1. Introduction
Throughout the geological history of the Earth, transgressions and regressions of the sea have occurred in various regions. The retreat of the sea basin, accompanied by the movement of the coastline towards the sea (regression), in some cases leads to the separation of a part of the sea area and its transformation into a residual, relict reservoir. At the same time, a crucial restructuring of all components of the ecosystem takes place. If an isolated basin freshens up, then marine species are replaced by brackish and then freshwater ones; if the reservoir remains saline, then the species composition of plankton and benthos change in accordance with changes in other hydrological parameters. Patterns of changes in the species composition allow reconstructing the history of the reservoir based on the remains of organisms in the layers of the bottom sediment formed at different stages of isolation. The plausibility of the reconstructions would be better if they could be corrected for direct observations of community change along with the gradual increase of the isolation. There is a unique opportunity for this in the western part of the White Sea, where, as a result of the post-glacial uplift, many coastal water bodies were formed, which are at different stages of isolation from the sea [
1,
2].
In the course of increasing isolation from the sea, the reservoir can pass through the meromictic stage with vertical stratification that persists year after year for a long time [
3]. The species composition of phytoplankton (PhP) and its quantitative characteristics in meromictic water bodies are determined by many factors, just as in holomictic water bodies (with seasonal mixing of the entire water column). They depend on the climate, chemical composition of water, its mineralization, trophic status of the reservoir, etc. Nevertheless, there are some common features in the structure of PhP in water bodies with stable stratification. Among them is a decrease in quantitative characteristics and species richness. This has also been noted in lakes that alternate periods of meromictic periods with complete mixing episodes [
4,
5,
6]. Stable stratification results in deposition of nutrients in the monimolimnion and they are inaccessible to aerobic PhP [
7]. This also causes a weakened spring algal blooms because of long-lasting nutrient deposition in deep layers.
In most continental meromictic lakes, PhP is represented by a small number of species that can reach high biomass, especially in the chemocline zone [
8,
9,
10,
11,
12,
13,
14]. The most abundant component of PhP is often cyanobacteria [
15,
16,
17], in some cases, diatoms [
14,
18,
19,
20], sometimes Chlorophyta [
7]. Diatoms often dominate also in the meromictic lakes of marine origin [
21,
23]. In stratified water bodies, a typical phenomenon is deep chlorophyll maximum [
23]. It is often associated with the development of cyanobacteria [
15,
16] or cryptophytes capable of mixotrophy [
23,
24,
25,
26,
27,
28].
In the surface horizon of meromictic water bodies, a different community than the chemocline community usually forms. For example, in Lake Svetloe (Arkhangelsk region, Russia), diatoms bloom in spring and early summer, while only cyanobacteria inhabit the chemocline [
29,
30]. In some cases, the highest intensity of oxygenic photosynthesis can be recorded near the lake surface [
17].
In meromictic water bodies of marine origin, there are also two zones of PhP concentration: one near the surface (more often consisted of diatoms, but often with the dominance of cyanobacteria), and the other in the depths of the water body, with cryptophytes being an important component there [
31,
32,
33,
34]. In the relict meromictic Lake Mogilnoye (Barents Sea, Kildin Island), the freshwater mixolimnion is usually dominated by diatoms and cyanobacteria, while the underlying salt layer is dominated by dinophyte order Gymnodiniales [
2,
35]. In coastal stratified marine water bodies, dinophytes also play an important role, reaching a high diversity there [
23,
32,
34,
36]. Flagellates, including cryptophytes, dominate in Antarctic meromictic lakes [
37].
Although the PhP of continental meromictic water bodies is well studied, the same cannot be said of coastal marine meromictic water bodies, especially in Russian Arctic where there are very few papers on phytoplankton and there only one research of seasonal succession. One of the most studied is Lake Mogilnoye on Kildin Island in the Barents Sea [
21,
35], but due to its geographical remoteness, research in it is limited to episodic expeditions in the summer. Much more convenient for monitoring studies are meromictic water bodies on the coast of the White Sea [
1,
2]. Nevertheless PhP is in the very initial phase of study. Among the water bodies that separate from the White Sea, the taxonomic composition, vertical distribution, and seasonal succession of PhP have been studied only in one - in the salt lagoon called Lake Kislo-Sladkoe (literally “Sour-and-Sweet”) near the White Sea Biological Station of the Lomonosov Moscow State University [
22]. The second example is the Lagoon on the Cape Zeleny [
38]. In this lagoon, benthos [
39], coastal vegetation [
40], microbial community [
41], and partly zooplankton [
42,
43] were studied, but information on PhP was only fragmentary [
1,
43,
44].The aim of this work is to trace the seasonal dynamics of PhP in the coastal meromictic Lagoon on the Cape Zeleny, partially isolated from the White Sea. The composition, abundance and biomass of PhP in different layers of water column were studied; their dynamics was compared to the sea PhP community; abiotic factors determining the dynamics of the PhP structure were identified.
