Submitted:
10 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
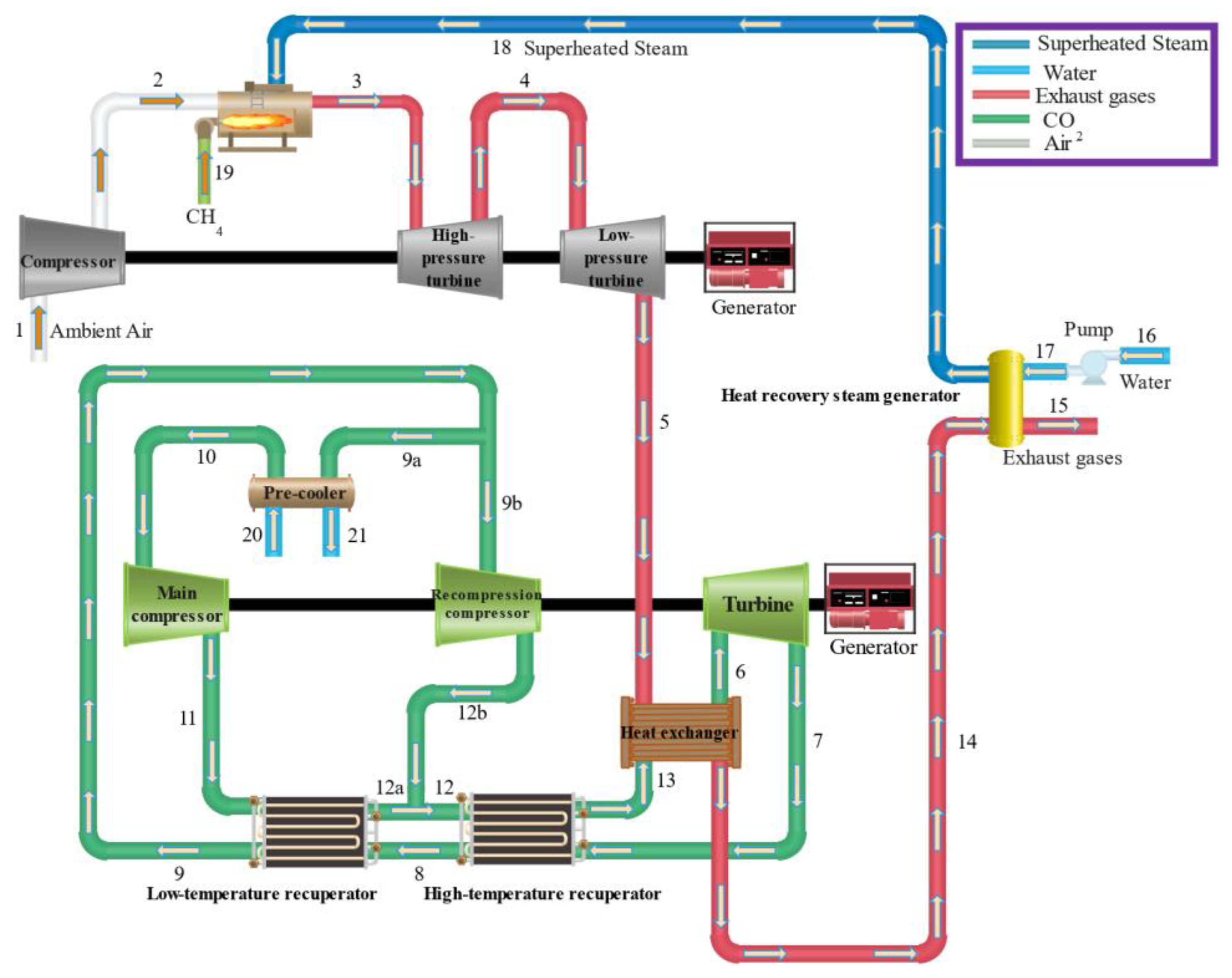
2. Description of system
- All gases are assumed ideal with specific heat and enthalpy changes depending on temperature, except for injected steam.
- Nitrogen and oxygen compression factors are assumed to be ideal even at the lowest temperature and highest pressure of the analysis.
- Due to thermodynamic restrictions, the turbine inlet temperature cannot exceed 1440 K.
- The air entering the compressor is considered completely dry and contains 21% oxygen and 79% kmol nitrogen on a molar basis.
- The combustion chamber efficiency in the gas turbines utilizing natural gas and methane in gas phases is very high and, in most studies, a value of 99% is considered.
- Combustion is considered to be steady and the CC is considered a well-stirred reactor (WSR).
- The temperature of combustion is based slightly on the stoichiometric rich side. This is done because Lefebvre [24] showed for a fixed enthalpy of reactants that the lower is the product mixture average specific heat, the higher is the resulting flame temperature because of the richer average specific heat for the products.
- Pressure drops due to friction are negligible [25].
- Pressure losses and heat losses in all heat exchangers and pipelines can be disregarded [22].
| T0=298.15 K | Pr1=10 | T10=305.15 K | ηc,is=0.87 |
| P0=101.325 kPa | Tfuel=298.15 K | P10=7400 kPa | ηcc=0.99 |
