1. Introduction
Obesity is a complex medical condition characterized by excessive accumulation of body fat due to consumption of high-calorie foods coupled with a sedentary lifestyle. According to the World Health Organization (WHO), being overweight or obese is defined as having a body mass index (BMI) equal to or greater than 30 kg/m². The global obesity prevalence has increased. It is expected to continue increasing [
1,
2,
3]. According to statistics, the global level of those who are overweight and obese reached 38% in 2020. It is anticipated to reach around 50% by 2035 [
4]. Being considered as a chronic disease, obesity is also known as a risk factor for metabolic disease. It can lead to the development of chronic diseases such as diabetes, cardiovascular diseases, and non-alcoholic fatty liver disease (NAFLD) [
5,
6]. Although a number of nature-derived ingredients have entered the market, there is still a need for efficient ingredients to reduce obesity with less adverse effects [
2,
3,
7].
The development of preventive and therapeutic agents against obesity is based on five different mechanisms of action, including stimulating thermogenesis, lowering lipogenesis, enhancing lipolysis, suppressing appetite, and decreasing lipid absorption [
8]. Among these mechanisms, decreasing lipid absorption is recognized as having the lowest adverse effects with limited application specifically in the duodenum rather than in the blood or brain [
8,
9]. Orlistat (tetrahydrolipstatin) isolated from
Streptomyces toxytricini is known as an efficient anti-obesity reagent with approval by United States Food and Drug Administration (FDA) [
10]. However, its adverse effects (such as steatorrhea, hepatotoxicity, kidney injury, osteoporosis and oncogenesis) and interactions with drugs (such as warfarin, amiodarone, and thyroxine) and fat-soluble vitamins should not be overlooked [
10,
11]. Natural extracts concentrated in bioactive compounds can serve as good candidates for safe anti-obesity reagents by targeting lipid absorption [
12,
13,
14].
Agaricus bisporus, commonly known as white button mushroom, is a type of edible mushroom that is widely consumed around the world. This mushroom is a good source of nutrients including protein, fiber, vitamins B and D, minerals, and bioactive compounds such as polysaccharides and polyphenols [
15].
A. bisporus has been studied for its potential health benefits including anti-carcinogenic, anti-microbial, anti-oxidant, anti-inflammatory and immunomodulatory activities [
16,
17]. Various kinds of bioactive compounds such as glycoprotein, β-glucan, chitin, and chitin derivatives are present in cell walls of
A. bisporus. Different extraction method of
A. bisporus can result in differential biological effects [
17,
18]. Currently, clinical trial results have revealed that
A. bisporus extract with a high density of polysaccharides can exert an anti-obesity effect [
19]. However, its exact mechanism of action has not been defined yet. Several studies have reported that
A. bisporus can exert anti-obesity effects through beta-oxidation and autophagy, although such effects depend on compositions of
A. bisporus extract [
20,
21].
In the present study, we attempted to investigate the anti-obesity effect of
A. bisporus extract (H2Oslim
®) containing a high density of polysaccharides with a unique extraction and concentration method [
18]. Possible mechanisms underlying this effect were also explored. For such purpose, we determined the effect of H2Oslim
® on pancreatic lipase activity
in vitro and oral lipid tolerance in Sprague-Dawley (SD) rats. Furthermore, we investigated the potential
in vivo effect of H2Oslim
® on body fat accumulation and obesity-related biomarkers in high-fat diet-induced obese C57BL/6N mice. Our results suggest that H2Oslim
® might act as an anti-obesity agent by inhibiting pancreatic lipase-mediated fat absorption, at least in part.
2. Materials and methods
2.1. Preparation of Agaricus bisporus extract (H2Oslim®)
H2Oslim®, in high density form of Agaricus bisporus extract, was kindly provided by Tradichem SL (Madrid, Spain).
2.2. Determination of chitosan contents in H2Oslim®
Chitosan content of H2Oslim
® was analyzed by measuring the content of total D-glucosamine following the analytical method listed in the Korean Health Functional Food Codex. Hydrolysis with hydrochloric acid (Junsei Chemical Co., Ltd., Tokyo, Japan) followed by quantification of glucosamine is a commonly used analytical method for chitosan quantification [
22,
23]. Briefly, H2Oslim
® was hydrolyzed by hydrochloric acid, resulting in the production of glucosamine. This glucosamine further reacted with acetylacetone (Junsei Chemical Co., Ltd.) under alkaline conditions, leading to the formation of chromogen 2-methyl-3-diacetylpyrrole derivatives. When the chromogen was exposed to p-Dimethylaminobenzaldehyde (Sigma-Aldrich Co. St. Louis, MO, USA) under acidic conditions, a purplish red compound was formed. The absorbance of this compound was measured at a wavelength of 530 nm. Within a specific concentration range, the absorbance was directly proportional to the concentration of glucosamine (Sigma-Aldrich Co. St. Louis), allowing for its quantification using spectrophotometry (Thermo Fisher Scientific, Vantaa, Finland). Total chitosan content was calculated using the following equation (1):
where Sc was the total glucosamine concentration in the standard (μg/mL), Ta was the absorbance of test material, Sa was the absorbance of standard, and Tw was the weight of test material (g).
2.3. In vitro pancreatic lipase activity assay
Pancreatic lipase inhibitory activity was assayed using a porcine pancreatic lipase and
p-nitrophenyl butyrate (
p-NPB) as a substrate according to a method reported previously [
24]. An enzyme buffer was prepared by adding porcine pancreatic lipase (Sigma-Aldrich Co. St. Louis) solution reconstituted with 10 mM morpholine propanesulphonic acid (MOPS) and 1 mM ethylenediaminetetraacetic acid (EDTA) (pH 6.8) to Tris buffer (100 mM Tris-HCl and 5 mM CaCl
2, pH 7.0). Then H2Oslim
® at various concentrations was mixed with enzyme buffer and incubated at 37°C for 15 min. After incubation, 10 mM
p-NPB was added and the enzyme reaction was allowed to proceed at 37°C for 15 min. Pancreatic lipase activity was determined by measuring hydrolysis of
p-NPB to
p-nitrophenol at 400 nm. Inhibition of pancreatic lipase activity (%) was calculated as [1 – (Absorbance of test sample with enzyme - Absorbance of test sample without enzyme)/ Absorbance of Blank] x 100.