3. Results
3.1. Hydrological Conditions
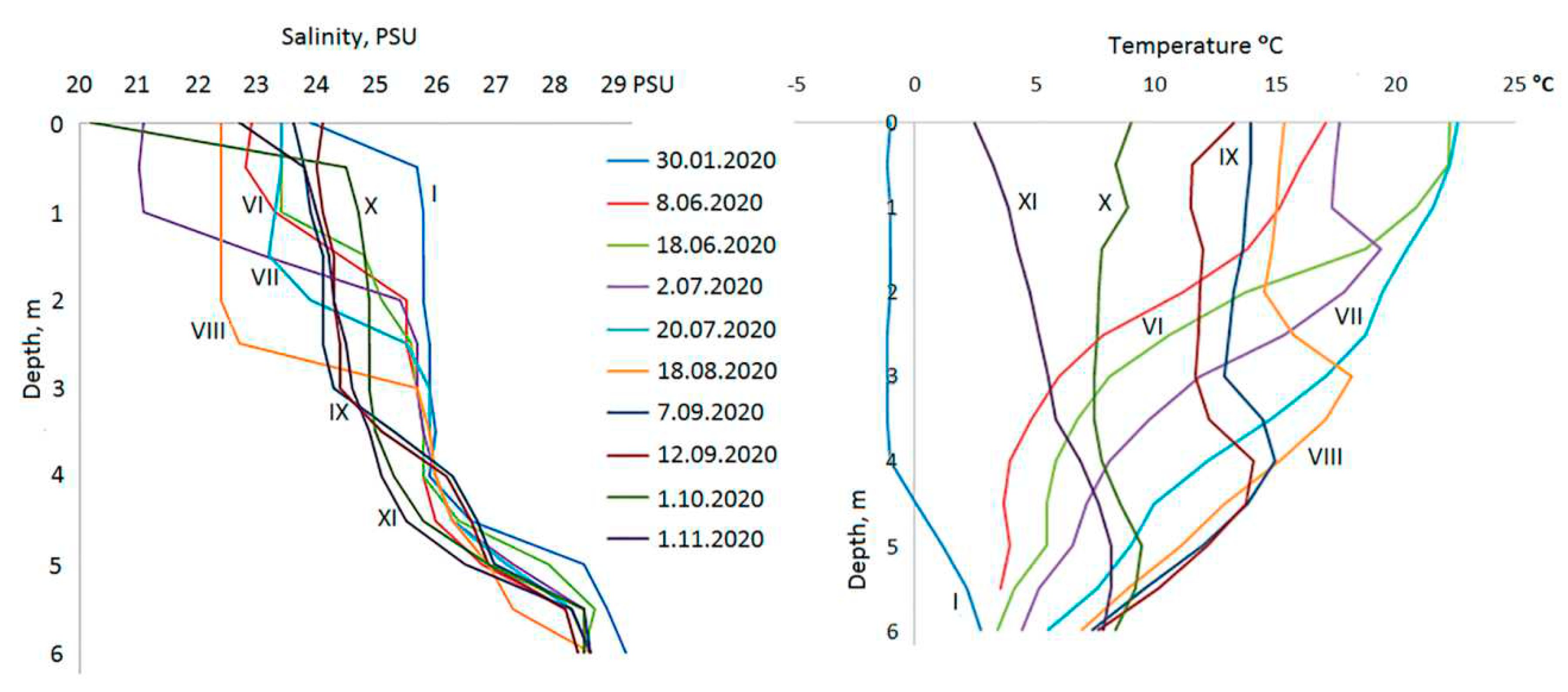
Entire water column in the Lagoon is salty throughout the year (
Figure 3). Despite the influence of the fresh runoff and precipitation the salinity of the surface layer doesn’t fall below 20‰, and during the freeze-up period it increased to 25.7‰.At a depth of 1 m the range of seasonal salinity fluctuations was 21.1-25.8‰, deeper they attenuated. Starting from a depth of 4 m, salinity variations did not exceed 1‰. According to salinity in the Lagoon, the water column can be divided into several zones: 1) the surface layer (0-1 m) characterized by widest range of seasonal variations; 2) halocline at 1-2.5 m; 3) 2.5-4 m layer with higher salinity; 4) 4-5 m second halocline; 5) from a horizon of 5.5 m and below is a near-bottom zone with high salinity (more than 28‰). In the sea, salinity values varied in the range of 20.2-24.6‰ in the summer-autumn period, and reached 27‰ in winter.