| T1=298.15 K | Ts=573.15 K | ε LTR =0.85 | ηt,is=0.89 |
| P1=101.325 kPa | s=5% | PRc=2.2-4.2 | ηp,is=0.70 |
| 1=1 kg/s | ϕ=0.4017 | ηt,is,Bottom=0.9 | ε HTR=0.85 |
| Pexh=101.325 kPa | TIT=1300 K | ηis,mc,rc=0.85 |
3. Modeling and simulation
3.1. Energy analysis
3.1.1. Combustion modeling
3.1.1.1. Combustion process with steam injection
3.1.1.2. Combustion process without steam injection
3.1.2. Analysis of expansion
3.2. Exergy analysis
4. Results and discussion
4.1. Validation
4.1.1. Combustion and chemical equilibrium equation
4.1.2. SCO2 subsystem
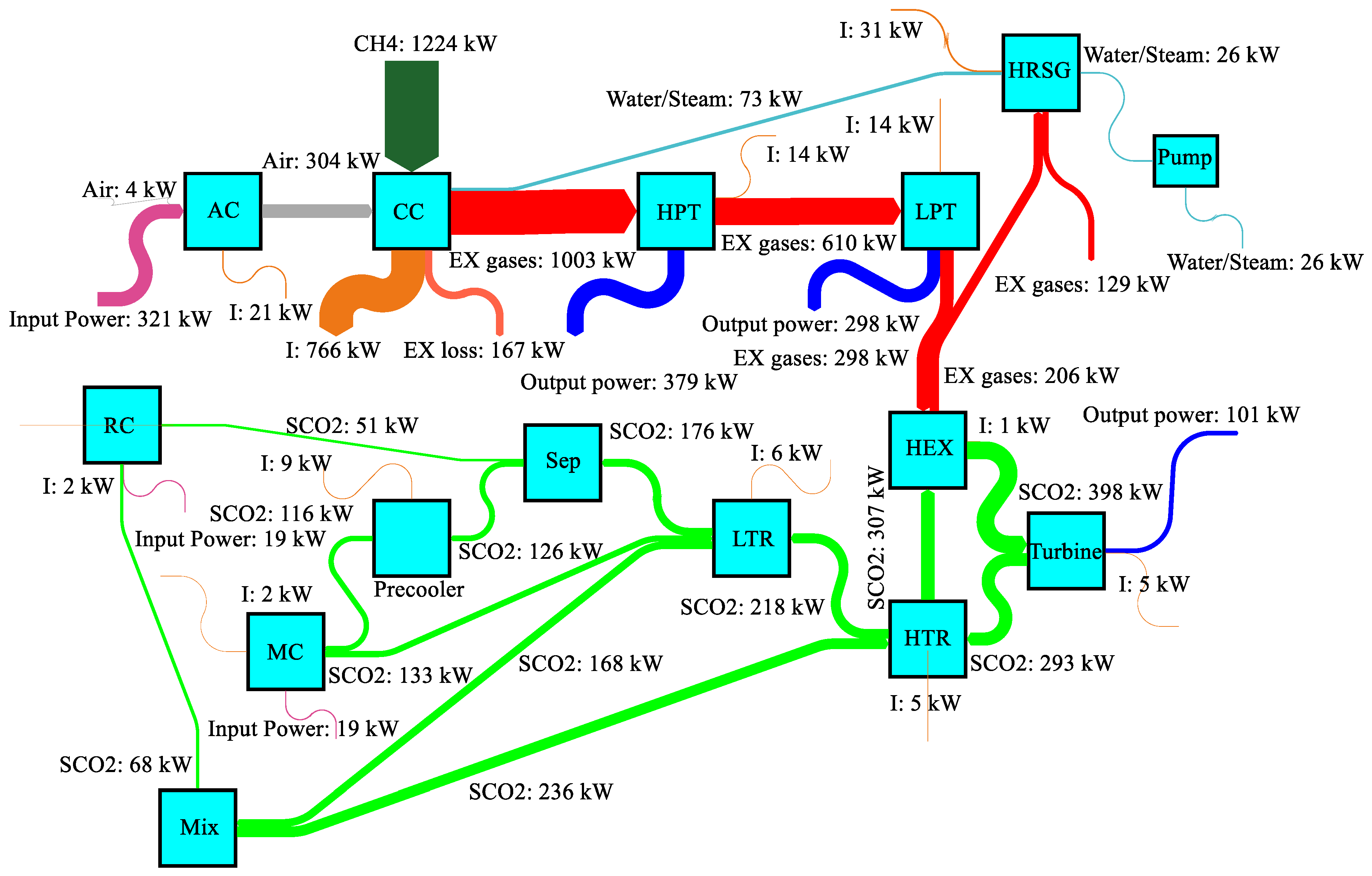
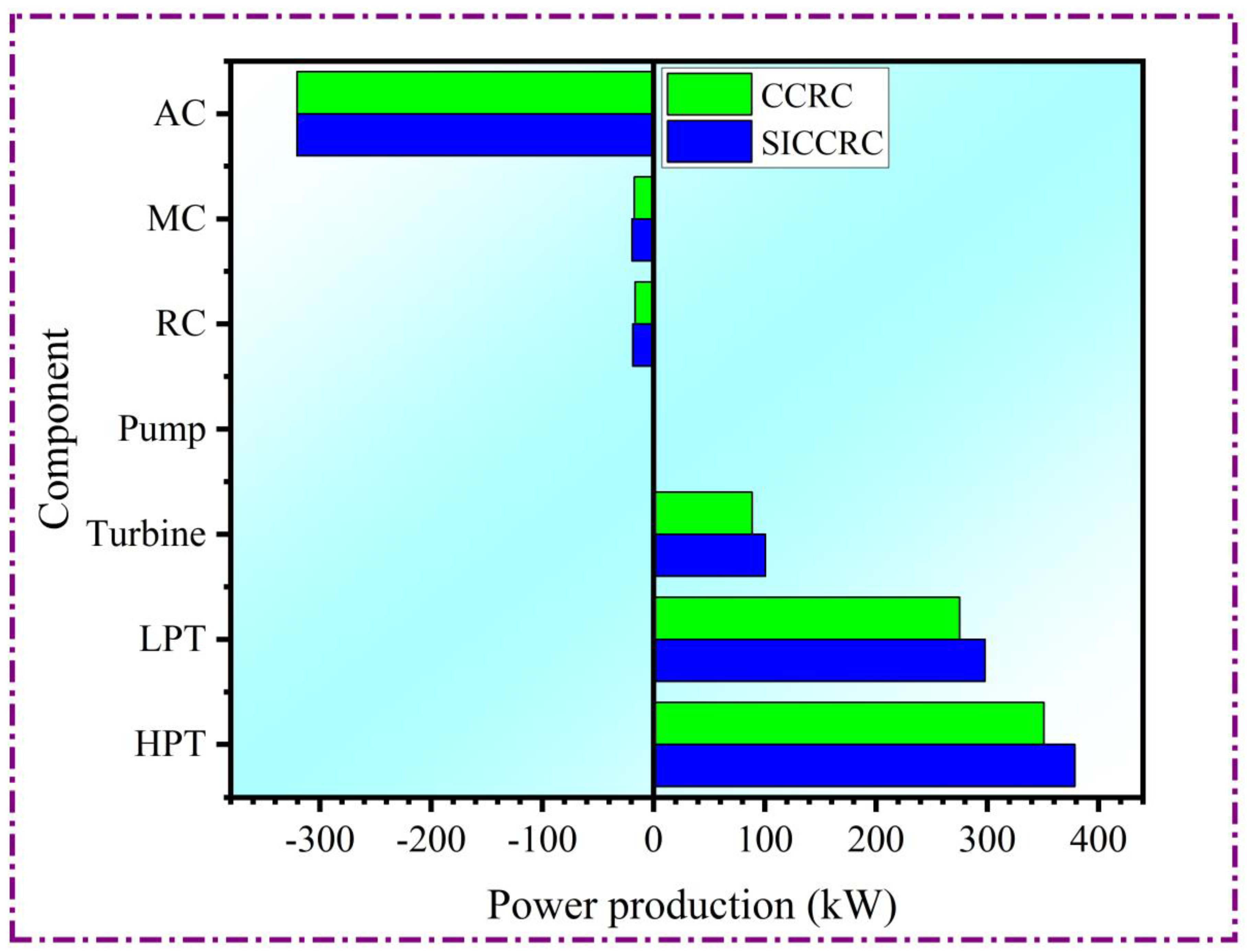
4.2. Power generation system case study
4.3. Parametric study
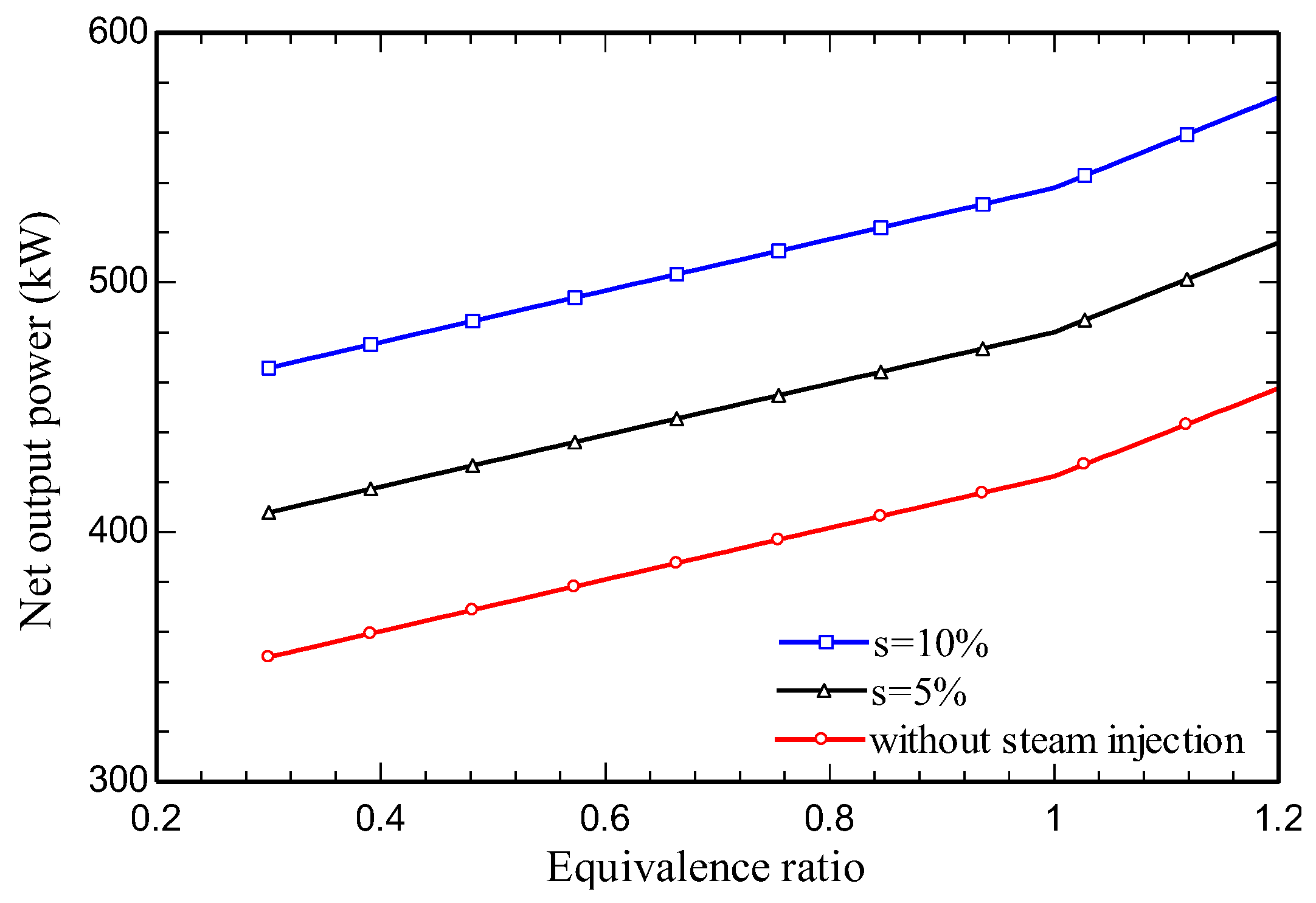
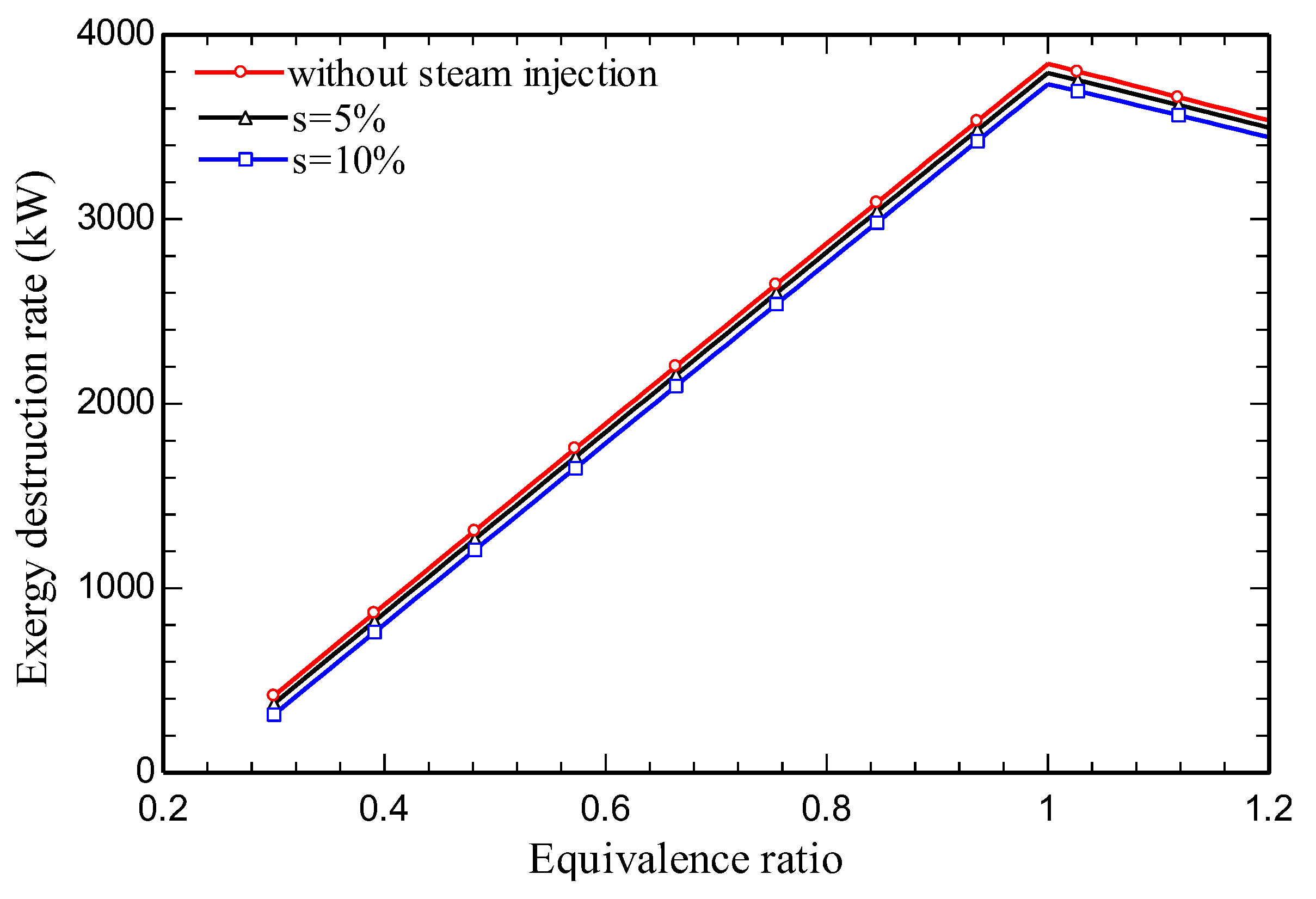
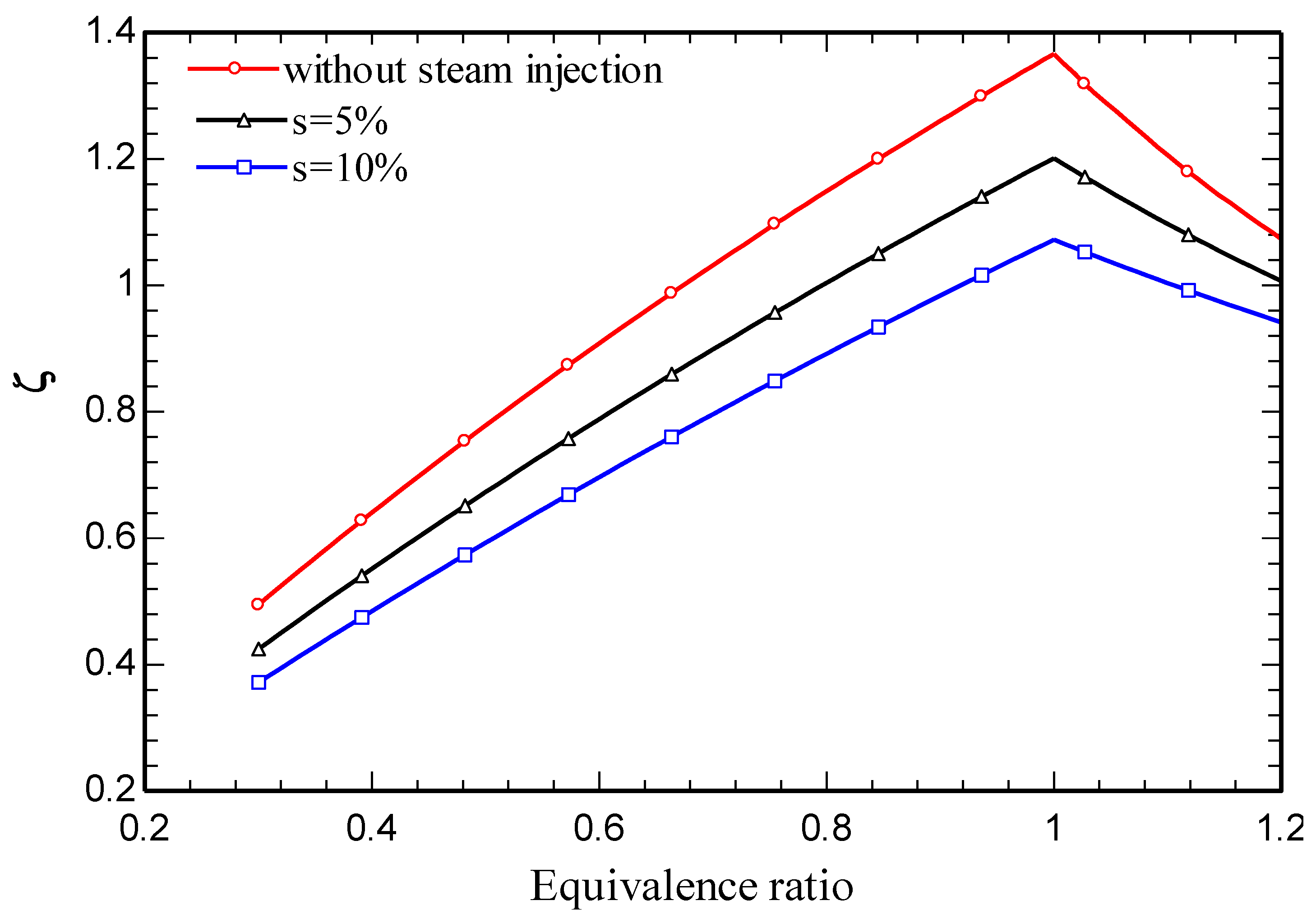
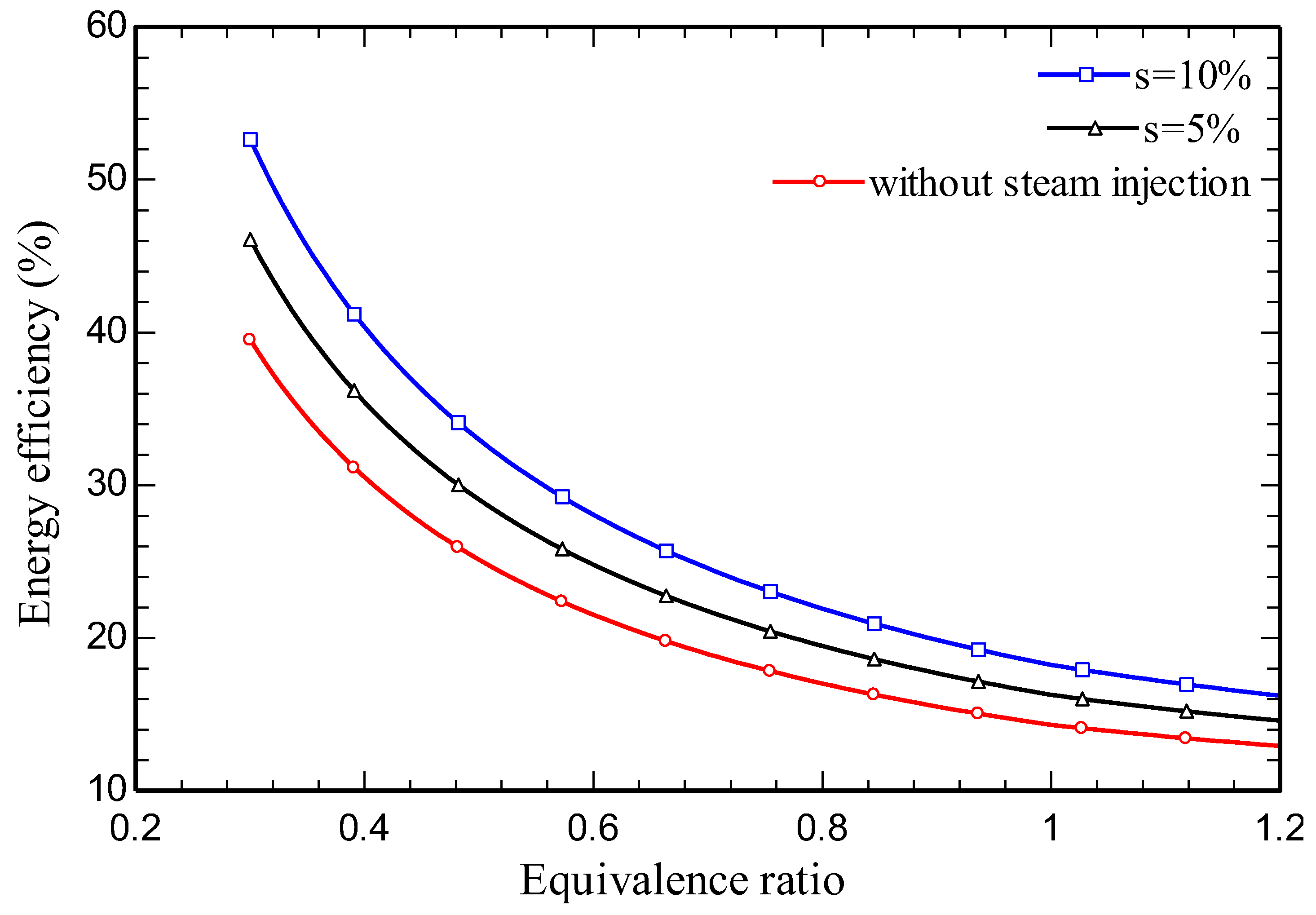
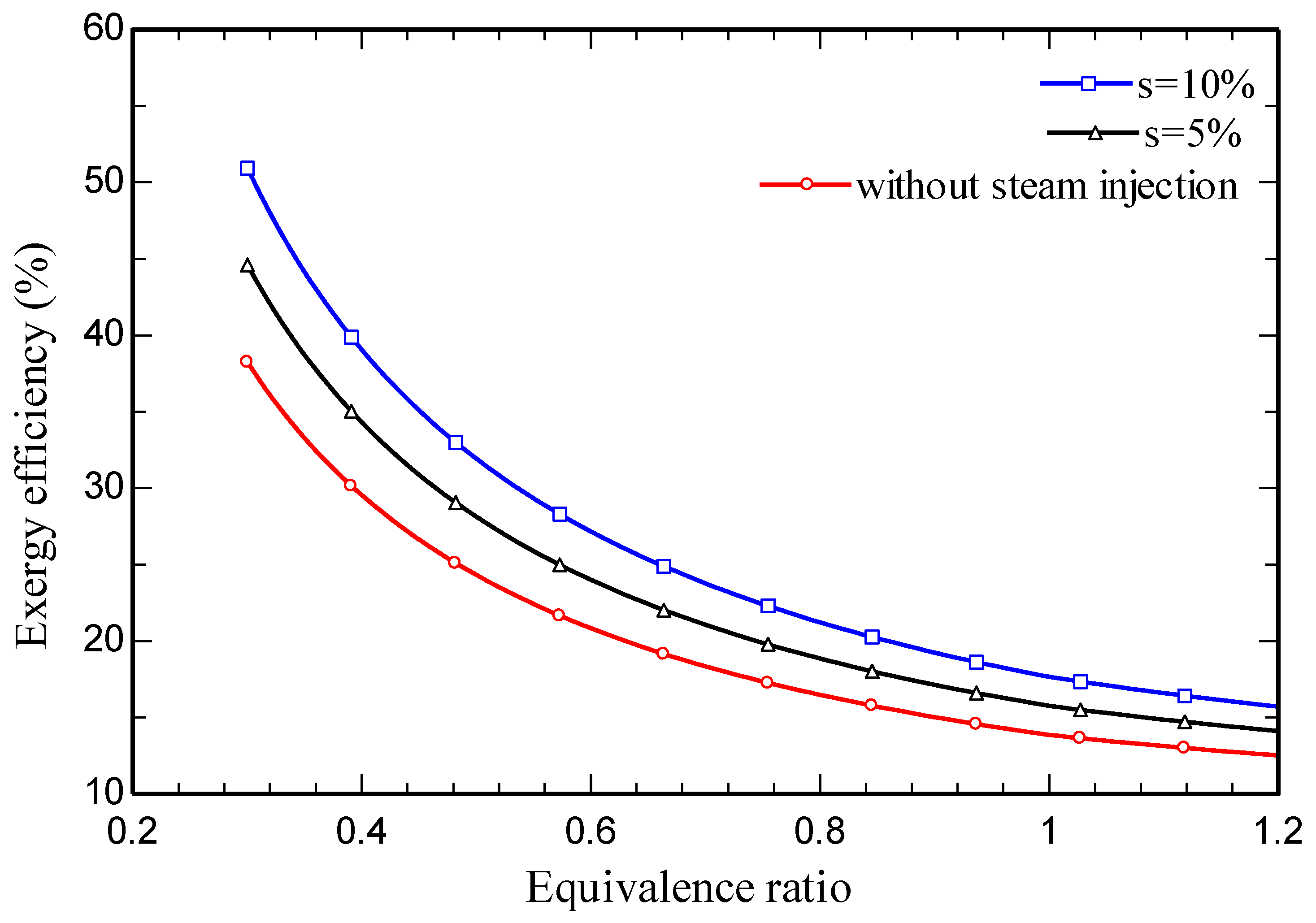
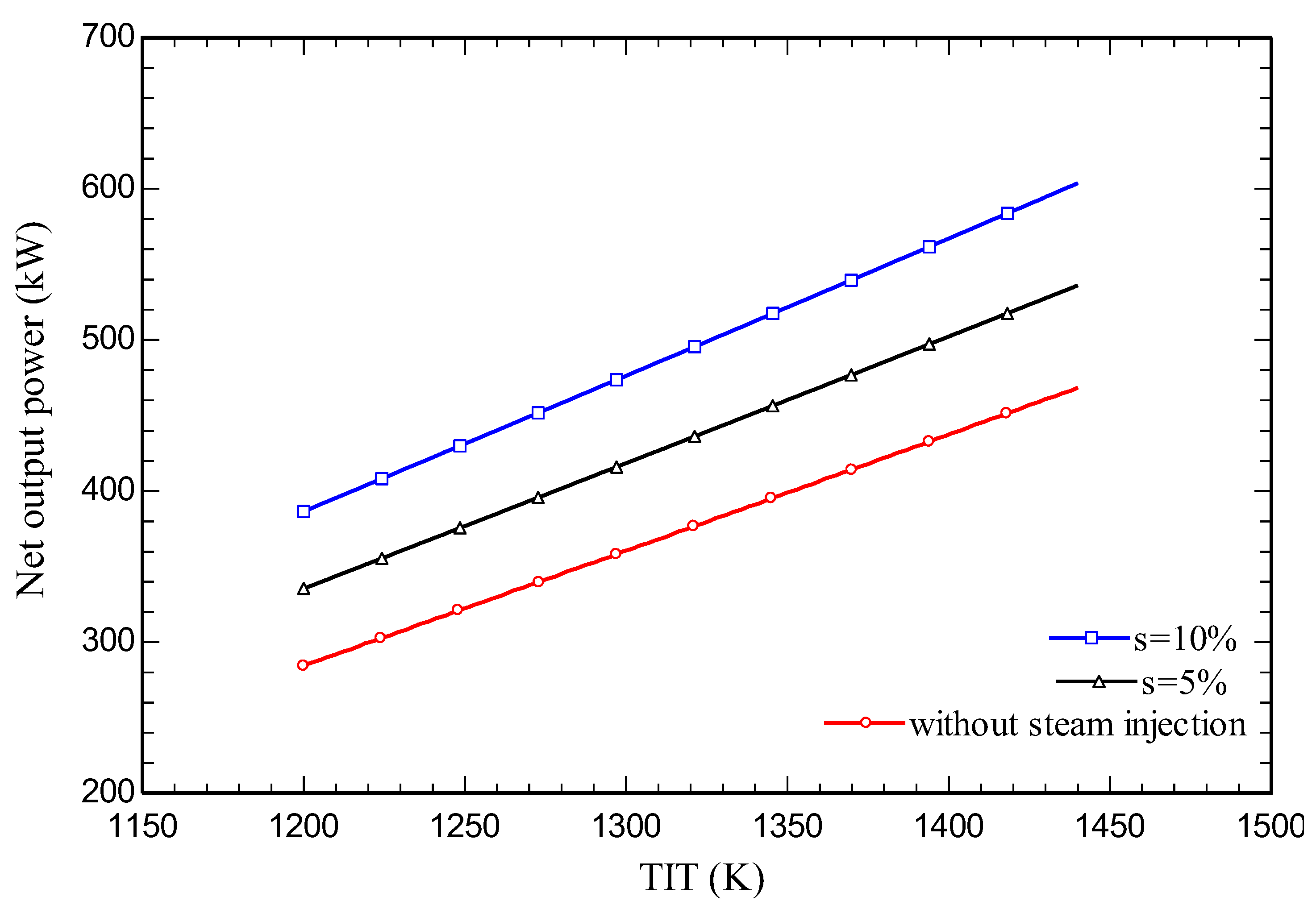
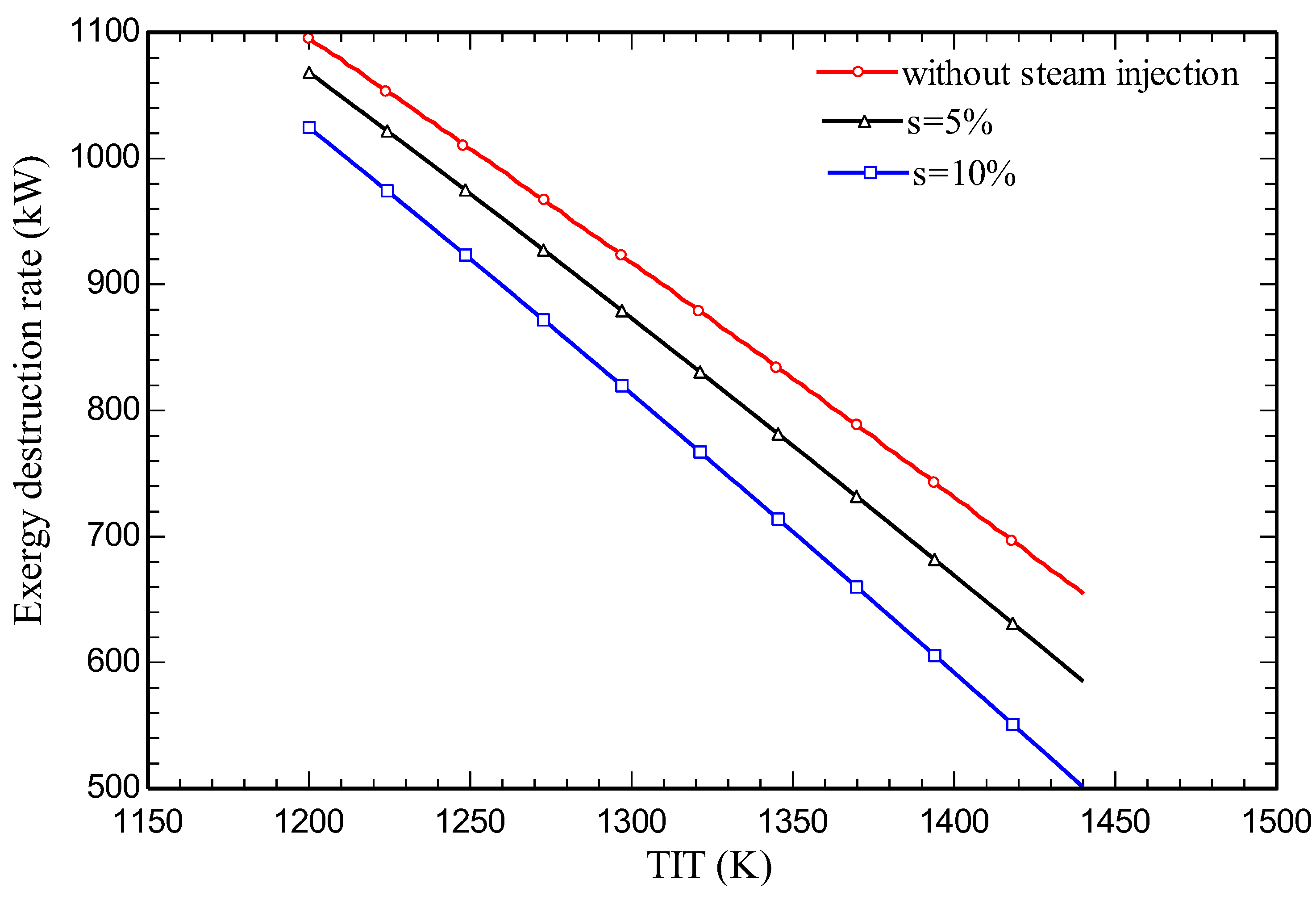
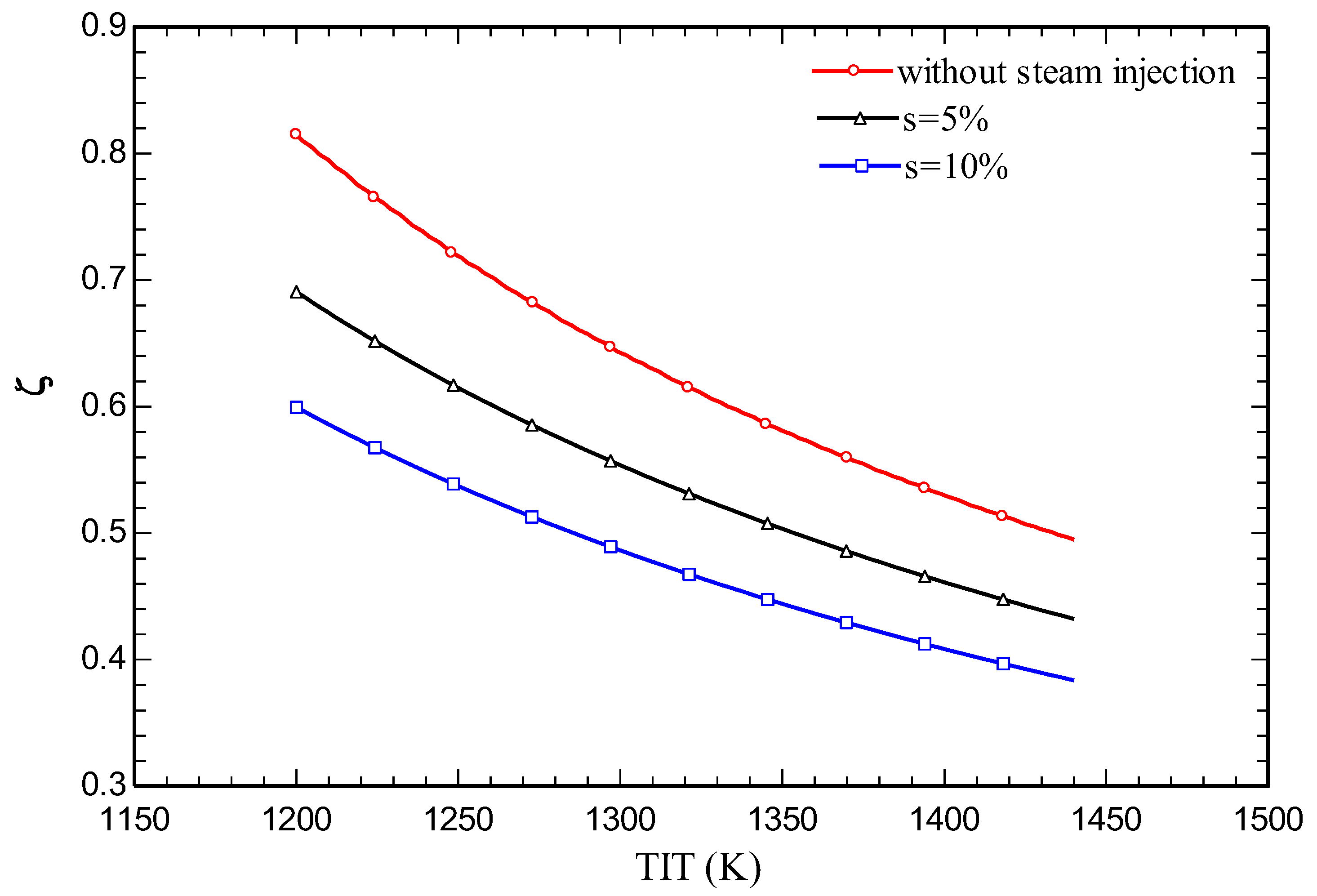
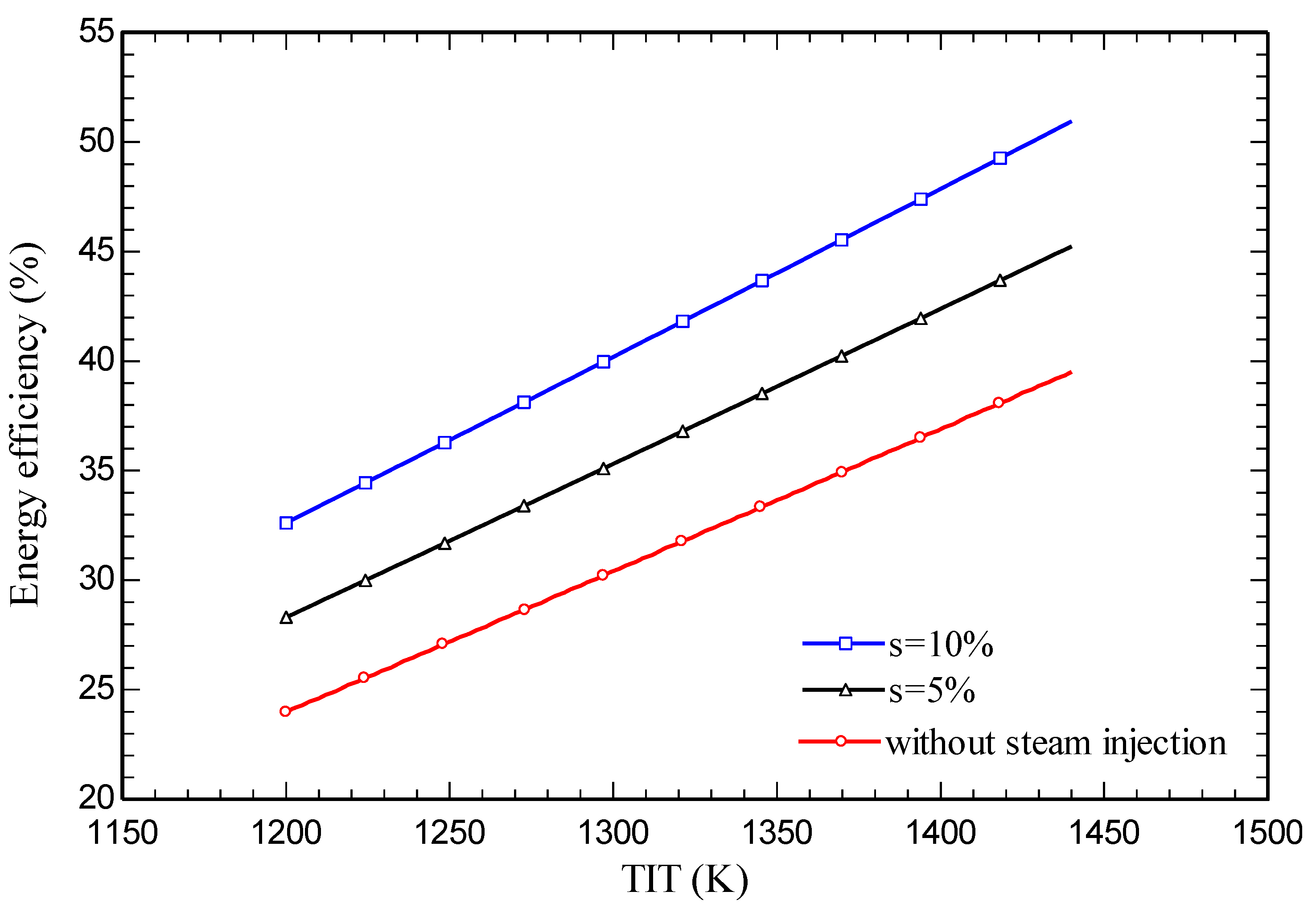
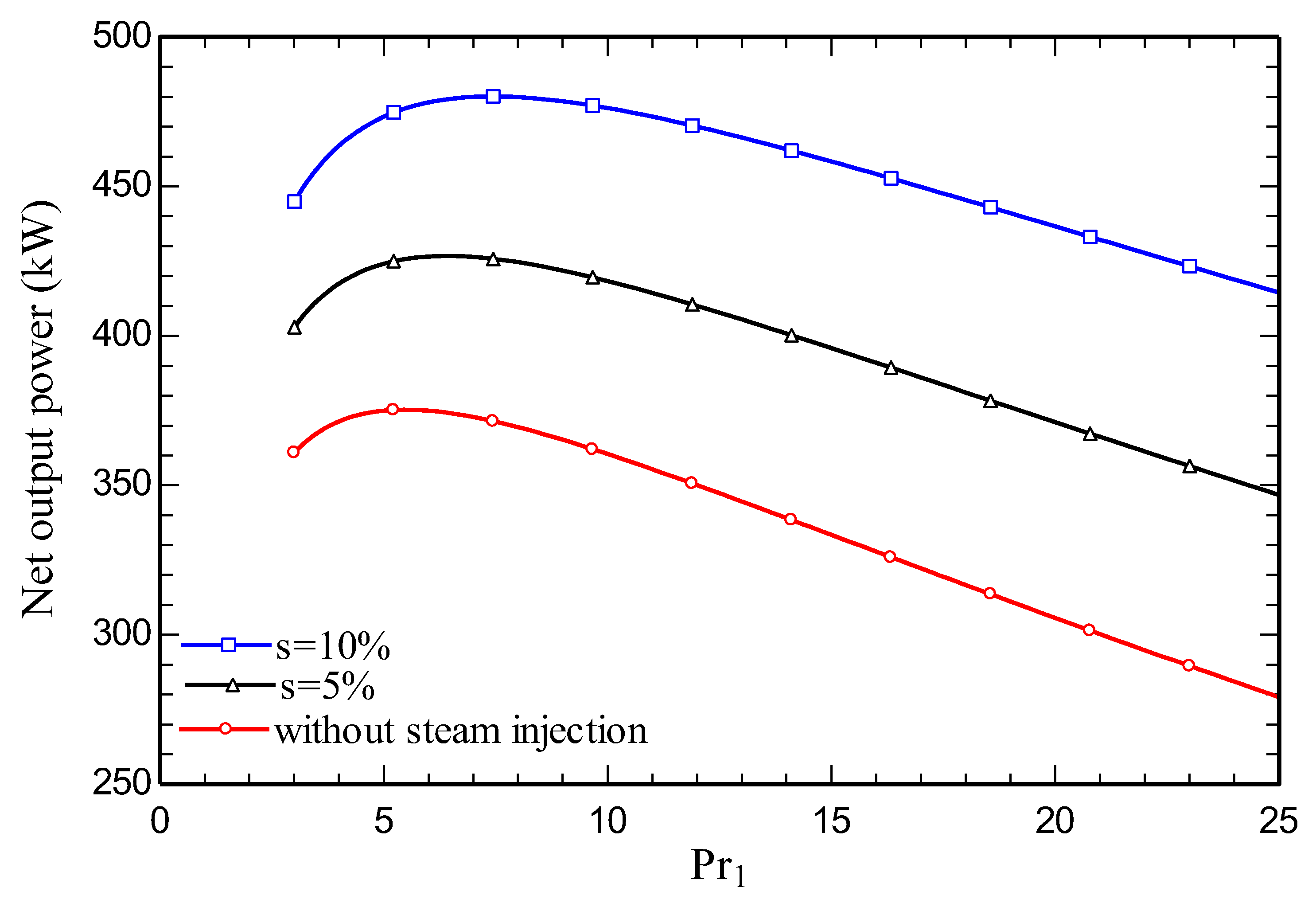
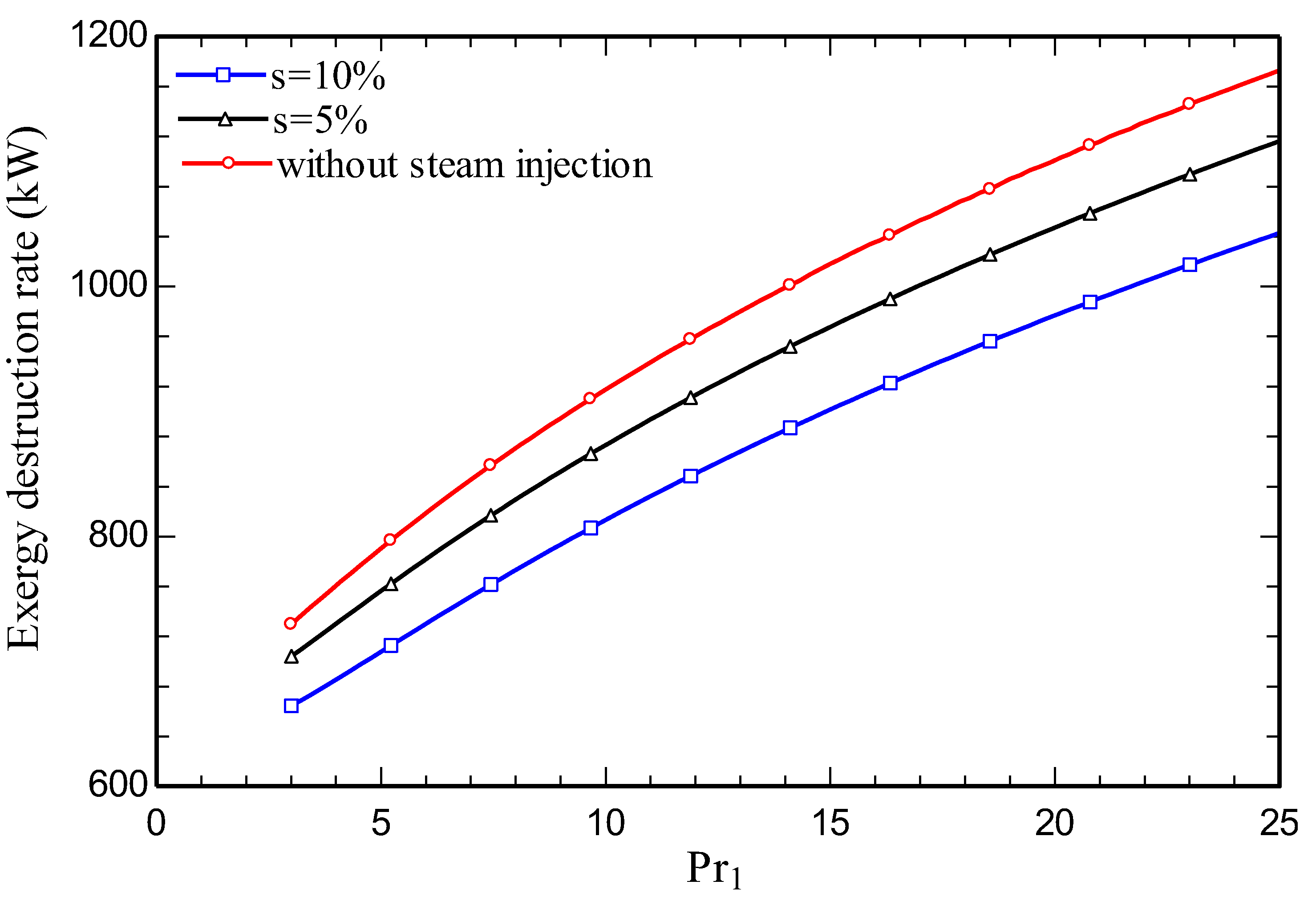
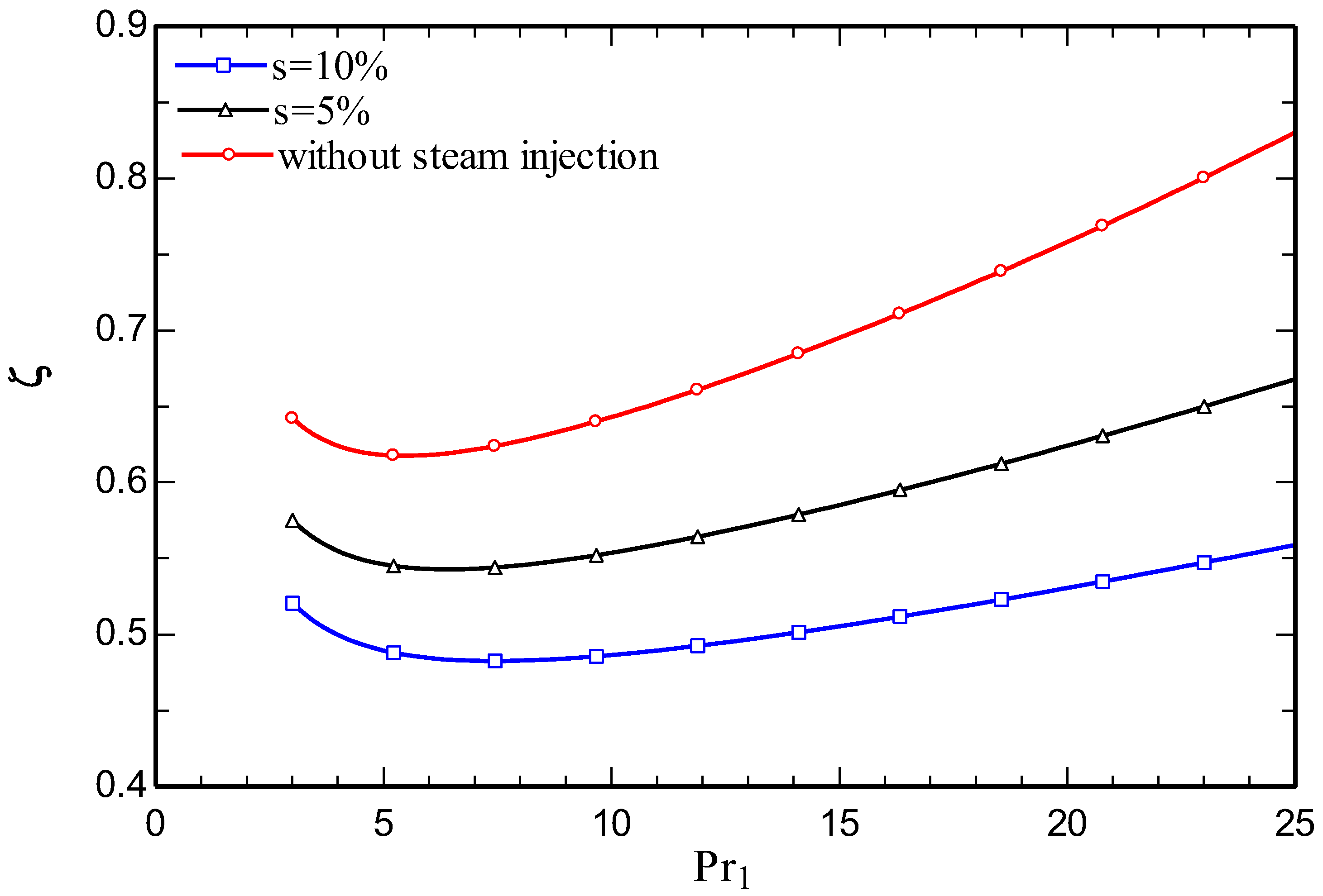
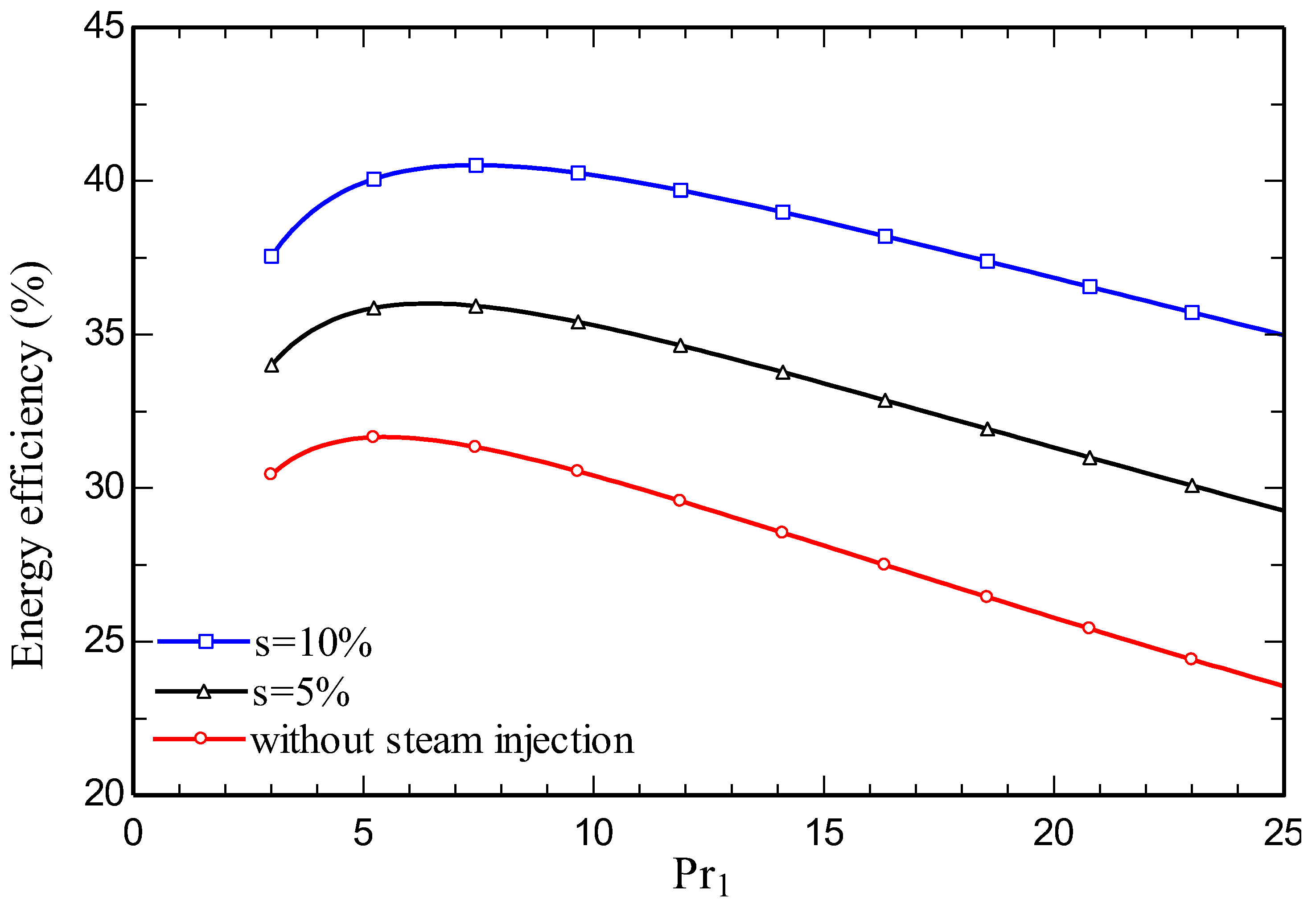
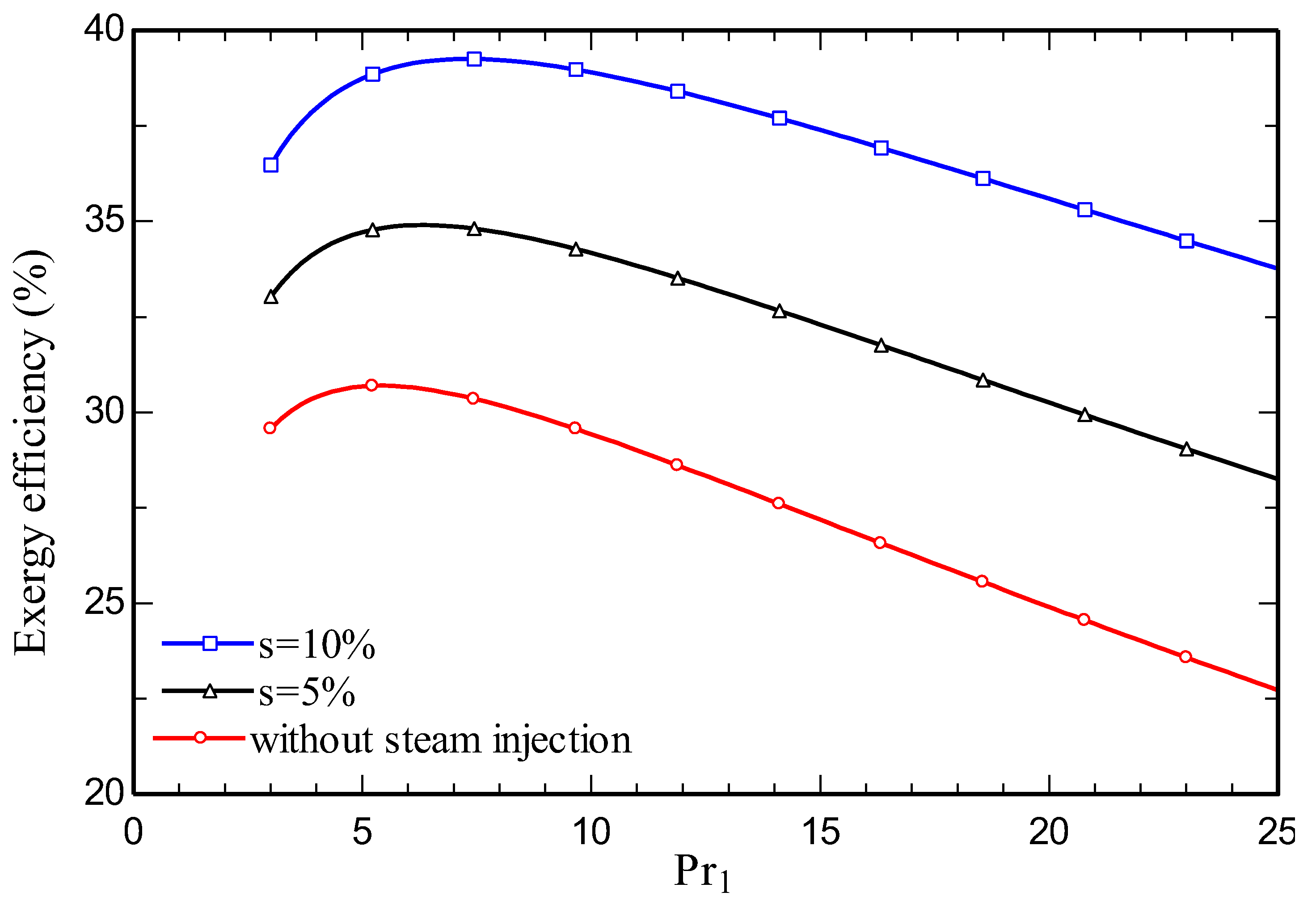

5. Conclusions
- Increasing the amount of steam injection improves the system net output power and lowers the exergy destruction rate. Moreover, it reduces the carbon dioxide emission index.