2.4. In vivo oral lipid tolerance test (OLTT)
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Hallym University (approved number: Hallym 2023-2). The animal study was conducted following the guidelines for the care and use of laboratory animals.
Five-week-old male Sprague-Dawley (SD) rats were purchased from Dooyeol Biotech Co. Ltd. (Seoul, Korea) and kept at the animal research facility of Hallym University. They were maintained at 23 ± 3°C and 50 ± 10% relative humidity with a 12-h light /dark cycle. During the acclimation period for one week, rats had free access to a commercial, non-purified rodent diet and tap water.
After the one-week acclimation period, rats were randomly allocated into three groups (n = 10 per group) as follows: (i) lipid emulsion control group (LC), (ii) lipid emulsion + 80 mg/kg body weight (BW) H2Oslim® group (L+H80), and (iii) lipid emulsion + 160 mg/kg BW H2Oslim® group (L+H160). After fasting for 16 h, rats in L+H80 and L+H160 groups were orally administered H2Oslim® at doses of 80 and 160 mg/kg BW, respectively. Rats in the LC group were orally administered sterile water as a vehicle. After 10 min, rats were administered a lipid emulsion consisting of 200 g/L soybean oil, 12 g/L lecithin from soybean, and 22.5 g/L glycerol by oral gavage at a dose of 10 mL/kg BW. All rats were anesthetized with isoflurane (Vspharm, Hanam, Korea). Blood was collected from the orbital vein before (0 h) and at 1, 2, 4, and 6 h after administration of the lipid emulsion. Concentrations of triglyceride and total cholesterol in serum were measured using ASAN SET TG-S and ASAN SET Total-Cholesterol kits (ASAN PHARM Co. Ltd., Hwaseong, Korea), respectively, according to the manufacturers’ instructions. The area under the concentration-time curve (AUC) of triglyceride or total cholesterol was calculated according to the trapezoid rule to evaluate exposure that integrated concentration across time.
2.5. Experimental design in high-fat induced obesity animal models
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Hallym University (approved number: Hallym 2023-2). The animal study was conducted following guidelines for the care and use of laboratory animals.
Four-week-old male C57BL/6N mice were purchased from Dooyeol Biotech Co. Ltd. (Seoul, Korea). They were kept at the animal research facility of Hallym University. They were maintained at 23 ± 3°C and 50 ± 10% relative humidity with a 12-h light /dark cycle. During the acclimation period for one week, mice had free access to a commercial, non-purified rodent diet and tap water.
After a one-week acclimation period, mice were randomly allocated into five groups (n = 10 per group) as follows: (i) control diet group (CD), (ii) high-fat diet group (HFD), (ⅲ) high-fat diet + 80 mg/kg BW/day H2Oslim® group (HFD+H80), (iv) high-fat diet + 160 mg/kg BW/day H2Oslim® group (HFD+H160), and (v) high-fat diet + 160 mg/kg BW/day chitosan group (HFD+C160). Mice in the CD group were fed a control diet (with 10% kcal from fat, 20% kcal from protein, and 70% kcal from carbohydrates; Cat. no. D124505B, Research Diets, Inc., New Brunswick, NJ, USA). Mice in other groups were fed a high-fat diet (with 60% kcal from fat, 20% kcal from protein, and 20% kcal from carbohydrates; Cat. no. D12452, Research Diets, Inc.). Food and water were provided ad libitum during the experiment. H2Oslim® or chitosan dissolved in distilled water was administered daily by oral gavage for eight weeks. An equal volume of distilled water was orally administered to mice in CD and HFD groups. Food intake was measured daily and body weight was measured weekly during the entire experimental period.
At the termination of experiment, mice were anesthetized with tribromoethanol diluted with amyl alcohol. Blood was then drawn from the orbital vein and serum was subsequently separated from the blood by centrifugation at 3,000 rpm for 20 min at 4 °C. After blood collection, mice were euthanized by cervical dislocation and four areas (epididymal, retroperitoneal, mesenteric, and inguinal) of white adipose tissue (WAT) were quickly excised, rinsed with physiological saline, and weighed.
2.6. Body composition assessment
One day before terminating the experiment, percentages of lean mass and fat mass were estimated using dual-energy X-ray absorptiometry (DEXA, PIXImusTM, GE Lunar, Madison, WI, USA).
2.7. Serum biochemical analysis
Serum levels of glucose, triglycerides (TG), and total cholesterol and activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the serum were measured with a blood chemistry autoanalyzer (KoneLab 20XT, Thermo Fisher Scientific).
2.8. Histological analysis
Epididymal adipose and liver tissues were fixed with 4% paraformaldehyde in phosphate buffer (0.5 M, pH 7.4), embedded in paraffin, and sectioned to a thickness of 5 μm. These tissue sections were then stained with hematoxylin and eosin (H&E). To observe fat deposition, liver tissues were embedded in Tissue-Tek OCT compound, serially sectioned to a thickness of 5 μm, and stained with Oil-Red O. Stained tissues were examined and photographed under a light microscope (AxioImager, Carl Zeiss, Jena, Germany) at 200x magnification. Adipocyte size in epididymal adipose tissue was analyzed using an AxioVision Imaging System (Carl Zeiss).
2.9. Measurement of lipids in livers and feces
Total lipids from liver tissues and feces were extracted according to a method described previously [
25] with a slight modification. Briefly, each sample was mechanically homogenized in phosphate buffered saline. Chloroform-methanol (2:1, v/v) was then added and the sample was homogenized. The homogenate was centrifuged at 2500 x g for 10 min. The upper phase was discarded. The lower chloroform phase containing lipids (triglyceride and cholesterol) was collected and evaporated in a rotary evaporator under vacuum. The lipid weight was measured. The lipid was dissolved in isopropanol and contents of triglyceride and total cholesterol were measured using a commercial kit (ASAN PHARM Co. Ltd.)
