Introduction
Proper adipose tissue (AT) maintenance through proliferation and differentiation of preadipocytes is essential for whole-body energy homeostasis (1,2). Accumulation of lipophilic persistent organic pollutants (POPs) and hypoxia are two factors that are recognized to adversely alter AT metabolism and to contribute to the development of obesity-related metabolic complications (3–5).
POPs are synthetic compounds that consist of many groups of halogenated compounds, such as polychlorinated biphenyls (PCBs). POPs, which bioaccumulate in the food chain and resist biodegradation, are commonly referred to as “metabolic disruptors” (6,7). Traditionally, 2,3,7,8-tetrachlorodibenzo- p- dioxin (TCDD) and dioxin-like PCBs have been reported to exhibit their toxicity by activating the aryl-hydrocarbon receptor (AhR), a transcription factor that regulates the expression of genes containing xenobiotic response element (XRE) in their promoter (8). TCDD and dioxin-like PCBs have been shown to alter the acquisition of adipocyte phenotype (also called “adipocyte differentiation”) and adipocyte metabolism (9,10)(9,10). For instance, it has been reported that 3T3-L1 cells and immortalized human preadipocytes (Normal Pre-Adipocytes, NPADs) exposure to dioxin and dioxin-like PCB126 prior to differentiation impaired subsequent adipocyte differentiation (11–13). In NPADS, impairment of adipocyte differentiation by PCB126 has later been associated to inflammation, specifically the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells activation (14).
Hypoxia, another important metabolic disruptor, is known to profoundly affect adipocyte functions (15,16). For instance, acute exposure (24h) to low oxygen tension is well known to decrease peroxisome proliferator-activated receptor γ (PPARγ) expression and to increase leptin secretion in differentiated human adipocytes (17–20). Acute hypoxia exposure also impacts the adipocyte’s lipid storage and mobilisation functions by reducing the activity of the lipoprotein lipase activity while increasing basal intracellular lipolysis (21,22). Moreover, exposure to acute hypoxia have been shown to increase GLUT1 facilitative glucose transporter gene and protein levels, glucose transport (17,23) as well as expression and release of inflammation-related adipokines (19,24,25) in human differentiated adipocytes. These cellular responses to hypoxia are mostly exerted via the genomic action of the hypoxia inducible factor 1a (HIF-1α), a transcription factor that is stabilized and activated when O2 levels drop below 4-5% (26–29).
Interestingly, hypoxia and dioxins signalling both exploit the ARNT transcription factor, which can be recruited by both AhR and HIF-1α thus establishing a significant foundation for a possible crosstalk between these two signalling pathways (5,30). We recently showed that differentiated human adipocytes co-exposed to hypoxia and PCB126 for 24h attenuates the transcription of CYP1A1, a detoxifying enzyme and a traditional marker of the xenobiotic response (19). In the same study, we reported that exposure to PCB126 or hypoxia alone or in combination increases IL-6 secretion in a dose dependent manner, which suggests that long-term co-exposure of adipocytes to hypoxia and POPs could favor the establishment of a pro-inflammatory state in the AT.
Considering the previously reported effects of dioxin-like PCBs and hypoxia on adipocytes, we exposed proliferating human preadipocytes to PCB126 and then differentiated them under hypoxia to determine how the combination of both stressors would affect adipocyte differentiation, glucose uptake and key inflammatory markers.
Methods
Pooled human subcutaneous preadipocytes from donors with a body mass index over 30 kg/m2 (SP-F-3) were obtained from Zenbio (Research Triangle Park, NC, USA) and cultured according to the manufacturer’s instructions. Cells suspended in Zenbio’s PM-1 culture media were seeded in culture-treated 96-well plates at a density of at least 40000 cells/cm2. Cells were then placed in a HeraCell 150iO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA) incubator maintained at 100% humidity and 5% CO2. After 3 days of proliferation, medium was replaced by Zenbio DM-2 to induce differentiation. After 7 days of differentiation, medium was partially replaced with Zenbio AM-1 in which cells remained until day 14 after induction.
During proliferation, preadipocytes were exposed to either 10uM PCB126 or DMSO (vector). Medium were not changed during the proliferation phase. Hypoxia exposure was initiated after 3 days of proliferation at the same time as culture media were switched to DM-2 containing no DMSO nor PCB126. Hypoxia exposures were carried out for 14 days in a HeraCell 150iO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA) maintained at 3% O2 with medical nitrogen and 5% CO2. During the differentiation phase, media were partially changed every other day to avoid acidification of the culture media by lactate accumulation under hypoxia. This procedure was also done for cells cultured under normoxia.
Cell viability was assessed daily by visual examination using a Zeiss Axiovert 40 C light microscope (Carl Zeiss, Göttingen, Germany) to detect anomalies such as cell detachment from culture ware. Twenty-four hours and 14 days following induction, cells expansion / viability was assessed by bioluminescent ATP quantification using CellTiterGlo 2 reagent from Promega (Madison, Wisconsin, USA). Because ATP content was affected by PCB126 and hypoxia, ATP measurement were refined by measuring the ADP:ATP ratio using the MAK135 assay kit from Millipore-Sigma (Winston Park Drive Oakville, ON, Canada).
Glucose uptake was measured using Promega bioluminescent GlucoseUptakeGlo assay based on 2-deoxyglucose (2-DG) uptake according to manufacturer instructions. Cells were first glucose starved by washing 3 times with Zenbio basal medium (BM-1) containing no glucose followed by incubation for 1.5 hour in BM-1 also without glucose. Insulin was added to relevant wells to a final concentration of 100nM 30 minutes before glucose uptake assessment. BM-1 was removed from wells and cells were incubated 12 minutes with 1mM 2-DG in PBS. The GLUT-specific inhibitor Glutor (31) was used at a final concentration of 1µM to confirm GLUT-specific glucose uptake. Glucose uptake was normalized to cellular ATP to account for the possible effects of treatments on cell expansion and/or energy metabolism. ATP measurements considered for this adjustment came from Glutor wells because 2-DG uptake is known to deplete cellular ATP as 2-DG is phosphorylated but not further metabolised (32).
TG concentrations were measured in glucose uptake cell lysate using the Wako L-type Triglyceride M (FUJIFILM Wako Chemicals U.S.A. Corporation, Richmond, VA) assay adapted for microplates.
Cells were rapidly washed 3 times with ice-cold PBS and lysed using Qiagen RLT buffer containing 1% Beta-mercaptoethanol. Lysates were spined on Qiagen Qiashredder columns and lysates were kept at –80
oC until further processed. Total ARN was extracted using Qiagen RNeasy mini kit and concentrated using Qiagen MinElute clean up kit when necessary. CDNA was obtained using Quantitect Reverse transcription kit on a T-personal Combi thermocycler (Biometra, Germany) and real-time polymerase chain reaction were conducted using Quantitect primers (Qiagen, Germantown, MD, USA) and MBI EVOlution EvaGreen rtPCR mix (Montreal Biotech Inc, Dorval, Qc, Canada) on a RG-3000 Rotor-Gene (Corbett Research Ltd, Mortlake, Australia). Melting curve analyses were performed to ensure that amplification yielded a single product. Amplification curves were analysed using the Rotor-Gene Q Series software version 2.2.3. Amplification efficiency was determined for each individual amplification reaction using the comparative quantitation feature of the Q software, which is based on the fluorescence increase over 4 cycles following “take-off” of the fluorescence signal. The “take-off” point corresponds to the point at which the second derivative of the amplification curve reach 20% of its maximum (determined automatically by the Rotor-Gene Q software). Average amplification factors were very stable within and across genes (averaging 1.7 to 1.8). Fluorescence thresholds were determined for each gene in the first experiments and assigned the value corresponding to half the average fluorescence at take-off cycles. Fold-changes were calculated using the formula (33):
where
E correspond to the average amplification efficiency of each gene within the same experiment (I.e.
E was averaged from individual reactions for each gene for each experiment). Fluorescence thresholds for CT determination were arbitrarily set for each gene after the first experiment as half the average fluorescence extrapolated at take-off.
Data were analysed by linear mixed modeling using restricted maximum likelihood with “proliferation” (PCB126 or DMSO during proliferation) and “differentiation” (21% or 3% O2 during differentiation) as main effects and “experiment” as cluster variable. Intercept was set as random across experiments to account for correlation between measurements within individual experiment. 3 independent experiments were conducted, and each combination of treatment was tested in duplicate (2 wells) within each experiment. We included individual data from replicate wells in the statistical design because replicate wells per experiment were minimal and because we believe such within-experiment replicates provide valuable information on the robustness of the biological effect. For clarity’s sake, purely technical replicates (i.e. duplicate measurements from a same well for a given variable) were averaged and contributed unique datum to the statistical model. For gene expression, statistical analyses were conducted on ΔΔCT values and for glucose uptake and triglyceride content, analyses were conducted on raw luminescence values or ratios of raw luminescence values. Figures represent main effects as averaged percent of control condition (proliferation in DMSO and differentiation under normoxia) within each experiment to better illustrate treatment effects by excluding part of the inter-experiment variability. Analyses were performed using the jamovi statistical software version 2.2.5 with the GAMJL module version 2.6.6.