- Steam injection in SIGTSC reduces the heat loss of the combustion chamber compared to the GTSC.
- Energy and exergy efficiencies of 35.3% and 34.1% respectively are obtained for the SIGTSC, which are greater than the corresponding values for the GTSC: 30.4% and 29.4%. Steam injection improves the thermodynamic efficiency.
- Due to this combustion chamber’s design temperature limitations for this configuration, TIT can only vary within a certain range. In addition, at 1440 K, the CC is considered almost adiabatic.
Nomenclature
| CC | Combustion chamber |
| Chemical flow exergy rate (kW) | |
| Outlet exergy flow rate (kW) | |
| Inlet exergy flow rate (kW) | |
| Standard chemical exergy of an ideal gas | |
| Thermodynamic flow exergy (kW) | |
| GTSC | Gas turbine with supercritical carbon dioxide |
| Molar Gibbs function (kJ/kmol) | |
| Enthalpy rate (kW) | |
| HEX | Heat exchange |
| HRSG | Heat recovery steam generator |
| HTR | High temperature recuperator |
| Internal irreversibility rate (kW) | |
| Equilibrium constant | |
| LHV | Lower heating value |
| LTR | Low temperature recuperator |
| Air mass flow rate (kg/s) | |
| P0 | Ambient pressure (kPa) |
| P1 | Compressor inlet pressure (kPa) |
| P10 | State 10 pressure (kPa) |
| Pexh | Exhaust pressure (kPa) |
| Pr1 | Air compressor pressure ratio |
| PRc | Bottom cycle pressure ratio |
| Heat transfer rate (kW) | |