2.10. Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). SAS for Windows version 9.4 (SAS Institute, Cary, NC, USA) was used to conduct statistical analyses. Student’s t-test was used to test difference between CD and HFD groups. Analysis of variance followed by Duncan’s multiple comparison test was used to compare means among HFD, HFD+H80, HFD+H160, HFD+C160 groups. P < 0.05 was considered significant.
4. Discussion
Growing evidence suggests that the use of nature-derived ingredients with specific manufacturing processes could be a promising approach for the prevention and treatment of multiple diseases [
12,
13,
14].
A. bisporus contains various nutrients, including essential and semi-essential amino acids, unsaturated fatty acids, proteins, vitamins, antioxidants such as phenolic compounds, flavonoids, and tocopherols, and polysaccharides [
15]. Along with its diverse range of nutrients,
A. bisporus extract is well-known for its antioxidant and immunomodulatory activities, cardiovascular health benefits, weight management properties, and positive effects on digestive health [
16,
17]. Several studies have investigated anti-obesity effects of
A.bisporus and their target molecules were varied according to the respective manufacturing methods. Li et al. [
20] have revealed that beta-glucan concentrated
A. bisporus extract can exert an anti-obesity effect through PPARγ-mediated autophagy. In addition, Maria et al. [
21] have demonstrated that
A. bisporus extract has an anti-obesity effect by promoting hepatic free fatty acid beta-oxidation.
Contents and types of polysaccharides in
A. bisporus extract vary depending on its deacetylation process.
A. bisporus extract contains polysaccharides such as glucan, chitin, and chitosan [
15,
26,
27]. Among these polysaccharides, chitosan is a type of dietary fiber that is derived from chitin. It is composed of randomly distributed β-(1,4) D-glucosamine and N-acetyl-D-glucosamine with a carbohydrate polymer. The deacetylation degree in chitosan, which indicates the presence of protonated -NH2 group, varies and affects its solubility [
28,
29]. Despite the widespread utilization of dietary chitosan for body fat reduction supported by experimental results globally, there remains a lack of consensus due to conflicting findings [
30,
31]. As previously reported [
19,
32], we obtained water-soluble polysaccharides containing H2Oslim
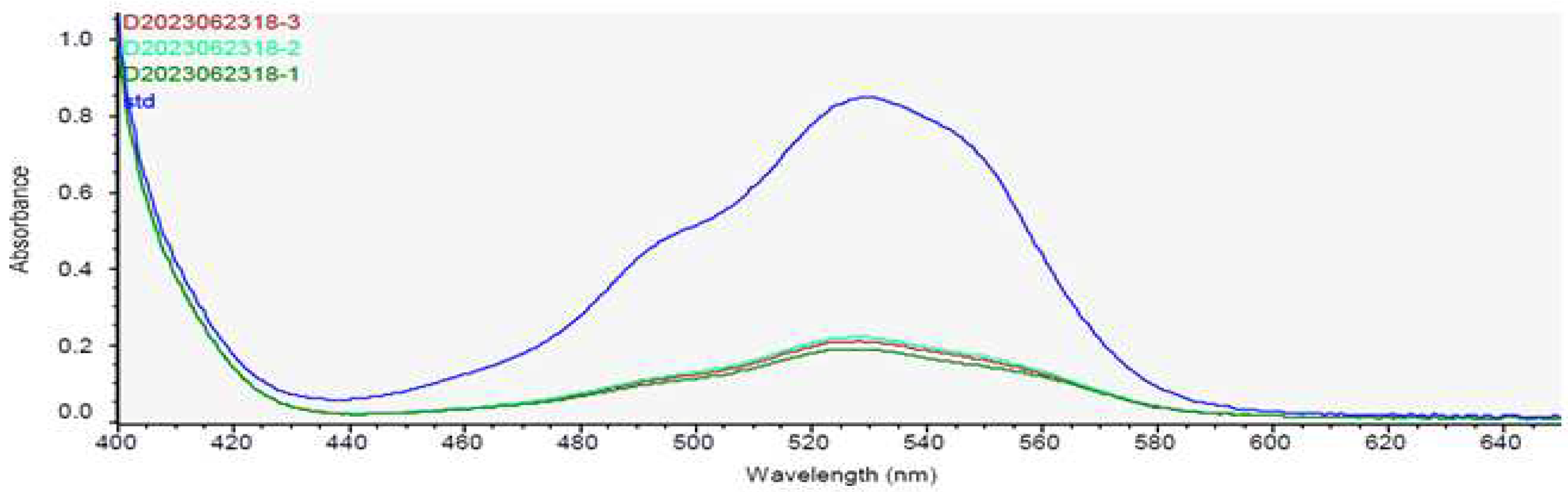
® using our extraction and deacetylation method and mushroom derived chitosan to confirm its biological effect on body fat reduction. The content of chitosan in the H2Oslim
® was 248.96 mg/g when extracted with our method (
Figure 1).
