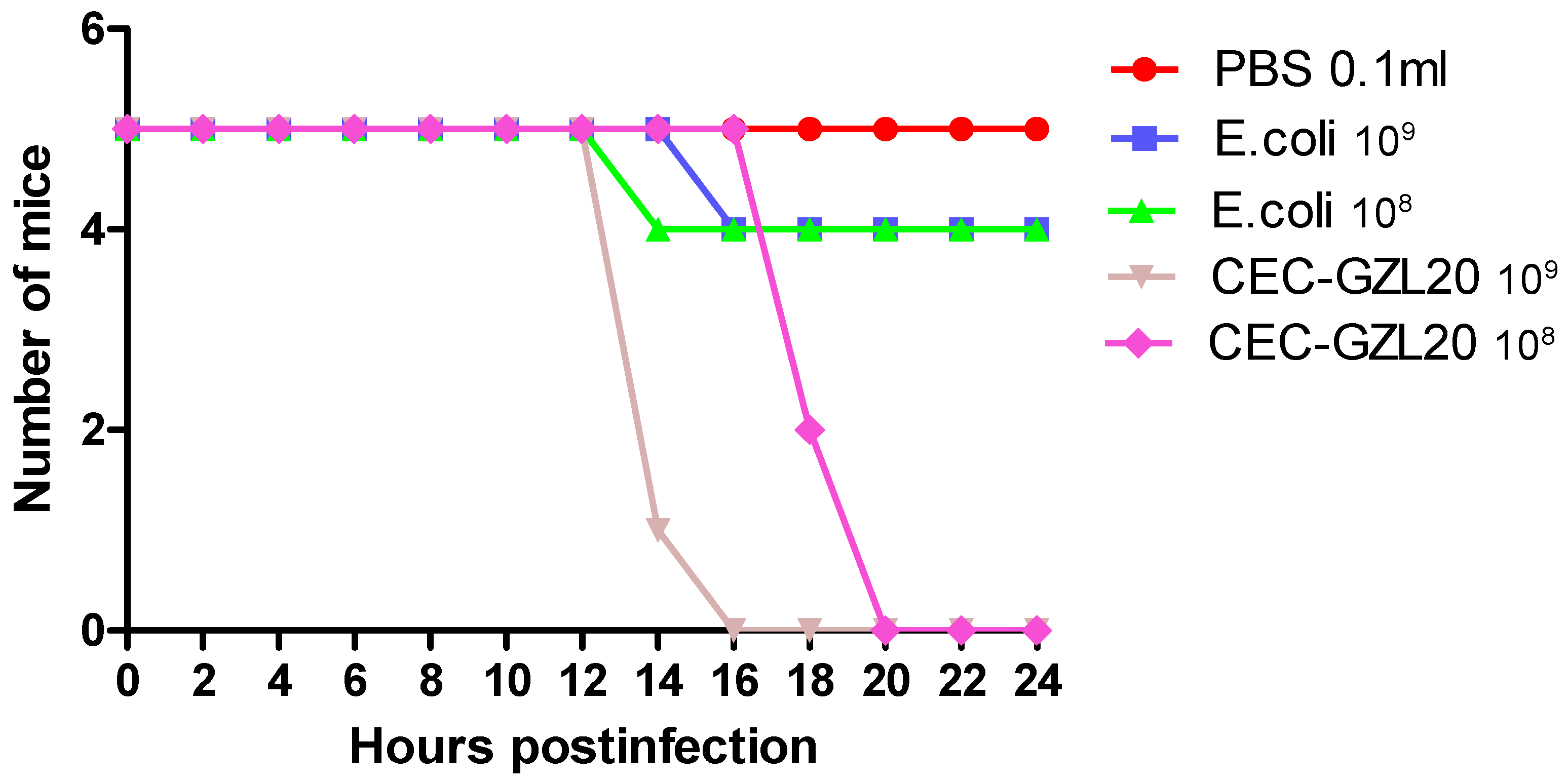
To assess the pathogenicity of the isolated bacteria, we employed a murine model system. Mice in groups I and II were intraperitoneally inoculated with different colony-forming units (cfu) of the isolated bacteria diluted in PBS, while mice in groups III and IV were inoculated with cfu of standard E. coli. The control mice in group V were inoculated with an equal volume of PBS. All mice in groups I and II died within 8-12 hours post-infection after inoculation with 1.0 × 10^8 and 1.0 × 10^9 cfu/0.1ml of the isolated bacteria, respectively. However, all mice in group V survived, with only one mouse dying after 16-18 hours post-infection after inoculation with 1.0 × 10^9 cfu/0.1ml of standard E. coli. (
Figure 6) presents the pathogenicity of CEC-GZL20 to mice, showing the calculated morbidity and mortality based on the presence of clinical signs and death post-infection. The X-axis represents the number of surviving mice, while the Y-axis represents the hours post-infection.