| s | Steam injection ratio (%) |
| SCO2 | Supercritical carbon dioxide |
| SIGTSC | Steam injection gas turbine with supercritical carbon dioxide |
| STIG | Steam injection gas turbine |
| T0 | Ambient temperature (K) |
| T1 | Air compressor inlet temperature (K) |
| T10 | State 10 temperature (K) |
| Tfuel | Fuel temperature (K) |
| Product temperature (K) | |
| Ts | Steam temperature (K) |
| TIT | Turbine inlet temperature (K) |
| Production or consumption power (kW) | |
| WSR | Well-stirred reactor |
| x | Molar injection ratio of H2O to air |
| Molar fraction of species i in a mixture | |
| ε | Molar air-fuel ratio |
| ϕ | Equivalence ratio |
| Number of moles of combustion products | |
| ε HTR | Heat exchange efficiency for HTR (%) |
| ε LTR | Heat exchange efficiency for LTR (%) |
| η c,is | Air compressor isentropic efficiency (%) |
| η cc | Combustion chamber efficiency (%) |
| η t,is | Turbine isentropic efficiency (%) |
| η p,is | Pump isentropic efficiency (%) |
| η t, is, Bottom | Bottom cycle turbine isentropic efficiency (%) |
| η is, mc,rc | Bottom cycle compressor isentropic efficiency (%) |
| ηⅠ | Energy efficiency (%) |
| ηⅡ | Exergy efficiency (%) |
| Carbon dioxide emission index |
References
- Competitiveness of US Gas Turbine Manufacturers. Gas Turbine Assoc 2013. https://gasturbine.org/docs/newdocs/YAGTP4602_GTA White Paper_041013 final.pdf (accessed April 13, 2023).