Results
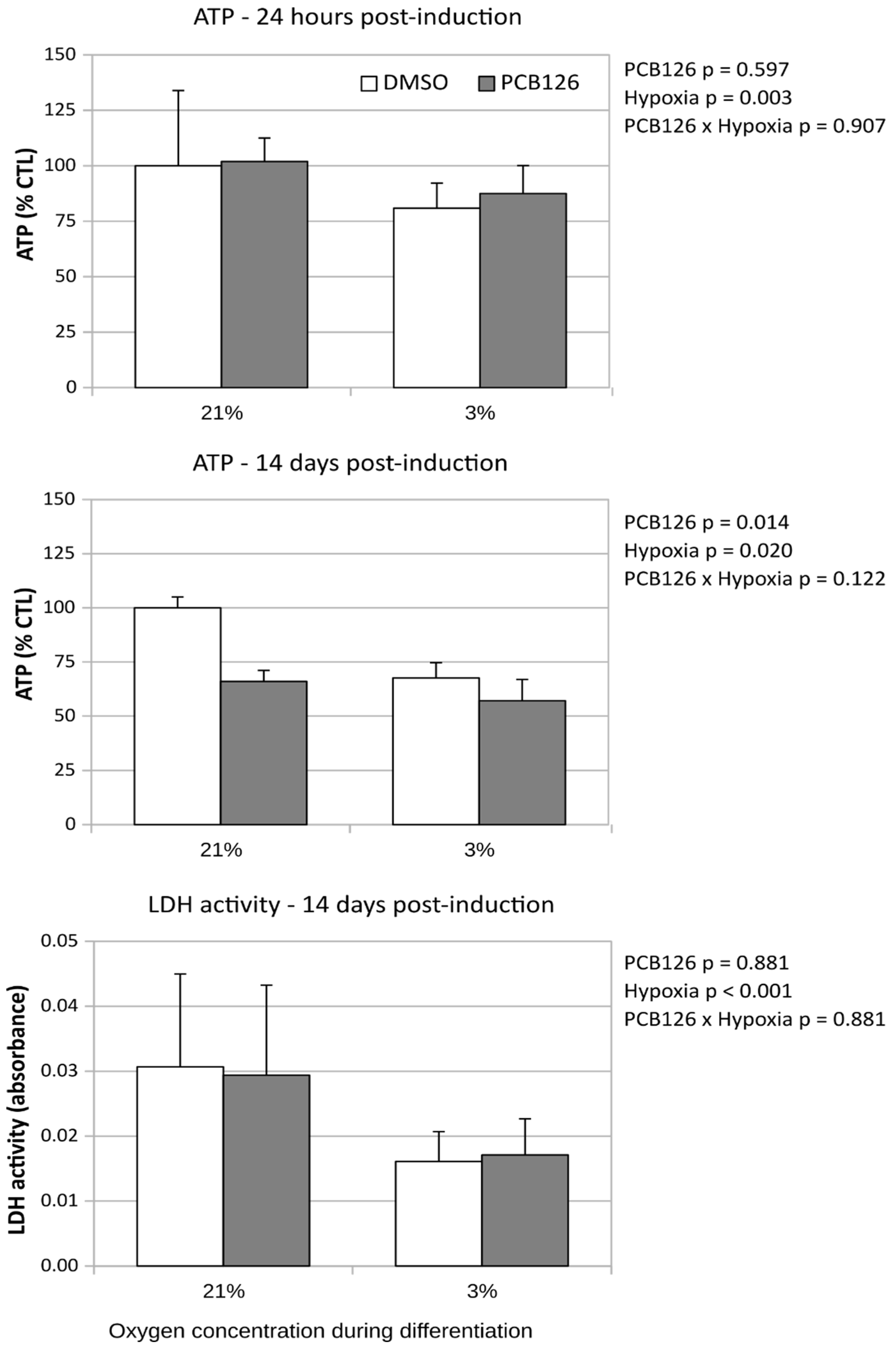
Daily visual examination of the cells showed no obvious anomaly such as reduced cell proliferation or cell detachment from culture ware in response to treatments throughout the experiment duration (17 days total) (Figure 1). After 72-hours of proliferation, cellular ATP was slightly lower (-7%, p=0.009) in PCB126 exposed cells but the ADP:ATP ratio and media LDH activity were similar between PCB126 and DMSO exposed cells (p=0.981 and 0.248, not shown), suggesting that PCB126 had only a minor effect on preadipocytes proliferation. After 24 hours of differentiation, ATP content was no more affected by PCB126 exposure while a 20% decrease in ATP (p<0.001) (Figure 2) and a minor increase in ADP:ATP ratio (0.247 vs 0.224, p=0.004, not shown) was observed in cells exposed to hypoxia. LDH activity in the media was not performed at that time. After 14 days of differentiation, both PCB126 and hypoxia treatments were associated to a significant ~30% decrease in ATP content (main effect of PCB126 p = 0.014, main effect of hypoxia p = 0.02) (Figure 2). Media LDH activity was not different between cells exposed to PCB126 or DMSO at that time, but media LDH activity (Figure 2) and ADP:ATP (not shown) ratio were on average reduced by ~50% in cells exposed to hypoxia (main effect of hypoxia p < 0.001 for both variables).
Figure 1.
Differentiating preadipocytes exposed to either DMSO or PCB126 uM during proliferation (3 days). Top 4 pictures were taken after the first 24 hours of differentiation under either 21% or 3% O2. Bottom 4 pictures were taken after 14 days of differentiation under either 21% or 3% O2. All pictures were taken using a light microscope at 5X.
Figure 1.
Differentiating preadipocytes exposed to either DMSO or PCB126 uM during proliferation (3 days). Top 4 pictures were taken after the first 24 hours of differentiation under either 21% or 3% O2. Bottom 4 pictures were taken after 14 days of differentiation under either 21% or 3% O2. All pictures were taken using a light microscope at 5X.
Figure 2.
Effect of PCB126 and hypoxia on cellular ATP content and media LDH activity. Top panel : Cell ATP content relative to control condition (DMSO, normoxia) 24 hours following induction. Mid panel : Cell ATP content relative to control condition after 14 days of differentiation. Bottom panel : Media LDH activity measured after 14 days of differentiation.
Figure 2.
Effect of PCB126 and hypoxia on cellular ATP content and media LDH activity. Top panel : Cell ATP content relative to control condition (DMSO, normoxia) 24 hours following induction. Mid panel : Cell ATP content relative to control condition after 14 days of differentiation. Bottom panel : Media LDH activity measured after 14 days of differentiation.
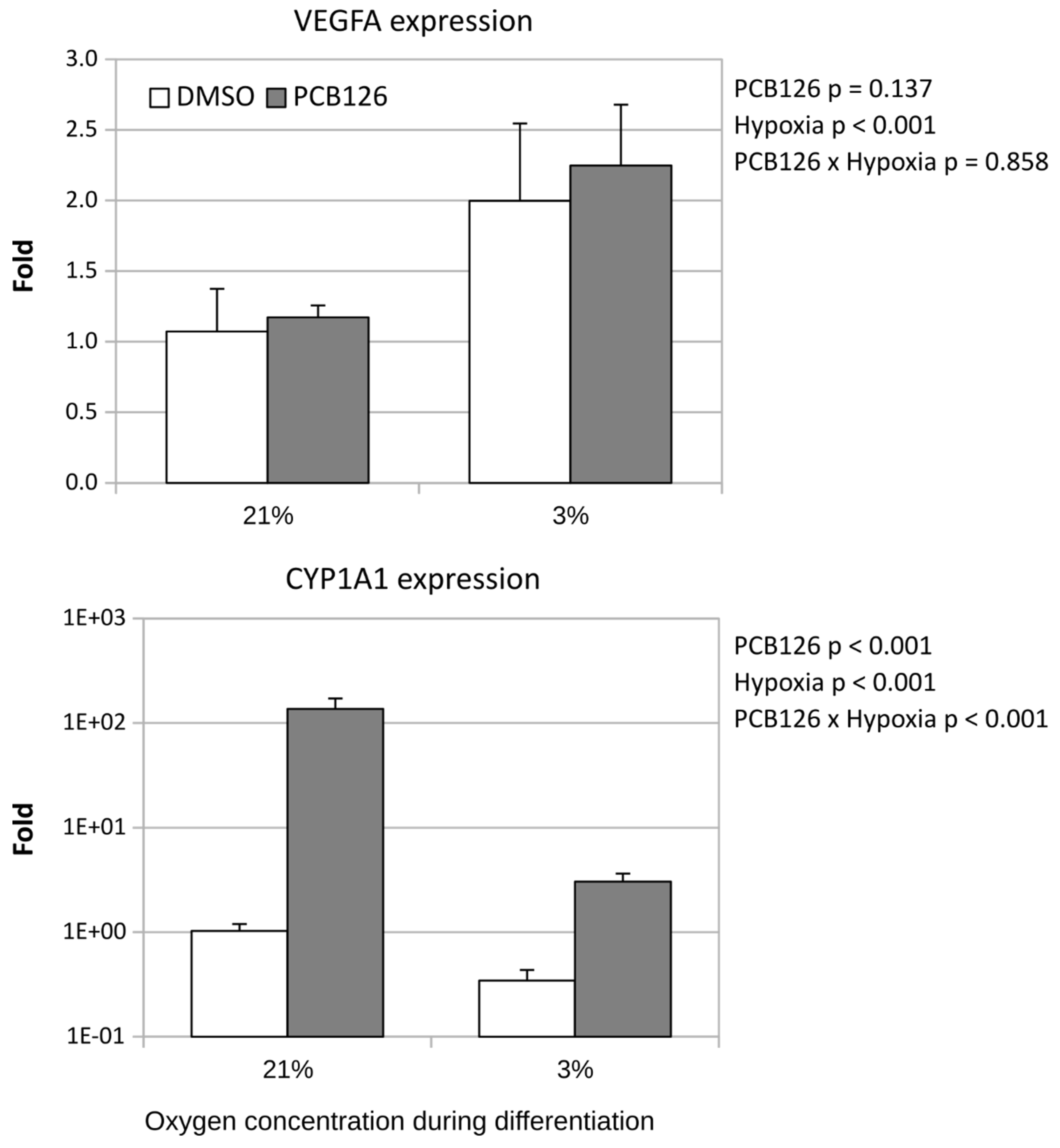
VEGFA expression was measured as an indicator of the induction of HIF signalling by hypoxia. VEGFA expression was increased 2-fold after 14 days of hypoxia (main effect of hypoxia p < 0.001) (Figure 3) and this effect was not altered by PCB126 exposure during proliferation. In contrast, there was a significant PCB126 x hypoxia interaction (p < 0.001) for CYP1A1 expression, a gene reflecting the induction of AhR signalling by PCB126. Specifically, following the initial PCB126 exposure, CYP1A1 expression was significantly increased after 14 days of PCB126-free normoxia or hypoxia treatment, this effect being greater upon normoxia (100-fold vs 3-fold) (Figure 3).
Figure 3.
Effect of PCB126 and hypoxia on VEGFA (top panel) and CYP1A1 (bottom panel) expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
Figure 3.