3.10. Pathological Lesions in Mice
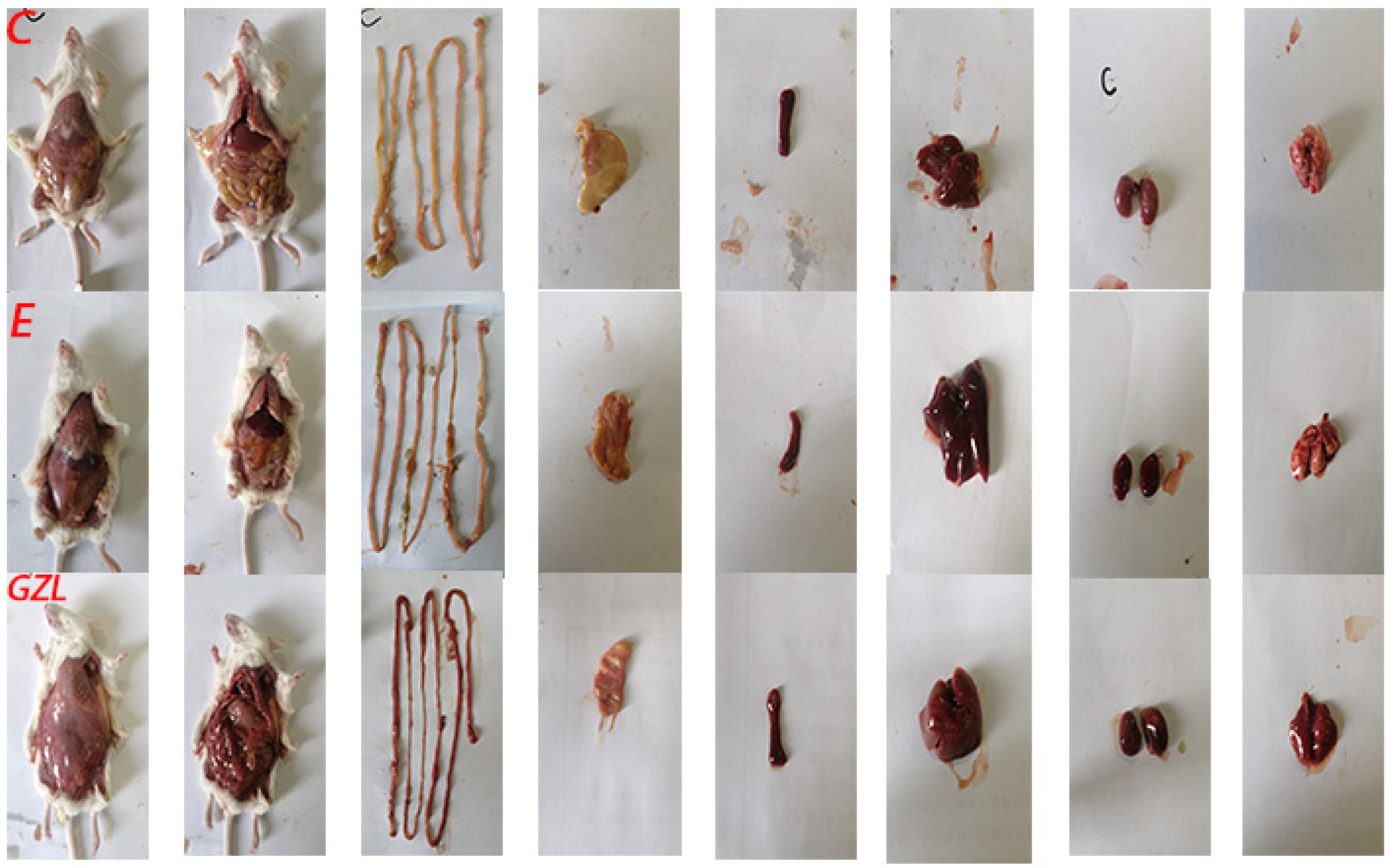
To further evaluate if mice infected with the isolated bacteria exhibited similar pathological characteristics, we performed autopsies following a standard protocol. The mice infected with CEC-GZL20 showed similar pathological changes as the deceased donkeys, as depicted in (
Figure 7). In the CEC-GZL20 group, the under-skin tissues were more hemorrhagic, the intestine tissue was fragile and hemorrhagic, and the stomach, spleen, liver, kidneys, and lung were swollen, fragile, and hemorrhagic (