- Jansohn P. Modern Gas Turbine Systems: High Efficiency, Low Emission, Fuel Flexible Power Generation. Elsevier Science; 2013.
- Kayadelen H, Ust Y. Performance and environment as objectives in multi-criterion optimization of steam injected gas turbine cycles. Appl Therm Eng 2014;71:184–196. [CrossRef]
- Lu M, Li D, Xie K, Sun G, Fu Z. Investigation of flame evolution and stability characteristics of H2-enriched natural gas fuel in an industrial gas turbine combustor. Fuel 2023;331:125938. [CrossRef]
- Ren J, Qian Z, Fei C, Lu D, Zou Y, Xu C, et al. Thermodynamic, exergoeconomic, and exergoenvironmental analysis of a combined cooling and power system for natural gas-biomass dual fuel gas turbine waste heat recovery. Energy 2023;269:126676. [CrossRef]
- Kayadelen H, Ust Y. Thermoenvironomic evaluation of simple, intercooled, STIG, and ISTIG cycles. Int J Energy Res 2018;42. [CrossRef]
- Kayadelen H, Ust Y, Başhan V. Thermodynamic performance analysis of state of the art gas turbine cycles with inter-stage turbine reheat and steam injection. Energy 2021;222:119981. [CrossRef]
- Yeranee K, Rao Y. A review of recent studies on rotating internal cooling for gas turbine blades. Chinese J Aeronaut 2021;34:85–113. [CrossRef]
- Javaherian A, Yari M, Gholamian E, Carton JG, Mehr AS. Proposal and comprehensive analysis of power and green hydrogen production using a novel integration of flame-assisted fuel cell system and Vanadium-Chlorine cycle: An application of multi-objective optimization. Energy Convers Manag 2023;277:116659. [CrossRef]