Temperature also differs in different layers of the Lagoon (
Figure 3). Winter cooling to negative temperature covers the upper 4 m of the water column; starting from 4.5 m and below, the temperature is positive throughout the year. Near the surface, the temperature varied from –1.1ºС (in January) to +22.6ºС (at the end of July), but in the near-bottom horizon at a depth of 6 m from +2.8 ºС to +8.4 ºС. The seasonal course of temperature varies at different horizons. In the layer from the surface to a depth of 2.5 m, maximum heating was recorded at the end of July, at the depth of 3-4 m – in August, 4.5-5.5 m – in September, and in the bottom zone – in October.
The seasonal course of temperature in the adjacent marine area is similar to that in the surface layer of the Lagoon, but summer temperature is 1-5 degrees lower in the sea. In November, the surface water layer in the Lagoon cools faster than in the sea, which favors outrunning freezing in it.
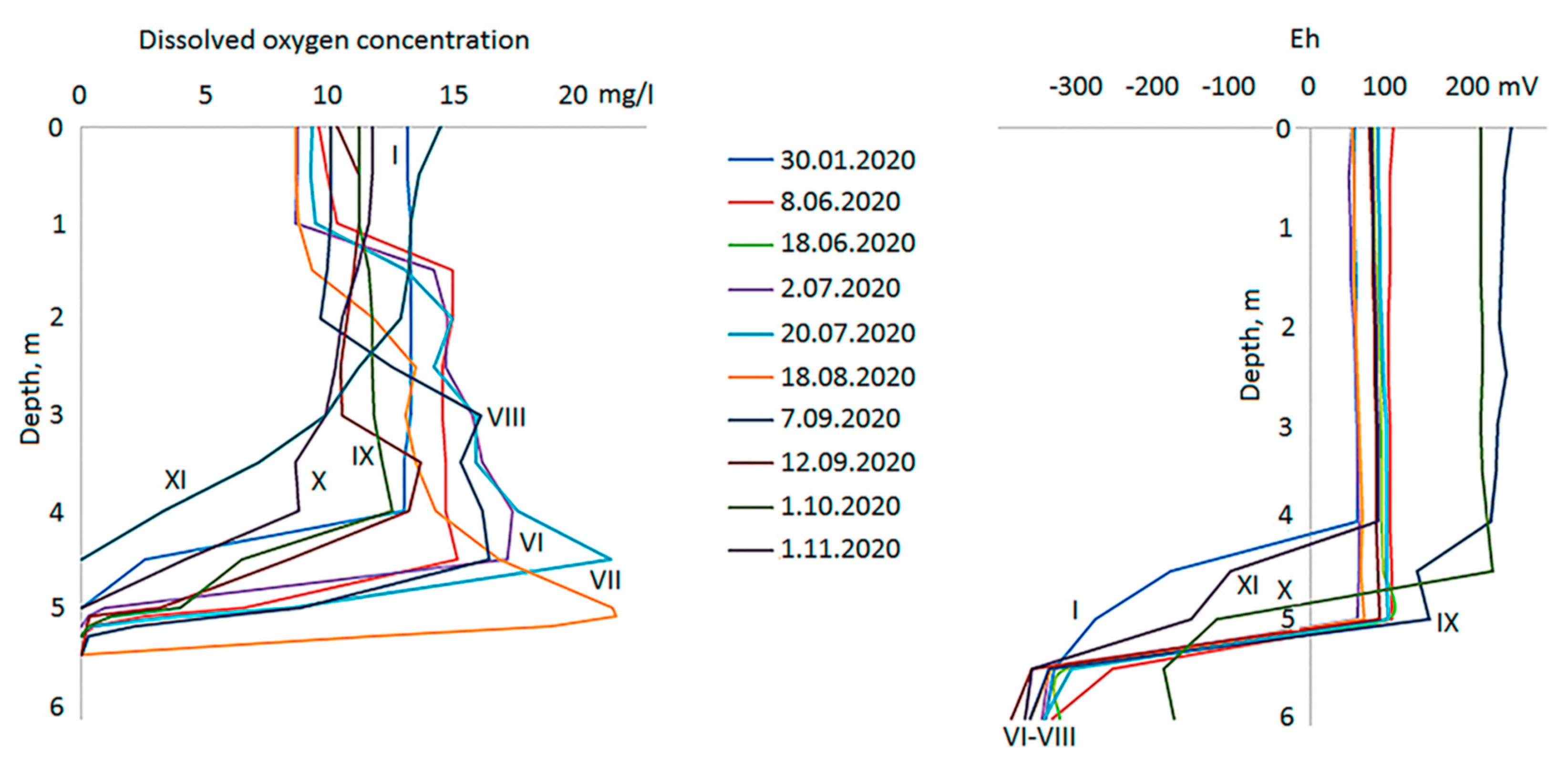
Oxygen regime differs significantly in the different water layers of the Lagoon (
Figure 4). In the surface layer of 0-1 m, the dissolved oxygen concentration corresponds to the saturation throughout the year; just slightly lower in January (88%). In January, October and November, the concentration of oxygen decreased with depth to zero at a depth of 4.5-5.0 m. In the summer months, a deep oxygen maximum amounted to 14-22 mg/l (up to 186% saturation) was observed in the middle part of the water column. Immediately below this horizon, only 0.5 m lower, oxygen dropped sharply. The upper border of anaerobic zone is situated a depth of 5.2-5.5 m in summer. The profiles of the redox potential clearly show how abruptly the transition from aerobic environment with positive ORP to anaerobic with negative values occurs in the chemocline (
Figure 4). Near the bottom, ORP is always less than -300 mV, while in the zone supersaturated with oxygen, its values are (+100)-(+200) mV. Thus, the potential difference recorded in the chemocline of the studied Lagoon can reach up to 0.4-0.5 V.