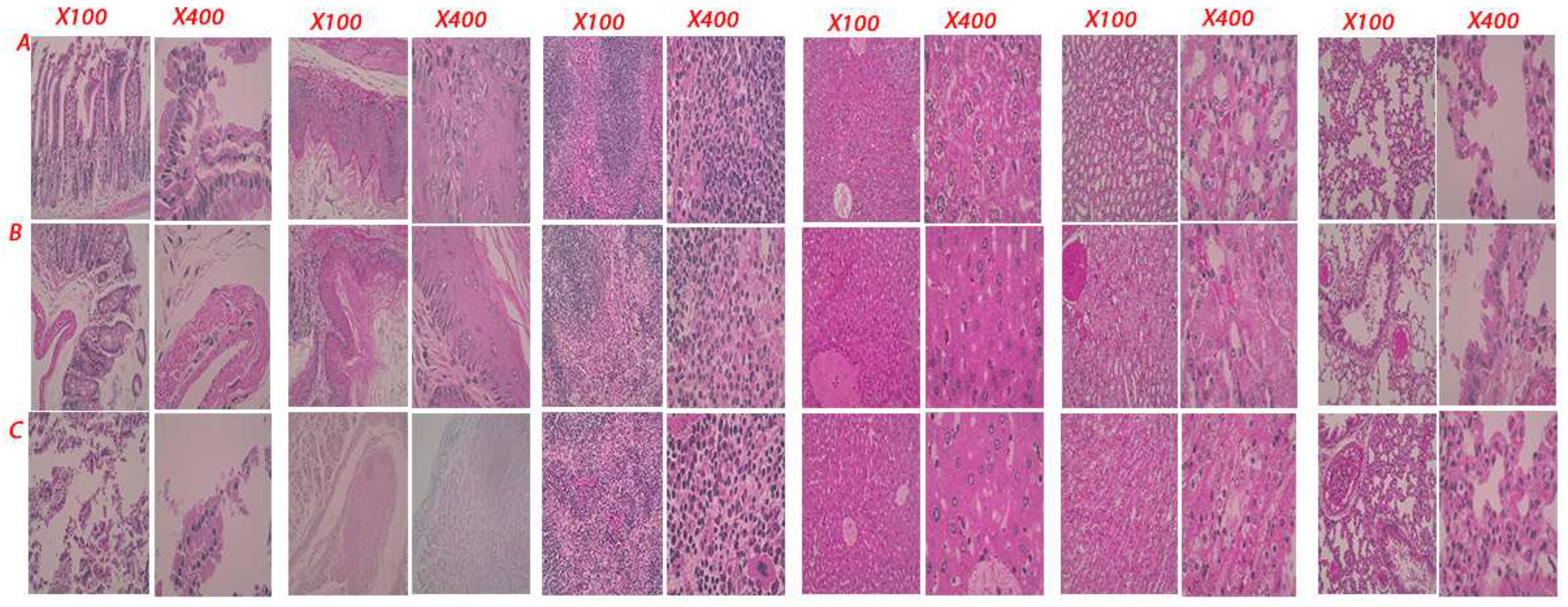
Figure 8). Histopathological examinations of mice in the control group, E. coli injected group, and the CEC-GZL20 infected group revealed varying results. The lesions observed in the CEC-GZL20 group were more severe and shared the most similarity with the lesions observed in the donkeys. The disrupted tissue structures were observed in the intestine and stomach, necrosis and congestion were present in the spleen and liver, the epithelial cells in distal and proximal convoluted tubules of the kidney were swollen, and the lung showed signs of inflammation.