- Sinha AA, Saini G, Sanjay, Shukla AK, Ansari MZ, Dwivedi G, et al. A novel comparison of energy-exergy, and sustainability analysis for biomass-fueled solid oxide fuel cell integrated gas turbine hybrid configuration. Energy Convers Manag 2023;283:116923. [CrossRef]
- Ryu B, Duong PA, Kang H. Comparative analysis of the thermodynamic performances of solid oxide fuel cell–gas turbine integrated systems for marine vessels using ammonia and hydrogen as fuels. Int J Nav Archit Ocean Eng 2023:100524. [CrossRef]
- Bolland O, Stadaas J. Comparative Evaluation of Combined Cycles and Gas Turbine Systems With Water Injection, Steam Injection, and Recuperation. J Eng Gas Turbines Power-Transactions Asme - J ENG GAS TURB POWER-T ASME 1995;117. [CrossRef]
- Jonsson M, Yan J. Humidified gas turbines - A review of proposed and implemented cycles. Energy 2005;30:1013–78. [CrossRef]
- Roumeliotis I, Mathioudakis K. Evaluation of water injection effect on compressor and engine performance and operability. Appl Energy 2010;87:1207–16. [CrossRef]
- Eshati S, Abu A, Laskaridis P, Khan F. Influence of water–air ratio on the heat transfer and creep life of a high pressure gas turbine blade. Appl Therm Eng 2013;60:335–47. [CrossRef]
- Renzi M, Riolfi C, Baratieri M. Influence of the Syngas Feed on the Combustion Process and Performance of a Micro Gas Turbine with Steam Injection. Energy Procedia 2017;105:1665–70. [CrossRef]