Effect of PCB126 and hypoxia on VEGFA (top panel) and CYP1A1 (bottom panel) expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
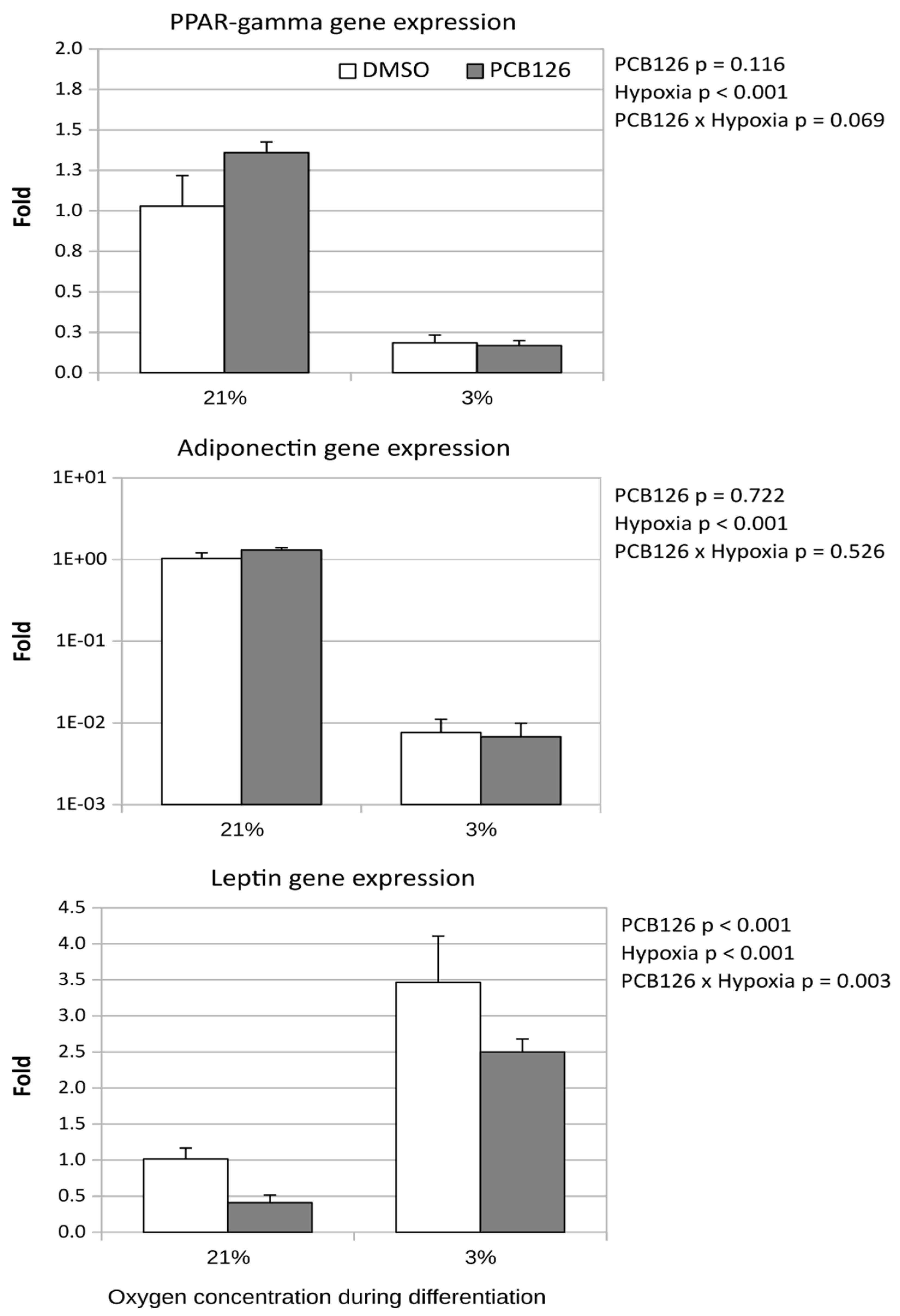
Adipose specific genes were affected differently by hypoxia and PCB126 pre-exposure. PPARγ and adiponectin expression were significantly supressed under hypoxia by 5- and 100-fold, respectively (main effect of hypoxia p < 0.001 in both cases) (Figure 4). PPARγ and adiponectin transcript levels were unaffected by PCB126 pre-exposure. In contrast, leptin expression was significantly increased by hypoxia, but was lowered by pre-differentiation PCB126 exposure. The leptin-lowering effect of PCB126 was significantly attenuated by hypoxia (PCB126 x hypoxia interaction p=0.003) (Figure 4).
Figure 4.
Effect of PCB126 and hypoxia on PPARγ (top panel), adiponectin (middle panel) and leptin (bottom panel) expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
Figure 4.
Effect of PCB126 and hypoxia on PPARγ (top panel), adiponectin (middle panel) and leptin (bottom panel) expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
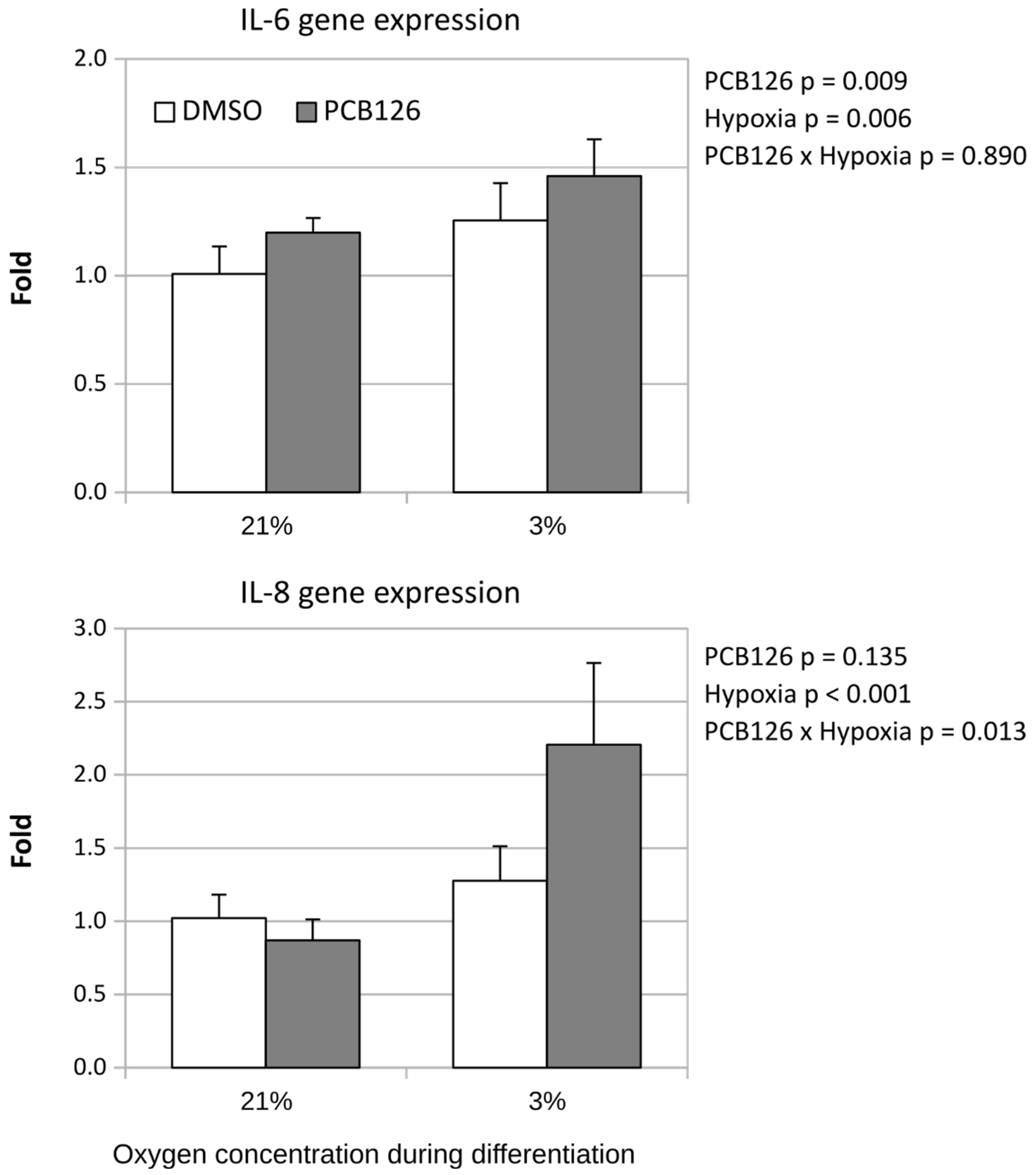
Expression of pro-inflammatory adipokine IL-6 was slightly but significantly increased by both PCB126 exposure during proliferation and hypoxia exposure during differentiation (p=0.009 and p=0.006 respectively) (Figure 5). IL-8 expression, on the other hand, was significantly increased by PCB126 exposure only in cells subsequently exposed to hypoxia during differentiation (PCB126 x hypoxia interaction p = 0.013) (Figure 5).
Figure 5.
Effect of PCB126 and hypoxia on Il-6 and IL-8 expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
Figure 5.