4. Discussion
This publication explores the findings and implications of the study on the mass mortality event caused by pathogenic Escherichia coli (E. coli) in a donkey farm. The results provide valuable insights into the pathogenesis, antibiotic resistance, and potential control strategies for E. coli infections in donkeys.
The identification of a unique strain of E. coli, named CEC-GZL20, as the causative agent of the mass mortality event is supported by various analyses. Clinical observation, histopathological examination, and 16S rRNA analysis confirmed that CEC-GZL20 shared a 99.98% sequence identity with E. coli, indicating its classification within this pathogen. This identification contributes to the understanding of E. coli infections and expands the knowledge of its potential virulence factors and genetic characteristics.
The study also conducted an antibiotic sensitivity test on CEC-GZL20, revealing high resistance to most antibiotics. Only Tetracycline and Gentamicin showed effectiveness against this strain. This finding highlights the concerning trend of antibiotic resistance in E. coli strains, which is consistent with the global issue of bacterial resistance to antibiotics. It emphasizes the urgent need for judicious use of antibiotics in animal husbandry and the development of alternative therapeutic strategies to combat this alarming rise in antibiotic resistance.
Furthermore, the study demonstrated the pathogenicity of CEC-GZL20 in a murine model. Mice inoculated with the isolated bacteria exhibited clinical symptoms and pathological changes similar to those observed in the affected donkeys. This suggests that CEC-GZL20 is highly virulent and capable of causing severe diarrhea and associated complications in susceptible animals. The use of a murine model provided valuable insights into the pathogenesis of the infection and serves as a basis for further research on the mechanisms underlying the disease.
The findings also highlight the importance of proper hygiene practices in preventing and managing similar outbreaks. The outbreak in the donkey farm was traced back to a change in feeding material, indicating the role of environmental factors in disease transmission. Implementing strict biosecurity measures, such as regular sanitization and monitoring of feeding materials, can help minimize the risk of bacterial infections in livestock.
In terms of clinical management, the study identified Tetracycline and Gentamicin as potentially effective antibiotics against CEC-GZL20. These findings provide guidance for clinicians and veterinarians in selecting appropriate treatment options for E. coli infections in donkeys. However, continued surveillance and monitoring of antibiotic resistance patterns are crucial to ensure the effectiveness of these antibiotics and facilitate the development of tailored treatment protocols.
Overall, this study contributes to the understanding of E. coli infections in donkeys and emphasizes the importance of effective control measures. It highlights the need for further research on the genetic characteristics and virulence factors of CEC-GZL20 to inform targeted control strategies. Additionally, it underscores the urgent need for responsible antibiotic use and the exploration of alternative therapeutic approaches. By considering the implications of these findings, stakeholders in animal husbandry and public health can work towards mitigating the impact of E. coli infections in donkeys and preventing future outbreaks.
5. Conclusion
In conclusion, this publication describes a mass mortality event in a donkey farm caused by pathogenic Escherichia coli (E. coli) infection. The outbreak resulted in severe diarrhea, septicemia, and respiratory infections in young donkeys, leading to significant morbidity and mortality. The characteristics of the E. coli strain, named CEC-GZL20, were identified through various analyses, including clinical observation, histopathological examination, 16S rRNA analysis, and antibiotic sensitivity testing.