- Mazzucco A, Rokni M. Thermo-economic analysis of a solid oxide fuel cell and steam injected gas turbine plant integrated with woodchips gasification. Energy 2014;76. [CrossRef]
- Amiri Rad E, Kazemiani P. Thermo-environmental and economic analyses of an integrated heat recovery steam-injected gas turbine. Energy 2017;141. [CrossRef]
- Salem Ahmed M, Mohamed Aly H. Performance characteristics of modified gas turbine cycles with steam injection after combustion exit. Int J Energy Res 2012;36. [CrossRef]
- Bahrami S, Ghaffari A, Thern M. Improving the Transient Performance of the Gas Turbine by Steam Injection during Frequency Dips. Energies 2013;6:5283–96. [CrossRef]
- Lei S, Wang D, Xie Y. Energy, Exergy and exergoeconomic analysis of two supercritical CO2 cycles for waste heat recovery of gas turbine. Appl Therm Eng 2021;196:117337. [CrossRef]
- Wu C, Wang S, Feng X, Li J. Energy, exergy and exergoeconomic analyses of a combined supercritical CO2 recompression Brayton/absorption refrigeration cycle. Energy Convers Manag 2017;148:360–77. [CrossRef]
- Akbari AD, Mahmoudi SMS. Thermoeconomic analysis & optimization of the combined supercritical CO2 (carbon dioxide) recompression Brayton/organic Rankine cycle. Energy 2014;78:501–12. [CrossRef]
- Lefebvre AH, Ballal DR. Gas Turbine Combustion: Alternative Fuels and Emissions, Third Edition. CRC Press; 2010.