It has been previously demonstrated that the cationic polymer formed by polyelectrolyte complexation of water-soluble polysaccharides from H2Oslim
® can form complexes with lipids, referred to as fat trapping ability [
19,
32,
33]. In the present study, we examined the inhibitory effect of H2Oslim
® on pancreatic lipase known to play an important role in digestion and absorption of triglycerides [
34]. As the treatment level of H2Oslim
® increased, there was a significant inhibition of pancreatic lipase activity, reaching 36.9% inhibition at 1.00 mg/mL (
Table 1). Additionally, we assessed the fat trapping ability of H2Oslim
® in vivo using an oral lipid tolerance test (OLTT), a standardized method to evaluate the body's ability to digest and absorb dietary fats [
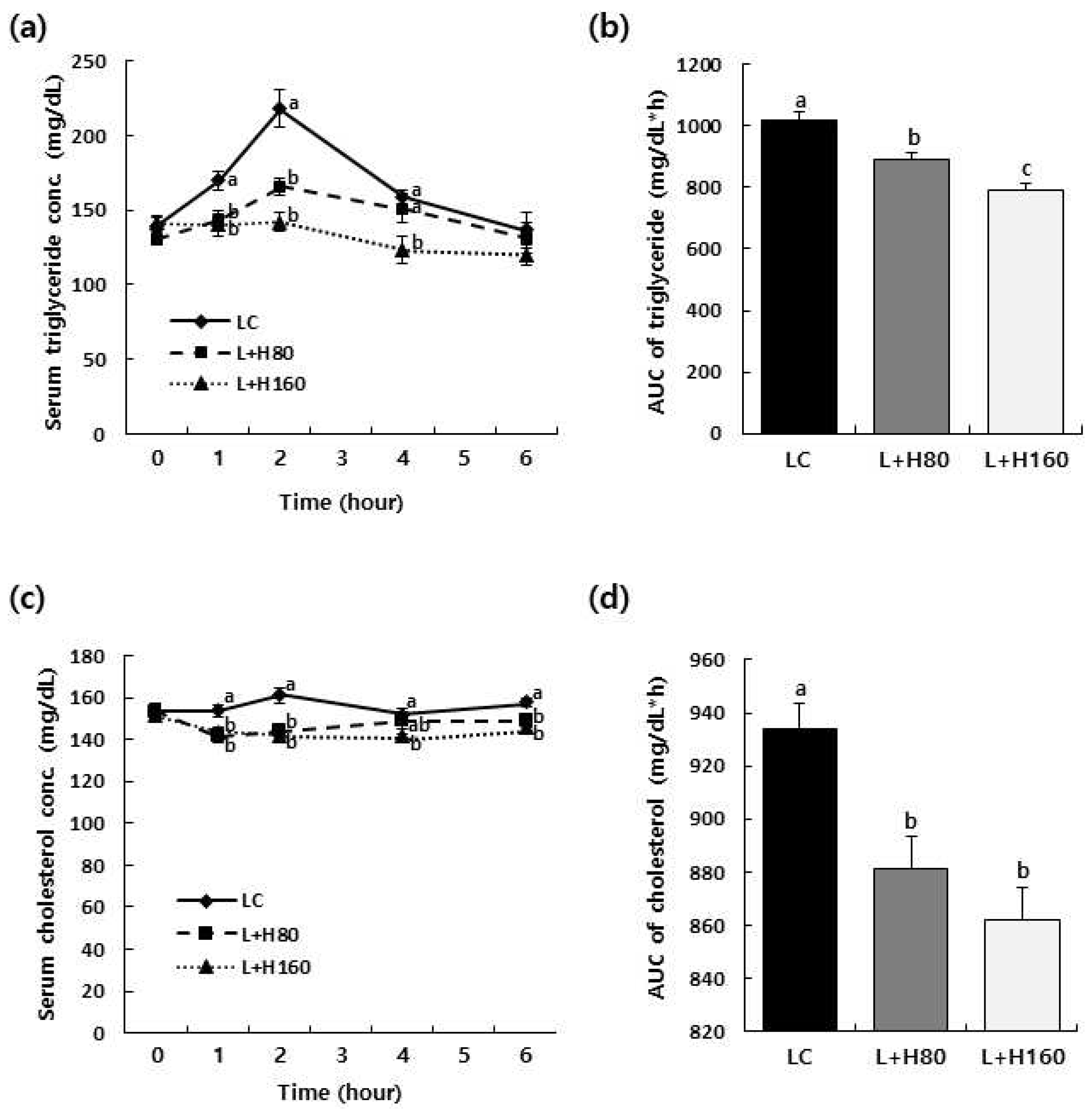
35]. Administration of H2Oslim
® dose-dependently reduced both AUC and maximum plasma levels of postprandial triglyceride and cholesterol, showing statistically significant differences compared to the LC group (
Figure 2). These findings suggest that H2Oslim
® can suppress lipid levels by modulating lipid metabolism by reducing pancreatic lipase activity, at least in part.
Our previous research has indicated that long-term administration of H2Oslim
® has preventive effects on obesity and hyperlipidemia in a randomized, double-blind, and placebo-controlled clinical trial involving overweight participants [
19]. A high-fat diet (HFD)-induced obese mouse model is commonly used to study metabolic syndrome, including obesity, hyperglycemia, and hyperlipidemia [
36,
37]. In our study, C57BL/6 mice with HFD-induced obesity were treated with H2Oslim
® for eight weeks. We observed that H2Oslim
® administration suppressed increases of body weight gain and fat mass caused by the HFD. Additionally, H2Oslim
® administration led to a reduction in food efficiency ratio (
Table 2). In terms of hematological parameters, elevated levels of glucose, ALT, and AST in the serum induced by HFD were suppressed by H2Oslim
® administration and increased level of triglycerides caused by HFD was diminished by administration of H2Oslim
® (
Table 3). These findings suggest that administration of H2Oslim
® can decrease body fat and weight and improve hematological parameters.
We also examined histological parameters associated with dyslipidemia and liver dysfunction in white adipose tissues and liver tissues. As depicted in
Figure 3, administration of H2Oslim
® effectively reversed the marked increase in weight of total WAT (epididymal, retroperitoneal, and mesenteric fat) induced by HFD. Moreover, hypertrophy of adipocytes, as measured by adipocyte size, was significantly reduced by H2Oslim
® administration compared to that in the HFD group. The liver plays a crucial role in lipid synthesis and distribution. Excessive accumulation of fats in the liver can lead to dyslipidemia and hyperglycemia, a condition known as NAFLD characterized by the presence of steatosis [
38,
39,
40]. H&E staining of the liver demonstrated that the high-fat diet induced hepatic steatosis, with numerous macrovesicles, lipid droplets, and hepatocellular ballooning. However, the administration of H2Oslim
® ameliorated these histopathological changes (
Figure 4A). Additionally, our study revealed that H2Oslim
® administration significantly reduced HFD-induced lipid accumulation in the liver, as detected by Oil-red O staining (
Figure 4B). Weight and lipid levels of the liver are known to follow hepatic steatosis [
39,
41]. Our study confirmed that liver weight and levels of lipids, triglycerides, and cholesterol were dramatically increased by HFD feeding. However, such increases were reversed by the administration of H2Oslim
® (
Figure 4C-F). Overall, treatment with H2Oslim
® and chitosan appears to have a preventive effect on obesity-related NAFLD by reducing lipid levels in the liver and improving histopathological changes associated with hepatic steatosis.