Effect of PCB126 and hypoxia on Il-6 and IL-8 expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
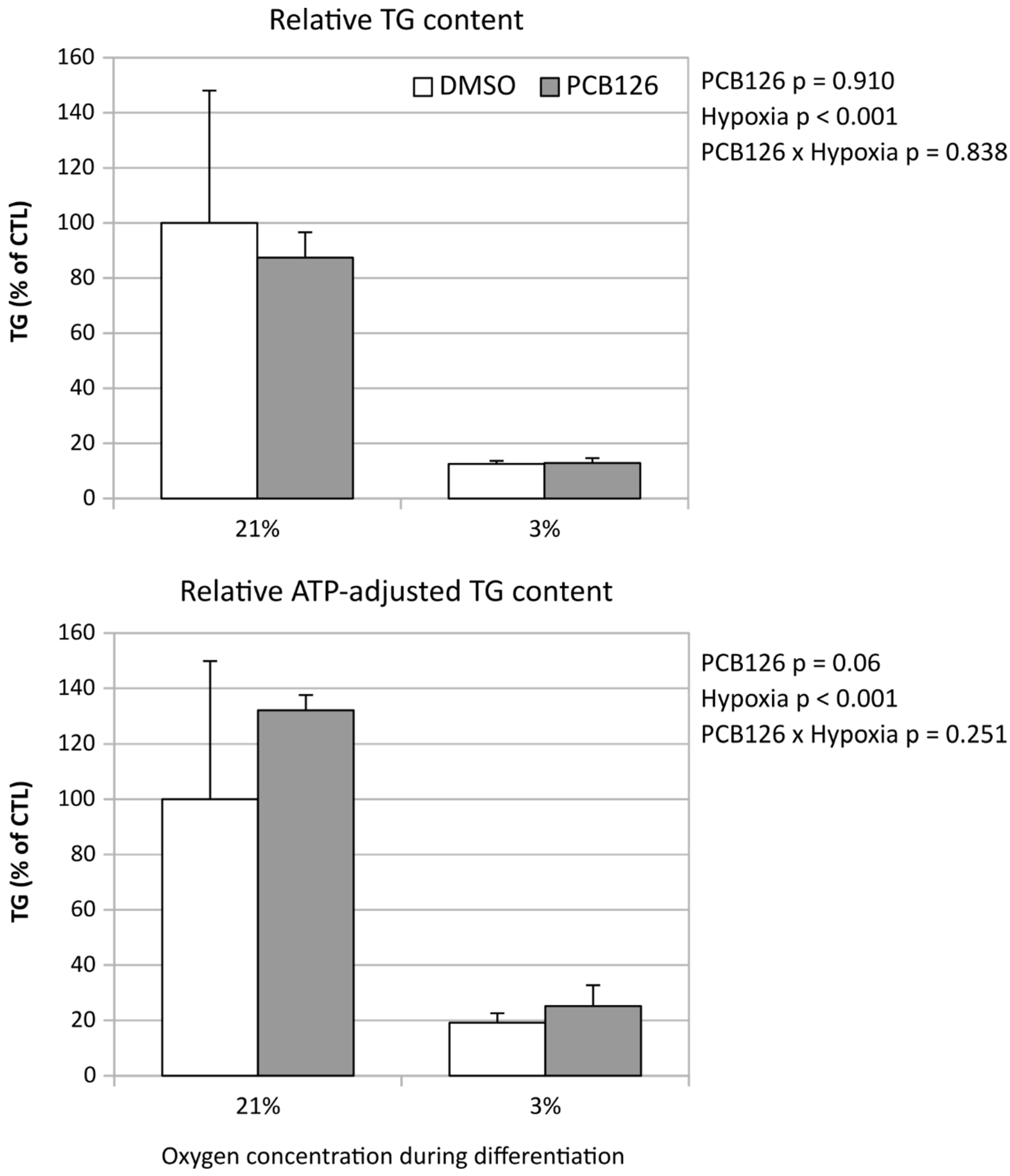
Adipocyte TG content decreased by more than 80% following 14 days of hypoxia exposure (main effect of hypoxia p < 0.001) (Figure 6). Interestingly, when TG content was normalized to ATP content, hypoxia was still associated with a reduction in TG content, but PCB126 exposure during proliferation tended to be associated to greater TG content, especially under normoxia (PCB126 x hypoxia interaction p=0.06) (Figure 6).
Figure 6.
Relative TG content of human differentiated preadipocytes exposed to PCB126 or DMSO during proliferation followed by differentiation for 14 days under either 21% or 3% O2. Top panel illustrates relative TG content compared to DMSO/normoxia. Bottom panel illustrates TG content normalized to ATP, relative to DMSO/normoxia.
Figure 6.
Relative TG content of human differentiated preadipocytes exposed to PCB126 or DMSO during proliferation followed by differentiation for 14 days under either 21% or 3% O2. Top panel illustrates relative TG content compared to DMSO/normoxia. Bottom panel illustrates TG content normalized to ATP, relative to DMSO/normoxia.
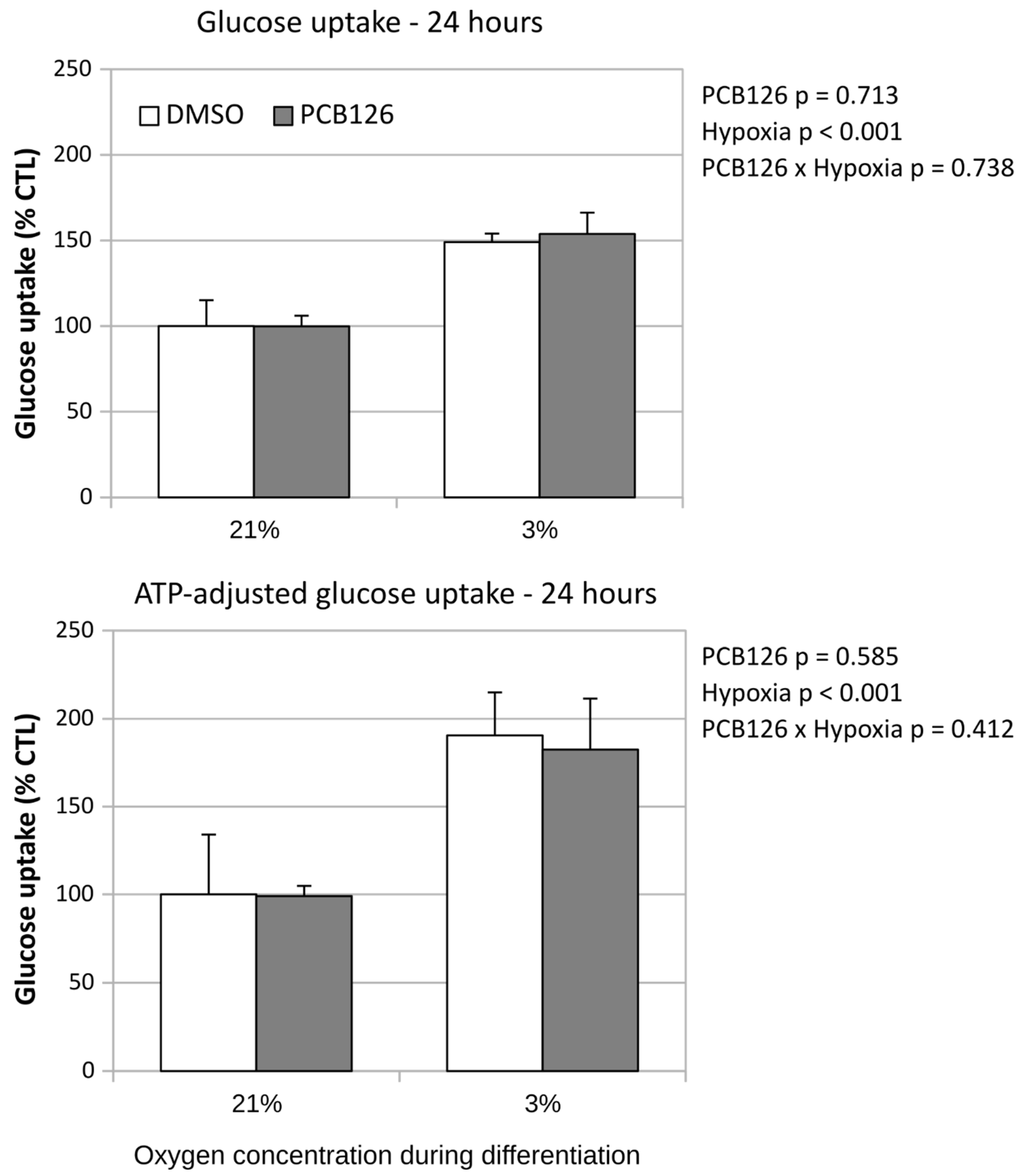
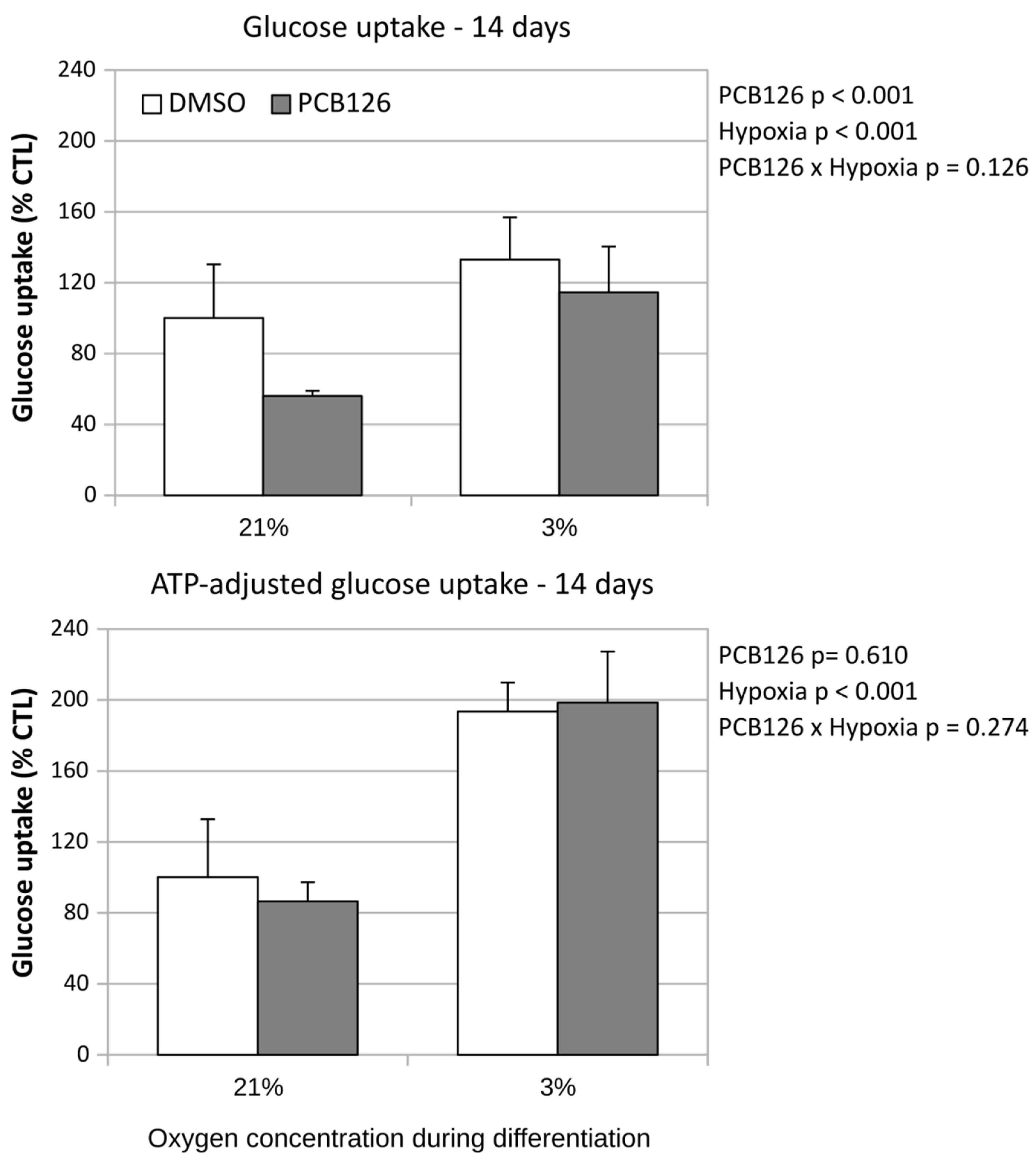
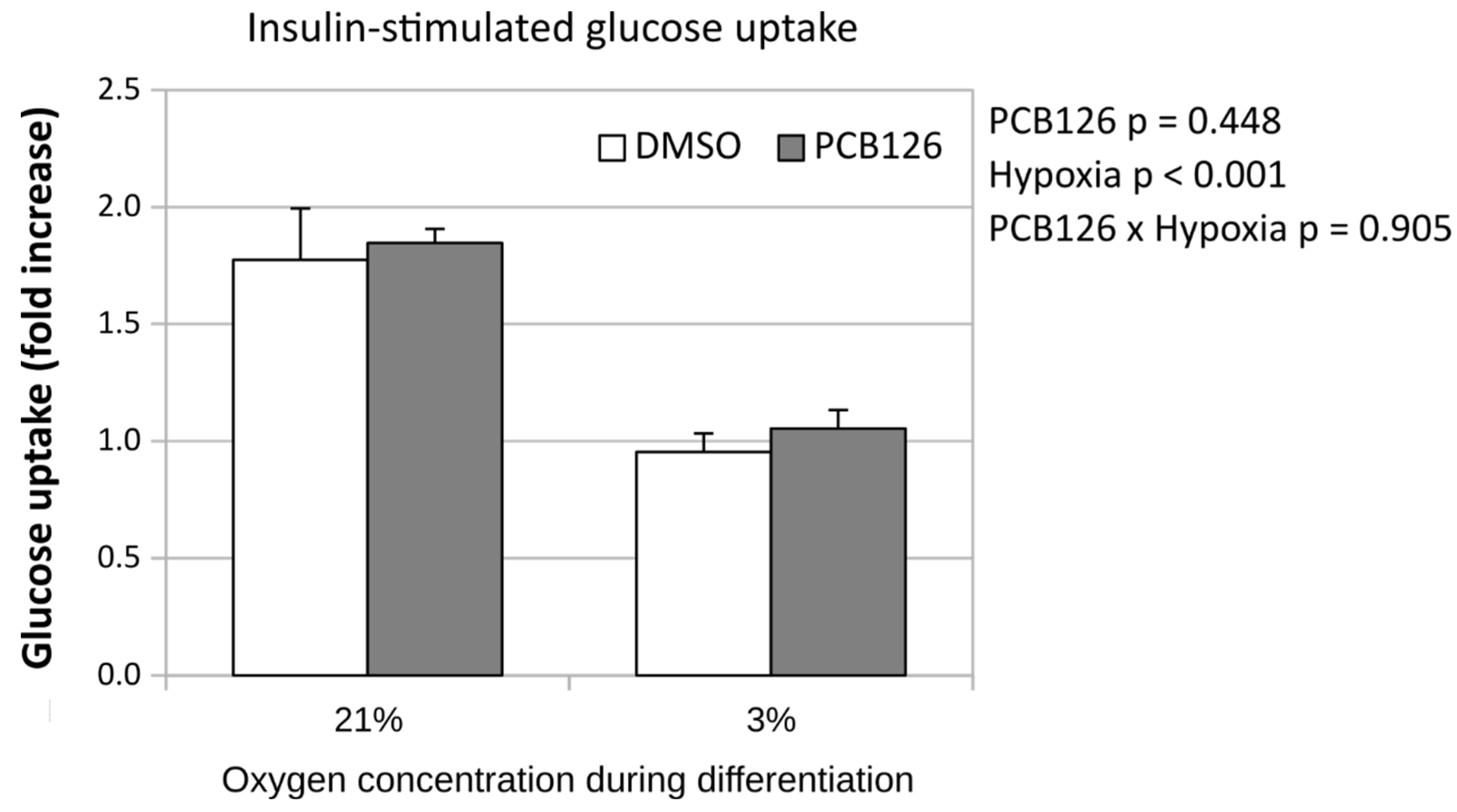
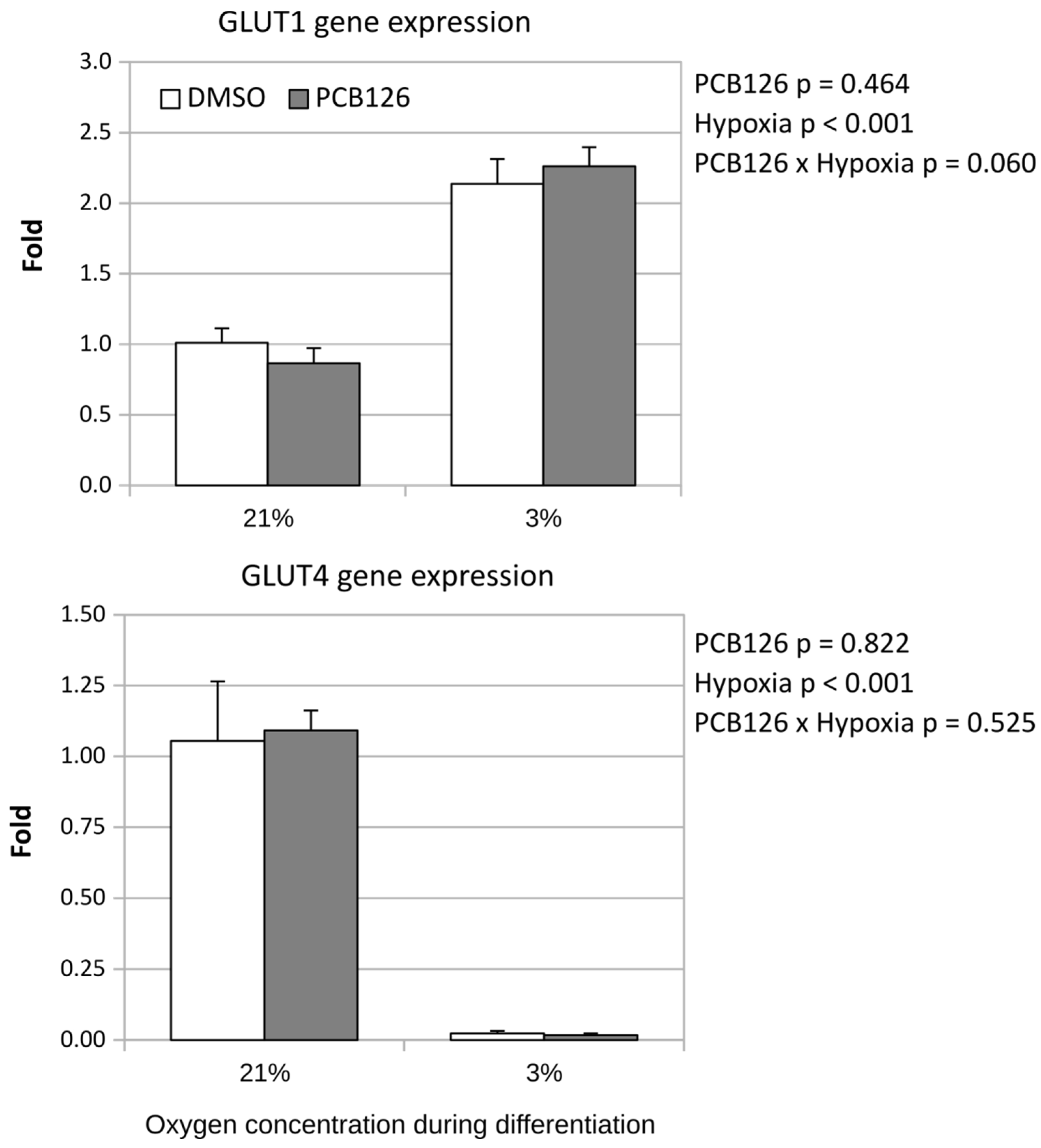