A mice experiment using the isolated bacteria replicated the clinical and pathological symptoms observed in the donkeys. The findings revealed that CEC-GZL20 exhibited high antibiotic resistance, with sensitivity only observed to Tetracycline and Gentamicin. The study highlights the importance of E. coli as a key component of the gut microbiota, responsible for digestion, nutrient absorption, and immune health in donkeys. The emergence of a pathogenic strain with high antibiotic resistance poses significant challenges for animal husbandry and human health. The development of effective control measures, including proper hygiene practices and appropriate antibiotic treatment, is crucial for managing and preventing similar outbreaks in the future. Further research on the genetic characteristics and virulence factors of CEC-GZL20 would provide valuable insights into its pathogenicity and aid in the development of targeted control strategies.
Overall, this publication contributes to the understanding of E. coli infections in donkeys and serves as a foundation for further investigations in this field.
References
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The Population Genetics of Commensal Escherichia Coli. Nat. Rev. Microbiol. 2010, 8, 207–217. https://doi.org/10.1038/nrmicro2298. [CrossRef]
- Riveros, M.; García, W.; García, C.; Durand, D.; Mercado, E.; Ruiz, J.; Ochoa, T.J. Molecular and Phenotypic Characterization of Diarrheagenic Escherichia Coli Strains Isolated from Bacteremic Children. Am. J. Trop. Med. Hyg. 2017, 97, 1329–1336. https://doi.org/10.4269/ajtmh.17-0066. [CrossRef]
- Hammerum, A.M.; Heuer, O.E. Human Health Hazards from Antimicrobial-Resistant Escherichia Coli of Animal Origin. Clin. Infect. Dis. 2009, 48, 916–921. https://doi.org/10.1086/597292. [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and Virulence in Escherichia Coli: An Evolutionary Perspective. Mol. Microbiol. 2006, 60, 1136–1151. https://doi.org/10.1111/j.1365-2958.2006.05172.x. [CrossRef]
- Pan, Z.; Ma, J.; Dong, W.; Song, W.; Wang, K.; Lu, C.; Yao, H. Novel Variant Serotype of Streptococcus Suis Isolated from Piglets with Meningitis. Appl. Environ. Microbiol. 2015, 81, 976–985. https://doi.org/10.1128/AEM.02962-14. [CrossRef]
- The Evolving Threat of Antimicrobial Resistance : Options for Action. World Health Organization 2012.
- von Baum, H.; Marre, R. Antimicrobial Resistance of Escherichia Coli and Therapeutic Implications. Int. J. Med. Microbiol. 2005, 295, 503–511. https://doi.org/10.1016/j.ijmm.2005.07.002. [CrossRef]
- Szmolka, A.; Nagy, B. Multidrug Resistant Commensal Escherichia Coli in Animals and Its Impact for Public Health. Front. Microbiol. 2013, 4, 258. https://doi.org/10.3389/fmicb.2013.00258. [CrossRef]
- Barry, A.L.; Coyle, M.B.; Thornsberry, C.; Gerlach, E.H.; Hawkinson, R.W. Methods of Measuring Zones of Inhibition with the Bauer-Kirby Disk Susceptibility Test. J. Clin. Microbiol. 1979, 10, 885–889. https://doi.org/10.1128/jcm.10.6.885-889.1979. [CrossRef]
Figure 1.
Clinical observation of a sick donkey.
Figure 1.
Clinical observation of a sick donkey.
Figure 2.
Gross lesions observed postmortem. A. A palled visible conjunctiva mucosa. B. A palled under skin tissue. C. abdominal cavity red-colored fuel with swollen tissue. D. A swollen heart and visible bleeding point on the liver. E. A swollen and fragile spleen. F. A long with tumor-like hard tissue on the surface. G. A swollen and fragile kidney. Visible hemorrhage, ulcers in intestine cavity(H) and stomach(I).
Figure 2.
Gross lesions observed postmortem. A. A palled visible conjunctiva mucosa. B. A palled under skin tissue. C. abdominal cavity red-colored fuel with swollen tissue. D. A swollen heart and visible bleeding point on the liver. E. A swollen and fragile spleen. F. A long with tumor-like hard tissue on the surface. G. A swollen and fragile kidney. Visible hemorrhage, ulcers in intestine cavity(H) and stomach(I).
Figure 3.
Histopathological lesions in the donkey. Representative figure showing the disrupted tissue structure in the intestine (A, a) and stomach (B, b). Necrosis and congestion in spleen (C, c) and liver (D, d). Necrosis and disrupted tissue structure in kidney (E, e). Inflammatory cells infiltrated in lung tissue (F, f).