We demonstrated that administration of H2Oslim
® exhibited a fat trapping ability both
in vitro and
in vivo. Fecal fat excretion is a well-established method for assessing fat malabsorption [
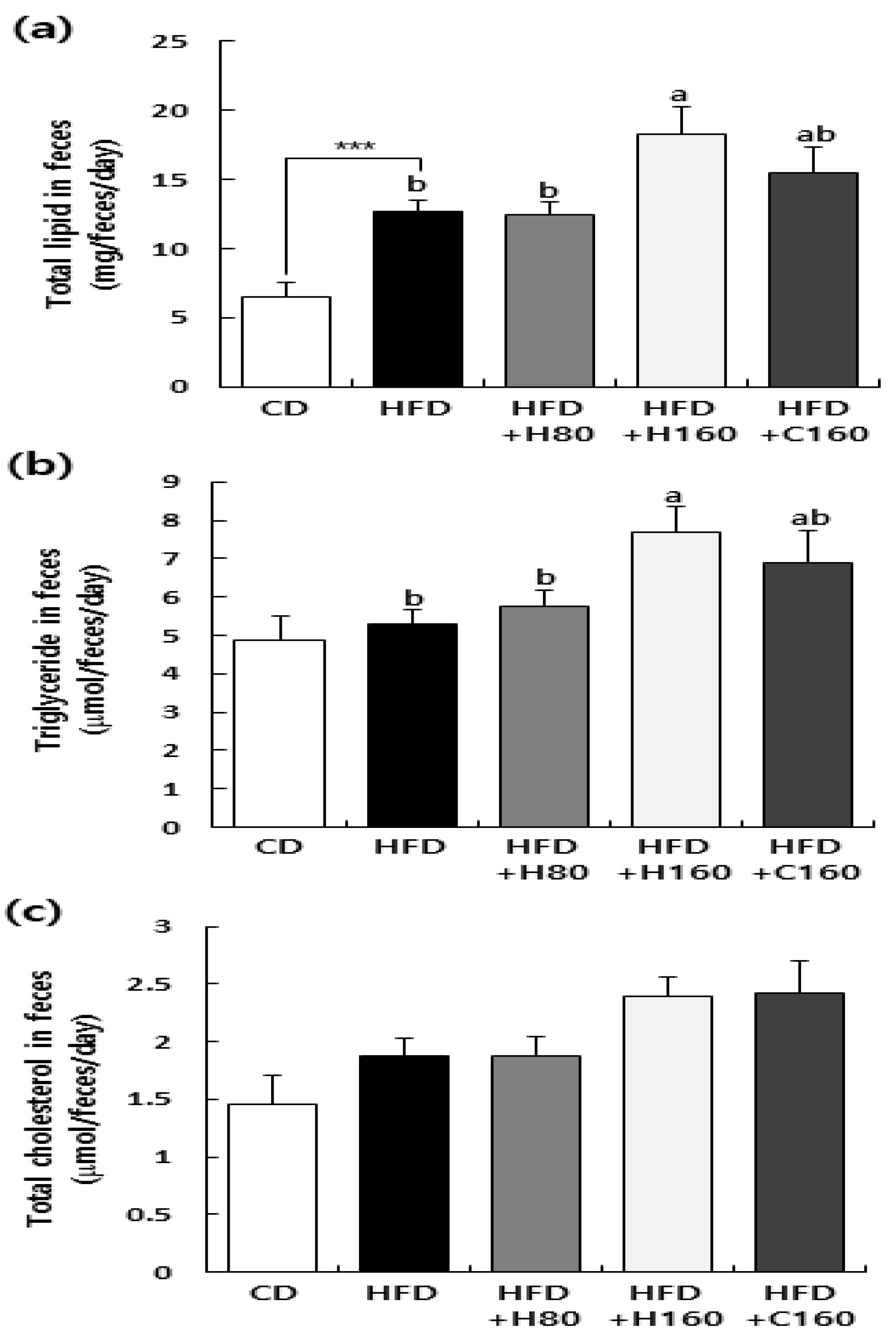
42]. In our study, we observed that administration of H2Oslim
® up-regulated lipid and triglyceride excretion in feces compared to the HFD group (
Figure 5). This suggests that H2Oslim
® might modulate the level of lipid absorption not only acutely, but also chronically.
To summarize results of our study, administration of H2Oslim® rich in polysaccharides showed promising effects in preventing obesity and its associated symptoms such as hyperlipidemia, hyperglycemia, and NAFLD. These effects were supported by reductions in body weight gain, fat mass percentage, and food efficiency ratio with improvements in glucose and triglyceride levels. Additionally, this treatment resulted in decreased weight of WAT and reduced lipid levels in the liver. These changes were associated with inhibition of pancreatic lipase activity in vitro and postprandial plasma lipids level in vivo, as observed in the OLTT and up-regulation of fecal excretion level of lipids in the HFD model. Consequently, H2Oslim® holds potential as a candidate for developing a functional food ingredient with anti-obesity properties.
Author Contributions
Conceptualization, H.K., D.K., E.J.K.; methodology, H.K., J.I.J., E.J.K.; formal analysis, H.K., Y.E.J., S.M.K., J.I.J., D.K.; data curation, H.K., Y.E.J., S.M.K., J.I.J.; writing—original draft preparation, H.K., E.J.K.; writing—review and editing, H.K., E.J.K.; visualization, J.I.J., E.J.K.; supervision, E.J.K.; project administration, H.K., D.K. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Quantitative confirmation of chitosan in H2Oslim®. Spectrophotometric profiles of D-glucosamine hydrolysate (Standard, 378.23 µg/mL) and H2Oslim® (Sample ID 1~3: 384, 450, and 424 µg/mL, respectively).
Figure 1.
Quantitative confirmation of chitosan in H2Oslim®. Spectrophotometric profiles of D-glucosamine hydrolysate (Standard, 378.23 µg/mL) and H2Oslim® (Sample ID 1~3: 384, 450, and 424 µg/mL, respectively).
Figure 2.