After 24 hours of differentiation, basal glucose uptake (either unadjusted or adjusted for ATP content) was increased by ~50% under hypoxia (main effect of hypoxia p<0.001), regardless of PCB126 exposure during proliferation or not (Figure 7). After 14 days of differentiation, basal glucose uptake unadjusted for ATP was still increased in response to hypoxia but was significantly reduced in cells exposed to PCB126 during proliferation (main effects of hypoxia and PCB126 both p<0.001) (Figure 8). However, when glucose uptake was adjusted for cells ATP content, only the effect of hypoxia remained significant (main effect of hypoxia p<0.001, main effect of PCB126 p=0.610) (Figure 8). Glucose uptake stimulation by insulin was only assessed at day 14 and was virtually completely abolished by differentiation under hypoxia while being unaffected by PCB126 exposure during proliferation (main effect of hypoxia p<0.001) (Figure 9). The expression of glucose transporters GLUT1, main transporter responsible for basal glucose uptake, and GLUT4, responsible for the increase in glucose transport in response to insulin, were consistent with the ATP-adjusted glucose uptake rates. PCB126 had no effect on either GLUT1 or GLUT4 expression, while hypoxia induced a 2-fold increase in GLUT1 expression and virtually abolished GLUT4 expression (main effect of hypoxia for both gene p < 0.001) (Figure 10).
Figure 7.
Early effect of PCB126 and hypoxia on basal glucose uptake in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days and measurement was done 1 day post induction.
Figure 7.
Early effect of PCB126 and hypoxia on basal glucose uptake in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days and measurement was done 1 day post induction.