- Kim Y, Kim CG, Favrat D. Transcritical or supercritical CO2 cycle using both low-and high-temperature heat sources. Energy 2012;43:402–15. [CrossRef]
- Kayadelen HK, Ust Y. Prediction of equilibrium products and thermodynamic properties in H2O injected combustion for CαHβOγNδ type fuels. Fuel 2013;113:389–401. [CrossRef]
- Kayadelen HK. A multi-featured model for estimation of thermodynamic properties, adiabatic flame temperature and equilibrium combustion products of fuels, fuel blends, surrogates and fuel additives. Energy 2018;143:241–56. [CrossRef]
- Poullikkas A. An overview of current and future sustainable gas turbine technologies. Renew Sustain Energy Rev 2005;9:409–43. [CrossRef]
- Moran MJ, Shapiro HN, Boettner DD, Bailey MB. Fundamentals of Engineering Thermodynamics, 9th Edition. Wiley; 2018.
- Bejan A, Tsatsaronis G, Moran MJ. Thermal Design and Optimization. Wiley; 1995.
- Wark K. Advanced Thermodynamics for Engineers. McGraw-Hill; 1995.
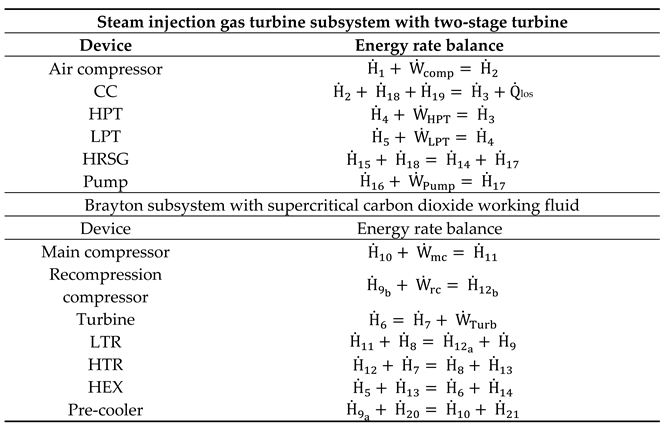
| Steam injection gas turbine subsystem with two-stage turbine | |
|---|---|
| Component | Exergy rate balance |
| Air compressor | |
| CC | |
| HPT | |
| LPT | |
| HRSG | |
| Pump | |
| Brayton subsystem with supercritical carbon dioxide working fluid | |
| Component | Exergy rate balance |
| Main compressor | |
| Recompression compressor | |
| Turbine | |
| LTR | |
| HTR | |
| HEX | |
| Pre-cooler | |
| Combustion product | Molar fraction obtained in current study with φ=0.6 | Molar percentage in reference [26] with φ=0.6 | Molar fraction obtained in current study with φ=1.2 | Molar percentage in reference [26] with φ=1.2 |
|---|---|---|---|---|
| CO2 | 0.05148 | 0.05151 | 0.0630 | 0.0631 |
| H2O | 0.2343 | 0.2338 | 0.2789 | 0.2786 |
| N2 | 0.6451 | 0.6455 | 0.5944 | 0.5948 |
| O2 | 0.06821 | 0.06824 | 4.12 E-06 | 1.42E-07 |
| CO | 1.76 E-06 | 3.22E-07 | 0.0317 | 0.0316 |
| H2 | 2.9 E-06 | 5.34E-07 | 0.0314 | 0.0315 |
| H | 4.927E-08 | 3.84E-09 | 2.37 E-04 | 4.35E-05 |
| O | 1.84 E-06 | 3.36E-07 | 1.08 E-06 | 3.69E-08 |
| OH | 1.52 E-04 | 5.46E-05 | 2.25 E-04 | 3.62E-05 |
| NO | 7.70 E-04 | 8.35E-04 | 2.71 E-05 | 5.39E-06 |
| State no. | Present work temperature [°C] |
Ref. [22] temperature [°C] |
Present work pressure [kPa] |
Ref. [22] pressure [kPa] |
Present work mass flow rate [kg/s] | Ref. [22] mass flow rate [kg/s] |
Present work exergy rate [MW] |
Ref. [22] exergy rate [MW] |
|
|---|---|---|---|---|---|---|---|---|---|
| 6 | 550 | 550 | 207.2 | 207.2 | 2939 | 2938.18 | 1557 | 1556.5 | |
| 7 | 428 | 428.01 | 74 | 74 | 2939 | 2938.18 | 1145 | 1144.5 | |
| 8 | 257.6 | 257.48 | 74 | 74 | 2939 | 2938.18 | 851.83 | 851.29 | |
| 9 | 119.5 | 119.36 | 74 | 74 | 2939 | 2938.18 | 690.45 | 690.05 | |
| 10 | 32 | 32 | 74 | 74 | 2098 | 2096.18 | 453.08 | 452.68 | |
| 11 | 97.03 | 96.88 | 207.2 | 207.2 | 2098 | 2096.18 | 520.13 | 519.55 | |
| 12 | 229.9 | 229.72 | 207.2 | 207.2 | 2939 | 2938.18 | 924.24 | 923.60 | |
| 13 | 384.4 | 384.36 | 207.2 | 207.2 | 2939 | 2938.18 | 1200 | 1199.13 | |
| State no. | Temperature [K] |
Pressure [kPa] |
Molar enthalpy [kJ/kmol] |
Molar entropy [kJ/kmol-K] |
Mass flow rate [kg/s] | Exergy rate [kW] |
|---|---|---|---|---|---|---|
| 1 | 298.2 | 101.3 | 0 | 198.6 | 1 | 4.473 |
| 2 | 608.7 | 1013 | 9246 | 200.6 | 1 | 303.9 |
| 3 | 1300 | 1013 | -2423 | 228.7 | 1.023 | 912.9 |
| 4 | 1026 | 320.4 | -12138 | 229.9 | 1.023 | 549.1 |
| 5 | 800.6 | 101.3 | -19755 | 231.1 | 1.023 | 261.1 |
| 6 | 823.2 | 20720 | 23230 | -0.241 | 0.01503 | 350.5 |
| 7 | 701.2 | 7400 | 17340 | 0.701 | 0.01503 | 257.8 |
| 8 | 530.8 | 7400 | 8789 | -13.26 | 0.01503 | 191.8 |
| 9 | 392.7 | 7400 | 1849 | -28.43 | 0.01503 | 155.4 |
| 10 | 305.2 | 7400 | -5628 | -50.71 | 0.01073 | 102 |
| 11 | 370.2 | 20720 | -4028 | -50.06 | 0.01073 | 117.1 |
| 12 | 503 | 20720 | 5695 | -27.27 | 0.01503 | 208.1 |
| 13 | 657.6 | 20720 | 14246 | -12.42 | 0.01503 | 270.1 |
| 14 | 686.2 | 101.3 | -23494 | 226.1 | 1.023 | 180.3 |
| 15 | 298.2 | 1013 | -74595 | 167.1 | 0.02346 | 1225 |
| 16 | 298.2 | 101.3 | 1889 | 6.61 | 0.1065 | 0 |
| 17 | 308.2 | 101.3 | 2642 | 9.096 | 0.1065 | 1.316 |
| State no. | Temperature [K] |
Pressure [kPa] |
Molar enthalpy [kJ/kmol] |
Molar entropy [kJ/kmol-K] |
Mass flow rate [kg/s] |
Exergy rate [kW] |
|---|---|---|---|---|---|---|
| 1 | 298.2 | 101.3 | 0 | 198.6 | 1 | 4.473 |
| 2 | 608.7 | 1013 | 9246 | 200.6 | 1 | 303.9 |
| 3 | 1300 | 1013 | -16709 | 229.7 | 1.073 | 1003 |
| 4 | 1029 | 320.4 | -26443 | 230.9 | 1.073 | 610.1 |
| 5 | 806.4 | 101.3 | -34104 | 232.1 | 1.073 | 298.2 |
| 6 | 823.2 | 20720 | 23230 | -0.241 | 0.01706 | 397.8 |
| 7 | 701.2 | 7400 | 17340 | 0.701 | 0.01706 | 292.5 |
| 8 | 530.8 | 7400 | 8789 | -13.26 | 0.01706 | 217.6 |
| 9 | 392.7 | 7400 | 1849 | -28.43 | 0.01706 | 176.4 |
| 10 | 305.2 | 7400 | -5628 | -50.71 | 0.01218 | 115.7 |
| 11 | 370.2 | 20720 | -4028 | -50.06 | 0.01218 | 132.9 |
| 12 | 503 | 20720 | 5695 | -27.27 | 0.01706 | 236.1 |
| 13 | 657.6 | 20720 | 14246 | -12.42 | 0.01706 | 306.5 |
| 14 | 687.3 | 101.3 | -38044 | 226.8 | 1.073 | 206.3 |
| 15 | 569.7 | 101.3 | -41829 | 220.8 | 1.073 | 129.1 |
| 16 | 298.2 | 101.3 | 1889 | 6.61 | 0.05 | 26.37 |
| 17 | 298.2 | 1013 | 1912 | 6.606 | 0.05 | 26.41 |
| 18 | 573.2 | 1013 | 54951 | 128.2 | 0.05 | 73.03 |
| 19 | 298.2 | 1013 | -74595 | 167.1 | 0.02345 | 1224 |
| 20 | 298.2 | 101.3 | 1889 | 6.61 | 0.1208 | 0 |
| 21 | 308.2 | 101.3 | 2642 | 9.096 | 0.1208 | 1.494 |
| Subsystem | Energy efficiency (%) |
Exergy efficiency (%) |
|---|---|---|
| GTSC system | ||
| STIG | 25.78 | 24.95 |
| SCO2 | 40.59 | 67.86 |
| Total | 30.41 | 29.43 |
| SIGTSC system | ||
| SISTIG | 30.06 | 29.09 |
| SCO2 | 40.59 | 67.65 |
| Total | 35.31 | 34.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