Effect of H2Oslim® administration on serum triglyceride and total cholesterol concentrations after oral administration of lipid emulsion in SD rats. SD rats were orally administered with H2Oslim®. After 10 min, rats were given lipid emulsion at a dose of 10 mL/kg BW by oral gavage. Blood was collected at 0, 1, 2, 4, and 6 h after lipid emulsion administration and serum was obtained from blood. Triglyceride (a) and total cholesterol (c) concentrations in serum were measured using relevant assay kits. AUCs for serum triglyceride (b) and total cholesterol (d) were calculated. Each bar represents the mean ± SEM (n = 10). Different letters indicate significant differences between LC, L+H80, and L+H160 groups at P < 0.05.
Figure 2.
Effect of H2Oslim® administration on serum triglyceride and total cholesterol concentrations after oral administration of lipid emulsion in SD rats. SD rats were orally administered with H2Oslim®. After 10 min, rats were given lipid emulsion at a dose of 10 mL/kg BW by oral gavage. Blood was collected at 0, 1, 2, 4, and 6 h after lipid emulsion administration and serum was obtained from blood. Triglyceride (a) and total cholesterol (c) concentrations in serum were measured using relevant assay kits. AUCs for serum triglyceride (b) and total cholesterol (d) were calculated. Each bar represents the mean ± SEM (n = 10). Different letters indicate significant differences between LC, L+H80, and L+H160 groups at P < 0.05.
Figure 3.
Effects of H2Oslim® administration on adipose tissue weight and morphological changes in epididymal adipose tissues of HFD-fed HFD C57BL/6N mice. Mice fed HFD were treated with H2Oslim® by oral gavage for eight weeks. (a) Adipose tissue weights in epididymal, retroperitoneal, mesenteric, and inguinal fat. Total white adipose tissue (WAT) weights calculated as the sum of epididymal, retroperitoneal, mesenteric, and inguinal fat. (b) Extracted epididymal adipose tissues were fixed, embedded in paraffin, and cut into 5 μm-thick slices. Tissue sections were stained with H&E. Representative H&E-stained images of epididymal adipose tissue (n = 5, 200x magnification) are shown. (c) The size of the adipocytes was quantified by measuring the longest diameter of adipocytes. Each bar represents the mean ± SEM (n = 10). ***, P < 0.001 significantly different from the CD group. Different letters indicate significant differences between HFD, HFD+H80, HFD+H160, and HFD+C160 groups at P < 0.05.
Figure 3.
Effects of H2Oslim® administration on adipose tissue weight and morphological changes in epididymal adipose tissues of HFD-fed HFD C57BL/6N mice. Mice fed HFD were treated with H2Oslim® by oral gavage for eight weeks. (a) Adipose tissue weights in epididymal, retroperitoneal, mesenteric, and inguinal fat. Total white adipose tissue (WAT) weights calculated as the sum of epididymal, retroperitoneal, mesenteric, and inguinal fat. (b) Extracted epididymal adipose tissues were fixed, embedded in paraffin, and cut into 5 μm-thick slices. Tissue sections were stained with H&E. Representative H&E-stained images of epididymal adipose tissue (n = 5, 200x magnification) are shown. (c) The size of the adipocytes was quantified by measuring the longest diameter of adipocytes. Each bar represents the mean ± SEM (n = 10). ***, P < 0.001 significantly different from the CD group. Different letters indicate significant differences between HFD, HFD+H80, HFD+H160, and HFD+C160 groups at P < 0.05.
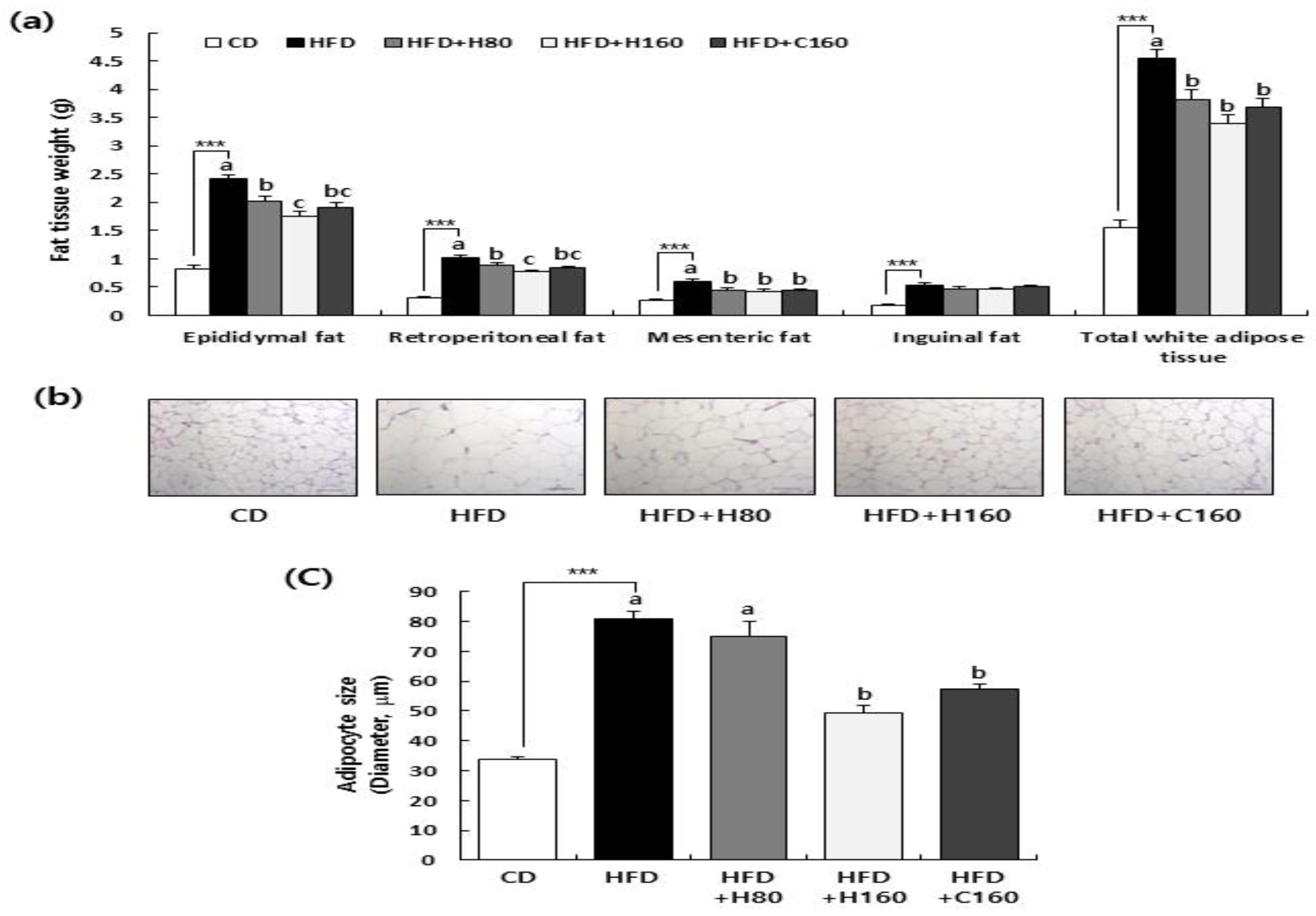
Figure 4.