Figure 8.
Effect of PCB126 and hypoxia on basal glucose uptake in human differentiated preadipocytes at day 14. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 3 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days. Glucose uptake was assessed at the end of the 14-day differentiation period.
Figure 8.
Effect of PCB126 and hypoxia on basal glucose uptake in human differentiated preadipocytes at day 14. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 3 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days. Glucose uptake was assessed at the end of the 14-day differentiation period.
Figure 9.
Effect of PCB126 and hypoxia on insulin stimulated glucose uptake % in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days and measurement was done at day 14 post induction.
Figure 9.
Effect of PCB126 and hypoxia on insulin stimulated glucose uptake % in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days and measurement was done at day 14 post induction.
Figure 10.
Effect of PCB126 and hypoxia on GLUT1 (top panel) and GLUT4 (bottom panel) expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
Figure 10.
Effect of PCB126 and hypoxia on GLUT1 (top panel) and GLUT4 (bottom panel) expression in human adipocytes. Preadipocytes were treated with DMSO (vector) or 10 µM PCB126 for 4 days (pre-differentiation exposure), then PCB126 was removed from media and differentiation was induced. At this point cells were subjected to 21 or 3% O2 for 14 days.
Discussion
A growing body of evidence shows that hypoxia and POPs accumulation in AT, two features observed when AT mass excessively expands, cause endocrine and metabolic alterations (6,7,9,10,15,16). A growing body of evidence shows that hypoxia and POPs accumulation in AT, two features observed when AT mass excessively expands, cause endocrine and metabolic alterations (10,34,35). Several studies suggested that AhR activation through exposure to chemical insults such as TCDD or PCBs induces inflammation in AT (36–39), and impairs adipocyte differentiation (7,40,41). However, these studies were conducted almost exclusively in guineapigs and rodents. When the effects of organic pollutants were assessed using cell cultures, exposure has almost always been conducted throughout or at the end of the differentiation phase despite the fact that it has been establish that, at least in the case of the murine 3T3-L1 adipogenic line, inhibition of adipocyte differentiation occurs exclusively when cells are exposed to TCDD before induction of differentiation (12,13). Work on a recently developed human adipogenic immortalized cell line (NPAD cells) indicated that PCB126 exposure during proliferation also impairs differentiation in these adipocyte precursors (11). Nonetheless, this effect was not easily reproduced in human mesenchymal stem cells using standard culture conditions (42). Since the effect of early dioxin-like organic pollutants exposure on the differentiation capacity of adipocyte precursor seems highly variable among cell lines, we investigated whether PCB126 exposure prevents the differentiation of human subcutaneous preadipocytes. Given our recent observation indicating that hypoxia strongly inhibits dioxin signalling, we also sought to examine how chronic hypoxia following early PCB126 exposure could affect adipocyte differentiation. Our results show that pre-differentiation exposure to PCB126 had no significant effect on adipocyte differentiation and glucose uptake in hSA. Instead, after 14 days of differentiation, the main effect of early PCB126 exposure was a minor decrease in cellular ATP content and significant modulation in the expression level of adipokines and adipose specific genes. Conversely, differentiation under continuous hypoxia strongly suppressed adipocyte differentiation, profoundly altered the expression of adipokines and adipose-specific genes and exacerbated the PCB126-induced inflammatory response.
CYP1A1 is a membrane-bounded hemoproteins part of the cytochrome P450 (CYP) enzyme family that plays a key role in the detoxification of xenobiotics (43,44). CYP1A1 induction is mostly transcriptional, and this mainly occurs by the AhR mechanism (43,44). We and others have previously shown that CYP1A1 expression was significantly induced by polychlorinated biphenyls exposure in hSA (11,19,45). In the current study, we found that CYP1A1 expression is still significantly induced (100-fold) in mature adipocyte obtained from preadipocytes that were exposed to PCB126 14 days earlier. Persistence of high CYP1A1 transcript levels in mature adipocytes following PCB126 exposure during preadipocyte proliferation has already been reported in NPAD cells (11). Together, the induction of CYP1A1 expression indicates that human preadipocytes are sensitive to xenobiotics compounds that activate the AhR signalling pathway.
In the present study, pre-differentiation exposure to PCB126 had no obvious consequence on adipocyte differentiation under normoxia, as demonstrated by the absence of alteration in PPARγ and adiponectin expressions as well as in TG content. This finding differs from the previous study showing that exposure of NPAD cells to PCB126 resulted in significant reduction in their ability to fully differentiate into adipocytes (11). This divergence could be attributed to several factors. First, in both studies, cells were exposed to PCB126 until confluence was reached, but this amounted to 3 days in the present study, compared to 6 days in the study of Gadupudi et al. (2015). Since PCBs have been shown to have different long term deleterious effects, such as DNA adducts formation, a longer incubation in the presence of PCB126 may have altered the cell cycle and the ability of NPAD cells to differentiate (46,47). Second, examination of the data reported by Gadupudi et al. suggests that NPAD cells seem to proliferate in a significant manner after confluence is reached (2015). NPAD cells could therefore be resistant to enter quiescence, which would be consistent with the seemingly low differentiation rates (25% in control conditions) that are concomitantly reported (11). This, in turn, suggests that NPAD cells may be natively less efficient at differentiating into adipocyte, making them, perhaps, more sensitive to disrupting factors like dioxin-like PCBs. Also, a more recent report by the same research group shows that treating human adipose mesenchymal/stromal cells in a similar fashion with Aroclor, a POP mixture containing dioxin-like PCB126 among others, does not impair their differentiation into adipocytes unless fetal bovine serum is almost completely removed from the culture media (42). This latter observation further supports the fact that different cell lines respond differently to POP exposure, which makes our observation that human subcutaneous preadipocytes behave differently from NPAD or 3T3-L1 cells less surprising.
Importantly, the inhibitory effect of PCB126 on NPAD differentiation reported by Gourronc et al. was accompanied by an inflammatory response characterized mainly by a strong upregulation of IL-8 expression (14). We and others showed previously that acute exposure to PCB126 increases the production of IL-8 in differentiated human adipocytes (19) and human multipotent adipose-derived stem cells (38). However, in the present study, the PCB126-normoxia sequence did not affect IL-8 expression, but instead led to a slight but significant increase in IL-6 expression, as previously observed in human monocytes or endothelial cells by others (47,48). While it cannot be excluded that IL-8 expression may have been transiently increased at any time point during proliferation or differentiation, this eventuality had no apparent impact on the capacity of human subcutaneous preadipocyte to differentiate into adipocytes under normoxic conditions.
Interestingly, pre-differentiation exposure to PCB126 significantly decreased leptin expression under normoxia. In our previous work (19), differentiated adipocytes acutely exposed to PCB126 for 48 hours had no significant change in leptin secretion. Lower serum levels of leptin were reported in the Yusho victims highly exposed to PCB and PCDF (49), but this effect was not observed in rats exposed to 50 g/kg of TCDD (50). How dioxin-like pollutants affect leptin secretion is currently poorly understood. Nonetheless, the current study provides preliminary evidence that human preadipocyte exposed to PCB126 appears to yield mature adipocytes that produce less leptin. Given the strong and positive association with leptin levels and body adiposity (51,52), whether dioxin exposure during AT development could, in the long term, alter leptin levels and therefore modulate the regulation of energy balance in humans remains to be explored.
Basal glucose uptake unadjusted for ATP was not affected by PCB126 after 24h of differentiation but was significantly lower after 14 days under normoxia. Absolute glucose uptake under insulin stimulation was also lower in cells that were exposed to PCB126 (not shown), however, when expressed as fold increase relative to basal glucose uptake, the insulin response appears not affected by PCB126 exposure. In vivo and in vitro studies have linked dioxin and dioxin-like PCBs to impaired glucose uptake and glucose intolerance, but these studies were all, as pointed out earlier, conducted in guineapigs, rodents, or rodent cell lines (37,41,53,54) which may behave differently in response to dioxin and other organic pollutants. The present study therefore suggests that preadipocyte exposure to PCB126 may produce populations of mature adipocytes displaying global reduced glucose uptake.
It is important to note that cells ATP content, although initially unaltered in cells exposed to PCB126, was significantly reduced 14 days post exposure. Interestingly, when glucose uptake on day 14 is normalized to ATP content, the reduction in glucose uptake induced by PCB126 disappears. The reason behind the decrease in cellular ATP is not obvious since neither media LDH activity nor ADP:ATP ratio provide evidence of apoptosis or necrosis in response to PCB126 exposure. It is however interesting to note that ATP correction makes glucose uptake measurements fall in line with the observation that GLUT1 and GLUT4 expression (the main basal and insulin stimulated glucose transporters) is not altered by PCB126. We believe that the concordance of these 2 relative measurements (glucose uptake relative to ATP and GLUTs relative to B-actin) is not trivial and, combined with the rest of our observations, suggest that human preadipocytes exposed to PCB126 produce normally differentiating adipocytes, but with a limited capacity for glucose uptake that could be attributable to lower ATP levels. Our study unfortunately provides no explanation for the delayed decrease in cellular ATP following PCB126 exposure beside that it does not appear to be apoptosis- or necrosis-related. However, dioxin-like pollutants have been shown to produce DNA adducts in the long term, which can lead to arrest in the cell cycle (55). Also, AhR have been identified as a factor that may also play a significant role in the regulation of the cell cycle and the commitment to differentiation (56). Since cell proliferation is exponential and has been reported to persist to some extent after differentiation induction (57), it is possible that PCB126/Ahr may induce slight impairments in human preadipocyte proliferation that may not produce noticeable effects after a few doubling times (i.e. 4 days) but may become significant after many (i.e. after 17 days) (11). This could eventually lead to a decrease in the number of otherwise healthy mature adipocyte, which could display a population phenotype like what was observed in the present study. Obviously, further investigations are required to test whether this paradigm is realistic.