Figure 3.
Histopathological lesions in the donkey. Representative figure showing the disrupted tissue structure in the intestine (A, a) and stomach (B, b). Necrosis and congestion in spleen (C, c) and liver (D, d). Necrosis and disrupted tissue structure in kidney (E, e). Inflammatory cells infiltrated in lung tissue (F, f).
Figure 4.
Identification of potential bacterial pathogen.
Figure 4.
Identification of potential bacterial pathogen.
Figure 5.
Evolution tree that showed the similarity of the isolated bacteria CEG-GZL20 to E. coli.
Figure 5.
Evolution tree that showed the similarity of the isolated bacteria CEG-GZL20 to E. coli.
Figure 6.
Pathogenicity of CEC-GZL20 to mice. Mice were experimentally infected with different dose of standard E. coli stain, and of isolated bacteria CEC-GZL20 strain. Morbidity and mortality were calculated based on the presence of clinical signs and death post infection, respectively. Axis X and Y presents the number of mice survived and the hours post infection, respectively.
Figure 6.
Pathogenicity of CEC-GZL20 to mice. Mice were experimentally infected with different dose of standard E. coli stain, and of isolated bacteria CEC-GZL20 strain. Morbidity and mortality were calculated based on the presence of clinical signs and death post infection, respectively. Axis X and Y presents the number of mice survived and the hours post infection, respectively.
Figure 7.
Pathological lesions in mice. C. Control; E. E. coli; GZL. CEC-GZL20. (Left to right: Under-skin view, whole view of inner organs, intestine, stomach, spleen, liver, kidneys, and lung).
Figure 7.
Pathological lesions in mice. C. Control; E. E. coli; GZL. CEC-GZL20. (Left to right: Under-skin view, whole view of inner organs, intestine, stomach, spleen, liver, kidneys, and lung).
Figure 8.
Histopathological lesions in the mice. Representative figure showing the disrupted tissue structure in the intestine, stomach, spleen, liver, kidney, and lung tissue.
Figure 8.
Histopathological lesions in the mice. Representative figure showing the disrupted tissue structure in the intestine, stomach, spleen, liver, kidney, and lung tissue.
Table 1.
Age distribution of the donkeys in the farm.
Table 1.
Age distribution of the donkeys in the farm.
| Age (Month) |
(0,2] |
(2,4] |
(<4) |
| Number |
| Total |
61 |
79 |
140 |
| Death |
20 |
0 |
0 |
| Portions |
32.79% |
0 |
0 |
Table 2.
Bacterial counts in different organs in the donkeys.
Table 2.
Bacterial counts in different organs in the donkeys.
| Sample |
Relative Bacterial Counts |
| Liver |
+++ |
| Lung |
++ |
| Spleen |
+++ |
| Kidney |
+ |
| Heart |
- |
Table 3.
Biochemical parameters of CEG-GZL20.
Table 3.
Biochemical parameters of CEG-GZL20.
| Reaction |
Indole |
Methyl Red |
VP |
Citrate |
| +/- |
- |
- |
- |
- |
| |
|
|
|
|
Table 4.
Results of Antibiotic-sensitivity test using disk diffusion susceptibility test with 20 common antimicrobial agents.
Table 4.
Results of Antibiotic-sensitivity test using disk diffusion susceptibility test with 20 common antimicrobial agents.
| Antimicrobial Agents |
Diameter of zone of inhibition (ZOI) (mm) |
Amikacin
Ampicillin
Bacitracin
Cephalothin
Chloramphenicol
Clindamycin
Gentamicin
Kanamycin
Lincomycin
Methicillin
Nalidixic acid |
|
8.10
9.20
8.15
8.50
9.10
7.90
15.80
9.45
7.00
8.20
7.65 |
Neomycin
Nitrofurantoin
Penicillin
Polymyxin
Streptomycin
Sulfonamides
Tetracycline
Vancomycin |
10.80
9.40
9.60
10.00
9.40
8.50
18.90
9.90 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).