Effect of H2Oslim® administration on fat accumulation in livers of HFD-fed HFD C57BL/6N mice. Mice fed HFD were treated with H2Oslim® by oral gavage for eight weeks. (a) Extracted liver tissues were fixed, embedded in paraffin, and cut into 5 μm-thick slices. Tissue sections were stained with H&E. Representative H&E-stained images of liver tissue (n = 5, 200x magnification) are shown. (b) Liver tissues were embedded in Tissue-Tek OCT compound, serially sectioned to a thickness of 5 μm, and stained with Oil-red O. Representative Oil-red O-stained images of liver tissue (n = 5, 200x magnification) are shown. (c) Liver weights. (d, e, f) Total lipids from liver tissues were extracted and contents of total lipids (d), triglycerides (e), and total cholesterol (f) in liver tissues were measured. Each bar represents the mean ± SEM (n = 10). *, P < 0.05 and **, P < 0.01, significantly different from the CD group. Different letters indicate significant differences between HFD, HFD+H80, HFD+H160, and HFD+C160 groups at P < 0.05.
Figure 4.
Effect of H2Oslim® administration on fat accumulation in livers of HFD-fed HFD C57BL/6N mice. Mice fed HFD were treated with H2Oslim® by oral gavage for eight weeks. (a) Extracted liver tissues were fixed, embedded in paraffin, and cut into 5 μm-thick slices. Tissue sections were stained with H&E. Representative H&E-stained images of liver tissue (n = 5, 200x magnification) are shown. (b) Liver tissues were embedded in Tissue-Tek OCT compound, serially sectioned to a thickness of 5 μm, and stained with Oil-red O. Representative Oil-red O-stained images of liver tissue (n = 5, 200x magnification) are shown. (c) Liver weights. (d, e, f) Total lipids from liver tissues were extracted and contents of total lipids (d), triglycerides (e), and total cholesterol (f) in liver tissues were measured. Each bar represents the mean ± SEM (n = 10). *, P < 0.05 and **, P < 0.01, significantly different from the CD group. Different letters indicate significant differences between HFD, HFD+H80, HFD+H160, and HFD+C160 groups at P < 0.05.
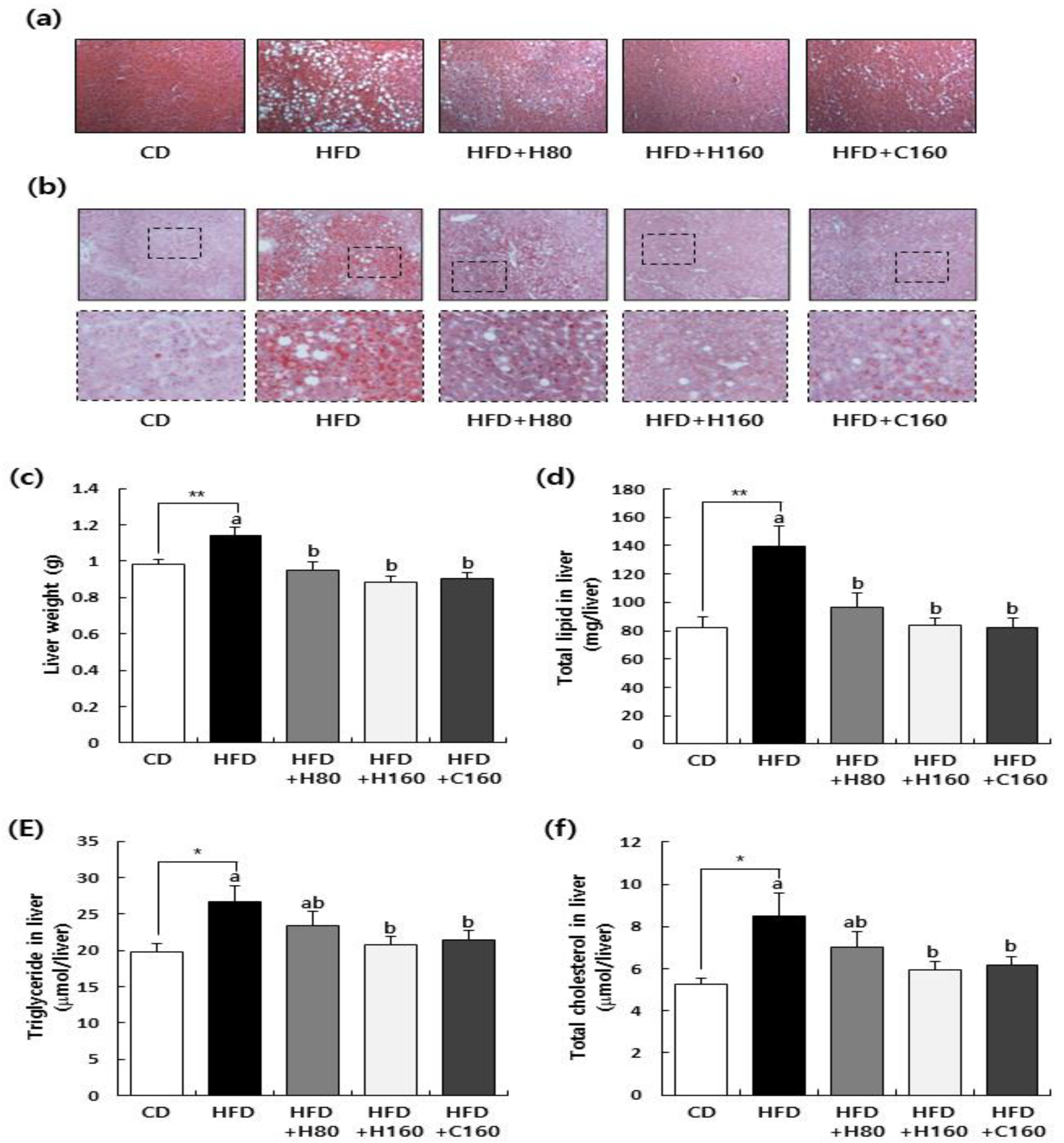
Figure 5.