Chronic exposure to hypoxia during differentiation was used to simulate the environment of hypoxic patches that may develop during the excessive development of AT mass (15). Expression of the VEGFA gene was increased significantly in adipocytes differentiated under hypoxia, indicating activation of the hypoxia response pathway persisting after 14 days. Cellular ATP levels were lower after both 24 hours and 14 days of hypoxia. However, both media LDH activity and the ADP:ATP ratio were also lower after 14 days of hypoxia, suggesting that depressed ATP levels may not reflect increased apoptosis or necrosis, but could instead indicate a state of cellular hypometabolism and alteration in adenosine metabolism that has been proposed to occur under hypoxia (58). Rapid decrease in cellular ATP unrelated to increase in ADP or cellular apoptosis/necrosis has also been reported in primary culture rat hepatocytes exposed to hypoxia (59). Such a mechanism could be critical for cell survival under hypoxia by preventing oxidative stress that could arise from ADP-stimulation of the oxygen-deprived oxidative phosphorylation. To the best of our knowledge, this effect of hypoxia on adipocyte ATP content has not been reported before and further studies will be required to better understand the mechanisms underlying this effect and how it may impact adipocyte function.
In the present study, hypoxia caused a clear visual reduction in adipocyte TG accumulation which was accompanied by a strong repression of PPARγ expression, indicating that continuous hypoxia greatly represses adipocytes differentiation. These results are in line with studies that utilized prolonged hypoxia exposure after differentiation induction (21,29,60). The decrease in adiponectin expression and increased in leptin and Il-6 expression under hypoxia are also consistent with previous observations (19,61). These alterations in lipid accumulation and adipokine profile further confirm that chronic severe hypoxia results in an impairment of differentiation in cultured human adipocytes.
Glucose uptake also behaved as expected in response to hypoxia exposure. After both 24 hours and 14 days of exposure, a strong increase in basal glucose uptake was observed, either adjusted or not for ATP content concomitant to an increase in GLUT1 expression, as observed by Wood et al. (23). However, the fact that glucose uptake remained significantly elevated under hypoxia following adjustment for ATP (contrary to the effect of PCB126 exposure where ATP adjustment abolished the effect of PCB126 on glucose uptake) is interesting and may suggest a shift in substrate partitioning toward glucose utilization and a greater contribution of the less efficient anaerobic glycolysis to energy metabolism under hypoxia. Finally, also as expected, hypoxia completely prevented insulin to promote glucose uptake in adipocyte, which was accompanied by a strong repression of GLUT4 expression, as previously observed.
We previously showed that hypoxia represses but does not completely abolish the induction of the dioxin response in human differentiated adipocytes acutely exposed to PCB126 (19). Consistently, in the present study most of the effects of PCB126 exposure, notably the decrease in leptin expression, were similarly obscured by 14 days of hypoxia. However, much like in our previous report, the induction of CYP1A1 by early exposure to PCB126 was strongly attenuated but still significantly present after 14 days of hypoxia. Together, these results confirm that hypoxia strongly interferes with, but does not completely abolish, the xenobiotic sensing pathway in adipocytes so that the combination of both factors could lead to interacting effects.
Such an interaction can be found in the fact that IL-8 expression was not increased by PCB126 exposure followed by normoxia but was significantly increased following the PCB126-hypoxia sequence (PCB126 x hypoxia p=0.013). PCB126 is increasingly well known to induce IL-8 expression in the short term (hours to days) in adipocyte cell lines, although this effect could be only transient (11,14). Hypoxia, on the other hand, has been shown to acutely induce IL-8 expression in several cell types in culture (lungs, ovarian cancer, macrophages) but, to the best of our knowledge, this has not been reported in human or rodent adipocytes. The present study seems to confirm that, even in the long term, hypoxia does not induce IL-8 expression in hSA. Therefore, a likely explanation for the increase in IL-8 expression by the PCB126-hypoxia combination is that continuous hypoxia may have allowed PCB126 to persistently stimulate IL-8 expression after several days of “PCB-free” culture. One way hypoxia could have achieved this is by preventing adipogenesis and impairing the “safe” deposit of remaining lipophilic PCB126 in lipid droplets, the major sink for adipose dioxin since adipocyte do not express CYP1A2, the protein responsible for the preferential sequestration of dioxins in organs such as the liver. Another contributing factor could be that remaining PCB126 molecules may have been more slowly metabolized and detoxified due to the strong repression of CYP1A1 expression by hypoxia. However, experimental data demonstrated that human cytochrome P450 does not metabolize PCB126 (62), which, if accurate, would make this later explanation unlikely. Regardless, while the interaction between acute and long-term hypoxia requires confirmation and the mechanisms responsible for this interaction need to be elucidated, the present observation suggest that hypoxia could potentiate the inflammatory response induce by dioxin-like pollutants. This is especially relevant knowing that 1) humans are exposed to dioxin-like pollutants at early age (breast milk), 2) that the proportion of individuals living with obesity, a condition increasingly recognized as inducing AT hypoxia, continue to rise worldwide and 3) that AT inflammation is increasingly recognized as one of the initiating factors in the metabolic cascade leading to obesity-related complications.
Some limitations and strengths of this study warrants discussion. First, the use of a single POP precludes extrapolation of our results to the more complex mixture of POPs preadipocytes/adipocytes are exposed to during their lifetime. Another limitation lies in the use of a single fixed hypoxia condition consisting of 3% O2. Despite that 3% O2 level is well recognized as being hypoxic in AT (15), this organ is likely exposed to fluctuations in O2 tensions of varying magnitudes, which may exert different effects on adipocyte metabolism. Hence, caution is warranted when extrapolating findings from our in vitro study to in vivo conditions and further investigation should be performed to examine the effect of PCB126 on mature adipocytes with longer period of exposure.
Conclusions
The present study suggests that human subcutaneous preadipocytes exposed to PCB126 yields relatively normal adipocytes, but the resulting adipocyte population is characterized by lower ATP content, absolute glucose uptake and leptin secretion. Exposure to hypoxia, on the other hand, exerts a much greater repression on adipocyte differentiation and lipid storage compared to PCB126 exposure during proliferation. Finally, our observations suggest that hypoxia and early PCB126 exposure have additive effects in terms of inflammatory response, a key feature of the development of chronic diseases such type 2 diabetes and cardiovascular diseases.
Funding
This study was funded by grants from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-04438). PI is a recipient of a research chair from the Institut du savoir Montfort (2016-018-Chair-PIMB).
Author contributions
ZEA, JF-M, and PI conceived and designed the experiments. ZEA and JF-M performed the experiments. ZEA, JF-M, and PI analyzed the data. ZEA, JF-M, and PI interpreted the data. All authors edited, revised, and approved the final version of the manuscript.
References
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–101. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3104761/pdf/JCI45887.pdf.
- Lee M-J, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13(4):371–376. [CrossRef]
- Trayhurn P. Hypoxia and Adipocyte Physiology : Implications for Adipose Tissue Dysfunction in Obesity. Annu Rev Nutr. 2014;34:207–36. Available from: 10.1146/annurev-nutr-071812-161156 Copyright. [CrossRef]
- Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H. Obesity and Persistent Organic Pollutants : Possible Obesogenic Effect of Organochlorine Pesticides and Polychlorinated Biphenyls. Obesity. 2011;19(4):709–14. Available from: http://dx.doi.org/10.1038/oby.2010.133/nature06264. [CrossRef]
- Myre M, Imbeault P. Persistent organic pollutants meet adipose tissue hypoxia: Does cross-talk contribute to inflammation during obesity? Obes Rev. 2014;15(1):19–28.
- Lee Y, Kim K, Jr DRJ, Lee D. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev. 2017;18:129–39. [CrossRef]
- Merrill M La, Emond C, Kim MJ, Antignac J, Bizec B Le, Birnbaum LS, et al. Review Toxicological Function of Adipose Tissue : Focus on Persistent Organic. Environ Health Perspect. 2013;162(2):162–9.
- Denison MS, Soshilov AA, He G, Degroot DE, Zhao B. Exactly the Same but Different: Promiscuity and Diversity in the Molecular Mechanisms of Action of the Aryl Hydrocarbon (Dioxin) Receptor. Toxicol Sci. 2011;124(1):1–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21908767. [CrossRef]
- Shan Q, Li H, Chen N, Qu F, Guo J. Understanding the Multiple Effects of PCBs on Lipid Metabolism. Diabetes, Metab Syndr Obes Targets Ther. 2020;13:3691–702. Available from: https://www.dovepress.com/understanding-the-multiple-effects-of-pcbs-on-lipid-metabolism-peer-reviewed-fulltext-article-DMSO.
- Nadal A, Quesada I, Tudurí E, Nogueiras R, Alonso-Magdalena P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat Rev Endocrinol. 2017;13:536–46. Available from: www.nature.com/nrendo. [CrossRef]
- Gadupudi G, Gourronc FA, Ludewig G, Robertson LW, Klingelhutz AJ. PCB126 Inhibits Adipogenesis of Human Preadipocytes. Toxicol Vitr. 2015;29(1):132–41. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4250299/pdf/nihms638572.pdf. [CrossRef]
- Phillips M, Enan E, Liu PCC, Matsumura F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Sci. 1995;108:395–402. [CrossRef]
- Chen C, Brodie AE, Hu CY, Brodie ANNE, Yuan C. CCAATEnhancer-Binding Protein p is not Affected by Tetrachlorodibenzo-p-dioxin Preadipocyte Differentiation. Obes Res. 1997;5(2):146–52.
- Gourronc FA, Robertson LW, Klingelhutz AJ. A Delayed Proinflammatory Response of Human Preadipocytes to PCB126 Is Dependent on the Aryl Hydrocarbon Receptor. Environ Sci Pollut Res Int. 2018;25(17):16481. Available from: /pmc/articles/PMC5764822/. [CrossRef]
- Trayhurn P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol Rev. 2013;93(1):1–21. Available from: http://physrev.physiology.org/cgi/doi/10.1152/physrev.00017.2012. [CrossRef]
- Netzer N, Gatterer H, Faulhaber M, Burtscher M, Pramsohler S, Pesta D. Hypoxia, Oxidative Stress and Fat. Biomolecules. 2015;5:1143–50. Available from: www.mdpi.com/journal/biomolecules/.
- Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch Eur J Physiol. 2007;455(3):479–92. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2040175/pdf/424_2007_Article_301.pdf. [CrossRef]
- Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol. 2008;198(1):127–34. Available from: https://joe.bioscientifica.com/view/journals/joe/198/1/127.xml. [CrossRef]
- Amine Z El, Mauger JF, Imbeault P. CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia. Cells 2022, Vol 11, Page 2282. 2022;11(15):2282. Available from: https://www.mdpi.com/2073-4409/11/15/2282/htm. [CrossRef]
- Grosfeld A, Zilberfarb V, Turban S, André J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia. 2002;45:527–30. [CrossRef]
- Mahat B, Mauger JF, Imbeault P. Effects of different oxygen tensions on differentiated human preadipocytes lipid storage and mobilisation. Arch Physiol Biochem. 2021;127(1):37–43. Available from:
https://doi.org/10.1080/13813455.2019.1609995. [CrossRef]
- O’Rourke RW, Meyer KA, Gaston G, White AE, Lumeng CN, Marks DL. Hexosamine Biosynthesis Is a Possible Mechanism Underlying Hypoxia’s Effects on Lipid Metabolism in Human Adipocytes. PLoS One. 2013;8(8):e71165. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0071165. [CrossRef]
- Wood IS, Wang B, Lorente-Cebrián S, Trayhurn P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-d-glucose uptake in human adipocytes. Biochem Biophys Res Commun. 2007;361(2):468–73. [CrossRef]
- Wood IS, Stezhka T, Trayhurn P. Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflugers Arch Eur J Physiol. 2011;462(3):469–77. [CrossRef]
- Famulla S, Horrighs A, Cramer A, Sell H, Eckel J. Hypoxia reduces the response of human adipocytes towards TNFα resulting in reduced NF-κB signaling and MCP-1 secretion. Int J Obes. 2012;36:986–92. Available from: www.nature.com/ijo.
- Mazzatti D, Lim F-L, Stuart Wood I, Trayhurn P. A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch Physiol Biochem. 2012;118(3):112–20. Available from: http://www.tandfonline.com/action/journalInformation?journalCode=iarp20. [CrossRef]
- Sheng X, Tucci J, Malvar J, Mittelman SD. Adipocyte differentiation is affected by media height above the cell layer. Int J Obes 2014 382. 2013;38(2):315–20. Available from: https://www.nature.com/articles/ijo201396. [CrossRef]
- Lin Q, Lee YJ, Yun Z. Differentiation Arrest by Hypoxia. J Biol Chem. 2006 Oct 13;281(41):30678–83. [CrossRef]
- Weiszenstein M, Musutova M, Plihalova A, Westlake K, Elkalaf M, Koc M, et al. Adipogenesis, lipogenesis and lipolysis is stimulated by mild but not severe hypoxia in 3T3-L1 cells. Biochem Biophys Res Commun. 2016;478:727–32. Available from: https://journals.scholarsportal.info/pdf/0006291x/v478i0002/727_alalisnshi3c.xml. [CrossRef]
- Vorrink SU, Domann FE. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem Biol Interact. 2014;0:82–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4091760/pdf/nihms594767.pdf.
- Reckzeh ES, Waldmann H. Development of Glucose Transporter (GLUT) Inhibitors. European J Org Chem. 2019;16:2321–9. [CrossRef]
- Chandramouli V, Carter JR. Metabolic effects of 2-deoxy-D-glucose in isolated fat cells. Biochim Biophys Acta - Gen Subj. 1977 Feb 28;496(2):278–91. [CrossRef]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):E45. Available from: https://pubmed.ncbi.nlm.nih.gov/11328886/. [CrossRef]
- Roos V, Rönn M, Salihovic S, Lind L, Bavel B van, Kullberg J, et al. Circulating Levels of Persistent Organic Pollutants in Relation to Visceral and Subcutaneous Adipose Tissue by Abdominal MRI. Obesity. 2012;21(2):413–8. Available from: http://doi.wiley.com/10.1038/oby.2012.123. [CrossRef]
- Vorkamp K. An overlooked environmental issue? A review of the inadvertent formation of PCB-11 and other PCB congeners and their occurrence in consumer products and in the environment. Sci Total Environ. 2016;541:1463–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26490526. [CrossRef]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated Biphenyl-77 Induces Adipocyte Differentiation and Proinflammatory Adipokines and Promotes Obesity and Atherosclerosis. Environ Health Perspect. 2008;116(6):761–8. [CrossRef]
- Baker NA, Shoemaker R, English V, Larian N, Sunkara M, Morris AJ, et al. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect. 2015;123(10):944–50. Available from: http://dx.doi.org/10.1289/ehp.1408594. [CrossRef]
- Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui L, Chevallier A, et al. Inflammatory Pathway Genes Belong to Major Targets of Persistent Organic Pollutants in Adipose Cells. Environ Health Perspect. 2012;120(4):508–14. [CrossRef]
- Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, et al. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139(2):653. Available from: /pmc/articles/PMC2910786/. [CrossRef]
- Barouki R, Antignac J-P, Emond C, Clément K, Birnbaum L, La Merrill M, et al. Adipose Tissue Pollutants And Obesity. ECOG’s Eb Child Adolesc Obes. 2015;1–22. Available from: ebook.ecog-obesity.eu.
- Loiola RA, Maria Dos Anjos F, Shimada AL, Soares Cruz W, Drewes CC, Fernandes Rodrigues S, et al. Long-term in vivo polychlorinated biphenyl 126 exposure induces oxidative stress and alters proteomic profile on islets of Langerhans. Sci Rep. 2016;6:27882. Available from: www.nature.com/scientificreports/. [CrossRef]
- Behan-Bush RM, Liszewski JN, Schrodt M V., Vats B, Li X, Lehmler H-J, et al. Toxicity impacts on human adipose MSCs acutely exposed to Aroclor and non-Aroclor mixtures of PCBs. BioRxiv. 2022;1–25. Available from: https://www.biorxiv.org/content/10.1101/2022.11.17.516943v1.article-info.
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–41. Available from: https://pubmed.ncbi.nlm.nih.gov/23333322/.
- Manikandan P, Nagini S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr Drug Targets. 2018;19(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28124606/. [CrossRef]
- Ellero S, Chakhtoura G, Barreau C, Langouët S, Benelli C, Penicaud L, et al. Xenobiotic-metabolizing cytochromes P450 in human white adipose tissue: Expression and induction. Drug Metab Dispos. 2010;38(4):679–86. [CrossRef]
- Dungen MW Van Den, Murk AJ, Kok DE, Steegenga WT. Toxicology in Vitro Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol Vitr. 2017;40:79–87. Available from: http://dx.doi.org/10.1016/j.tiv.2016.12.011. [CrossRef]
- Liu D, Perkins JT, Petriello MC, Hennig B. Exposure to coplanar PCBs induces endothelial cell inflammation through epigenetic regulation of NF-κB subunit p65. Toxicol Appl Pharmacol. 2015;289(3):457–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26519613.
- Wang C, Petriello MC, Beibei Z, Bernhard Hennig B. PCB 126 Induces Monocyte/Macrophage Polarization and Inflammation through AhR and NF-κB pathways. Physiol Behav. 2019;176(5):139–48.
- Uchi H, Yasukawa F, Furue M. [Adipokine profile of Yusho patients]. Fukuoka Igaku Zasshi. 2013;104(4):85–7. Available from: https://pubmed.ncbi.nlm.nih.gov/23858783/.
- Lindén J, Korkalainen M, Lensu S, Tuomisto J, Pohjanvirta R. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and leptin on hypothalamic mRNA expression of factors participating in food intake regulation in a TCDD-sensitive and a TCDD-resistant rat strain. J Biochem Mol Toxicol. 2005;19(3):139–48. [CrossRef]
- Considine R V., Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. Available from: https://pubmed.ncbi.nlm.nih.gov/8532024/. [CrossRef]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. Available from: https://pubmed.ncbi.nlm.nih.gov/7584987/.
- Olsen H, Enan E, Matsumura F. Regulation of glucose transport in the NIH 3T3 L1 preadipocyte cell line by TCDD. Environ Health Perspect. 1994;102(5):454–8. [CrossRef]
- Enan E, Liu PCC, Matsumura F. TTetrachlorodibenzo-p-dioxin Causes Reduction of Glucose Transporting Activities in the Plasma Membranes of Adipose Tissue and Pancreas from the Guinea Pig*. J Biol Chem. 1992;267(28):197–202. Available from: http://www.jbc.org/content/267/28/19785.full.pdf.
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6(12):947–60. Available from: https://www.nature.com/articles/nrc2015. [CrossRef]
- Girer NG, Tomlinson CR, Elferink CJ. The Aryl Hydrocarbon Receptor in Energy Balance: The Road from Dioxin-Induced Wasting Syndrome to Combating Obesity with Ahr Ligands. Int J Mol Sci. 2020;22(1):49. Available from: https://www.mdpi.com/1422-0067/22/1/49/htm. [CrossRef]
- Fajas L. Adipogenesis: a cross-talk between cell proliferation and cell differentiation. Ann Med. 2003;35(2):79–85. Available from: https://www.tandfonline.com/doi/abs/10.1080/07853890310009999. [CrossRef]
- Gu C, Jun JC. Does Hypoxia Decrease the Metabolic Rate? Front Endocrinol (Lausanne). 2018;13;9(668).
- Hayashi K, Ochiai T, Ishinoda Y, Okamoto T, Maruyama T, Tsuda K, et al. Relationship between cellular ATP content and cellular functions of primary cultured rat hepatocytes in hypoxia. J Gastroenterol Hepatol. 1997;12(3):249–56. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1440-1746.1997.tb00417.x. [CrossRef]
- Hashimoto T, Yokokawa T, Endo Y, Iwanaka N, Higashida K, Taguchi S. Modest hypoxia significantly reduces triglyceride content and lipid droplet size in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2013;440:43–9. Available from: https://journals-scholarsportal-info.proxy.bib.uottawa.ca/pdf/0006291x/v440i0001/43_mhsrtcldsi3a.xml. [CrossRef]
- Chaiban JT, Bitar FF, Azar ST. Effect of chronic hypoxia on leptin, insulin, adiponectin, and ghrelin. Metabolism. 2008;1;57(8):1019–22. [CrossRef]
- Yamazaki K, Suzuki M, Itoh T, Yamamoto K, Kanemitsu M, Matsumura C, et al. Structural basis of species differences between human and experimental animal CYP1A1s in metabolism of 3,3 0 ,4,4 0 ,5-pentachlorobiphenyl. J Biochem. 2011;149(4):487–94. Available from: https://academic.oup.com/jb/article/149/4/487/968025. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).