Effect of H2Oslim® administration on fat excretion in feces of HFD-fed HFD C57BL/6N mice. Mice fed HFD were treated with H2Oslim® by oral gavage for eight weeks. One day before the end of experiments, samples of feces were obtained from each mouse for 24 h. Total lipids from feces were extracted and contents of total lipids (a), triglycerides (b), and total cholesterol (c) in feces were measured. Each bar represents the mean ± SEM (n = 10). ***, P < 0.001, significantly different from the CD group. Different letters indicate significant differences between HFD, HFD+H80, HFD+H160, and HFD+C160 groups at P < 0.05.
Figure 5.
Effect of H2Oslim® administration on fat excretion in feces of HFD-fed HFD C57BL/6N mice. Mice fed HFD were treated with H2Oslim® by oral gavage for eight weeks. One day before the end of experiments, samples of feces were obtained from each mouse for 24 h. Total lipids from feces were extracted and contents of total lipids (a), triglycerides (b), and total cholesterol (c) in feces were measured. Each bar represents the mean ± SEM (n = 10). ***, P < 0.001, significantly different from the CD group. Different letters indicate significant differences between HFD, HFD+H80, HFD+H160, and HFD+C160 groups at P < 0.05.
Table 1.
Effect of H2Oslim® on pancreatic lipase activity in vitro .
Table 1.
Effect of H2Oslim® on pancreatic lipase activity in vitro .
| H2Oslim® (mg/mL) |
Inhibition of pancreatic lipase activity (%) |
| 0 |
0 d
|
| 0.01 |
21.4 ± 2.0 c
|
| 0.05 |
29.1 ± 2.2 b
|
| 0.1 |
32.2 ± 2.4 ab
|
| 0.5 |
33.2 ± 2.1 ab
|
| 1.0 |
36.9 ± 1.7 a
|
| 1.5 |
35.1 ± 2.0 a
|
| 2.0 |
34.5 ± 1.7 ab
|
| 3.0 |
32.3 ± 1.7 ab
|
Table 2.
Effect of H2Oslim® administration on body weight, body composition, and food intake in HFD-fed C57BL/6N mice.
Table 2.
Effect of H2Oslim® administration on body weight, body composition, and food intake in HFD-fed C57BL/6N mice.
| |
CD |
HFD |
HFD+H80 |
HFD+H160 |
HFD+C160 |
| Initial body weight (g) |
19.2 ± 0.7 |
20.7 ± 0.4 |
21.1 ± 0.2 |
20.7 ± 0.5 |
21.1 ± 0.2 |
| Final body weight (g) |
29.4 ± 0.4 |
40.6 ± 0.7***,a
|
34.8 ± 0.5 b
|
33.5 ± 0.9 b
|
35.5 ± 1.0 b
|
| Body weight gain (g) |
10.2 ± 0.9 |
20.0 ± 0.6***,a
|
13.7 ± 0.4 b
|
12.8 ± 1.2 b
|
14.4 ± 0.9 b
|
| Lean mass percentage (%) |
75.8 ± 0.8 |
60.4 ± 0.7***,b
|
62.4 ± 0.8 ab
|
64.1 ± 1.0 a
|
63.2 ± 0.8 a
|
| Fat mass percentage (%) |
24.2 ± 0.9 |
39.6 ± 0.7***,a
|
37.6 ± 0.8 ab
|
35.9 ± 1.0 b
|
36.8 ± 0.8 b
|
| Food intake (g/day) |
2.85 ± 0.04 |
2.46 ± 0.04***,a
|
2.18 ± 0.02 c
|
2.24 ± 0.02 c
|
2.32 ± 0.01 b
|
| Food efficiency ratio1
|
0.065 ± 0.006 |
0.148 ± 0.005***,a
|
0.115 ± 0.004 b |
0.104 ± 0.010 b
|
0.112 ± 0.007 b
|
Table 3.
Effect of H2Oslim® administration on serum glucose and lipid levels and serum ALT and AST activities in HFD-fed C57BL/6N mice .
Table 3.
Effect of H2Oslim® administration on serum glucose and lipid levels and serum ALT and AST activities in HFD-fed C57BL/6N mice .
| |
CD |
HFD |
HFD+H80 |
HFD+H160 |
HFD+C160 |
| Glucose (mg/dL) |
197.2 ± 6.6 |
222.6 ± 8.4*,a
|
186.3 ± 6.5 b
|
178.0 ± 10.8 b
|
195.6 ± 13.0 ab
|
| Triglyceride (mg/dL) |
38.0 ± 2.5 |
54.5 ± 2.8***,a
|
47.2 ± 3.2 ab
|
41.1 ± 2.7 b
|
39.0 ± 2.3 b
|
| Total cholesterol (mg/dL) |
145.4 ± 9.2 |
176.2 ± 3.8**
|
171.2 ± 5.6 |
166.4 ± 9.5 |
160.0 ± 4.6 |
| ALT (U/L) |
51.4 ± 7.4 |
122.1 ± 21.6**,a
|
70.4 ± 8.9 b
|
55.9 ± 5.6 b
|
90.2 ± 22.4 ab
|
| AST (U/L) |
118.8 ± 12.1 |
192.1 ± 19.6**,a
|
138.3 ± 9.5 b
|
139.2 ± 11.1 b
|
169.3 ± 17.8 ab
|