In the sea, concentration of dissolved oxygen corresponded to saturation at a given temperature. The minimum value of 10.2 mg/l was recorded during the warmest time and the maximum value of 13 mg/l in November. In January, the oxygen concentration in the sea was not measured.
The pH profiles are in good agreement with vertical zonation for other hydrological parameters (
Figure 5). The surface layer stands out, where the pH is, at times, slightly less than in the underlying water. Throughout the layer between 0.5 m and 3.5 m pH is almost uniform, but varies seasonally, gradually shifting to the alkaline during the PhP growing season. In summer, at a depth of 4-5 m the pH is noticeably higher than in the overlying stratum. Below the 5 m horizon pH sharply decreases, the lowest values are recorded near the bottom. In this water body pH never fall below the neutral value of 7.0.
Table 4. 5-5 m and sharp drop at a depth of 5.0-5.5 m, where the chemocline was located (Figure
5). A suspension of microorganisms in a chemocline does not let light down, and the near-bottom 1-1.5 m of the water column are in the aphotic zone. When the Lagoon is covered with ice, the light intensity underwater is less than in summer. In January, the maximum depth of light propagation
was 3 m, and in November it was 5 m.
The compensation depth, where 1% of sunlight reaches, from June to November fell to a depth of 5.1-5.3 m, that is, it was located in the upper part of the chemocline; in January it was at a horizon of 1.5 m. According to measurements of underwater illuminance, all the samples fell on the euphotic zone, but the ones from chemocline at a depth of 5.0-5.4 m, were taken at its lower boundary.
The daytime length in January was 6 hours, in June-early July 24 hours, then gradually decreased and reached 7.2 hours by the beginning of November.
3.2. Taxonomic Composition of Algal Flora
Totally 293 species and taxa ranks of supraspecific level of algae and cyanobacteria were found, of which 227 were identified to species or genus belonging to 18 classes and 11 phyla (table 1).
Table 1.
Taxonomic composition of phytoplankton in the lagoon on the Cape Zeleny.
Table 1.
Taxonomic composition of phytoplankton in the lagoon on the Cape Zeleny.
| Phylum |
Class |
Number of taxa |
| Miozoa |
Dinophyceae |
68 |
| |
Oxyrrhidophyceae |
1 |
| Bacillariophyta |
Bacillariophyceae |
61 |
| |
Mediophyceae |
34 |
| |
Coscinodiscophyceae |
9 |
| Cyanobacteria |
Cyanophyceae |
25 |
| Chlorophyta |
Pyramimonadophyceae |
7 |
| |
Chlorophyceae |
3 |
| |
Chlorodendrophyceae |
2 |
| |
Trebouxiophyceae |
1 |
| Ochrophyta |
Chrysophyceae |
4 |
| |
Dictyochophyceae |
4 |
| Euglenozoa |
Euglenophyceae |
3 |
| Cercozoa |
Filosa |
1 |
| |
Thecofilosea |
1 |
| Charophyta |
Zygnematophyceae |
1 |
| Haptophyta |
Coccolithophyceae |
1 |
| Katablepharidophyta |
Katablepharidophyceae |
1 |
| Cryptophyta |
|
not identified |
Most of the identified algal flora is represented by marine species, but there were also freshwater species – 38 species belonging to 7 classes: Cyanophyceae (25), Bacillariophyceae (5), Coscinodiscophyceae (2), Chlorophyceae (2), Chrysophyceae (2), Trebouxiophyceae (1), Zygnematophyceae (1).
3.3. Biomass and Dominant Taxa in the Lagoon
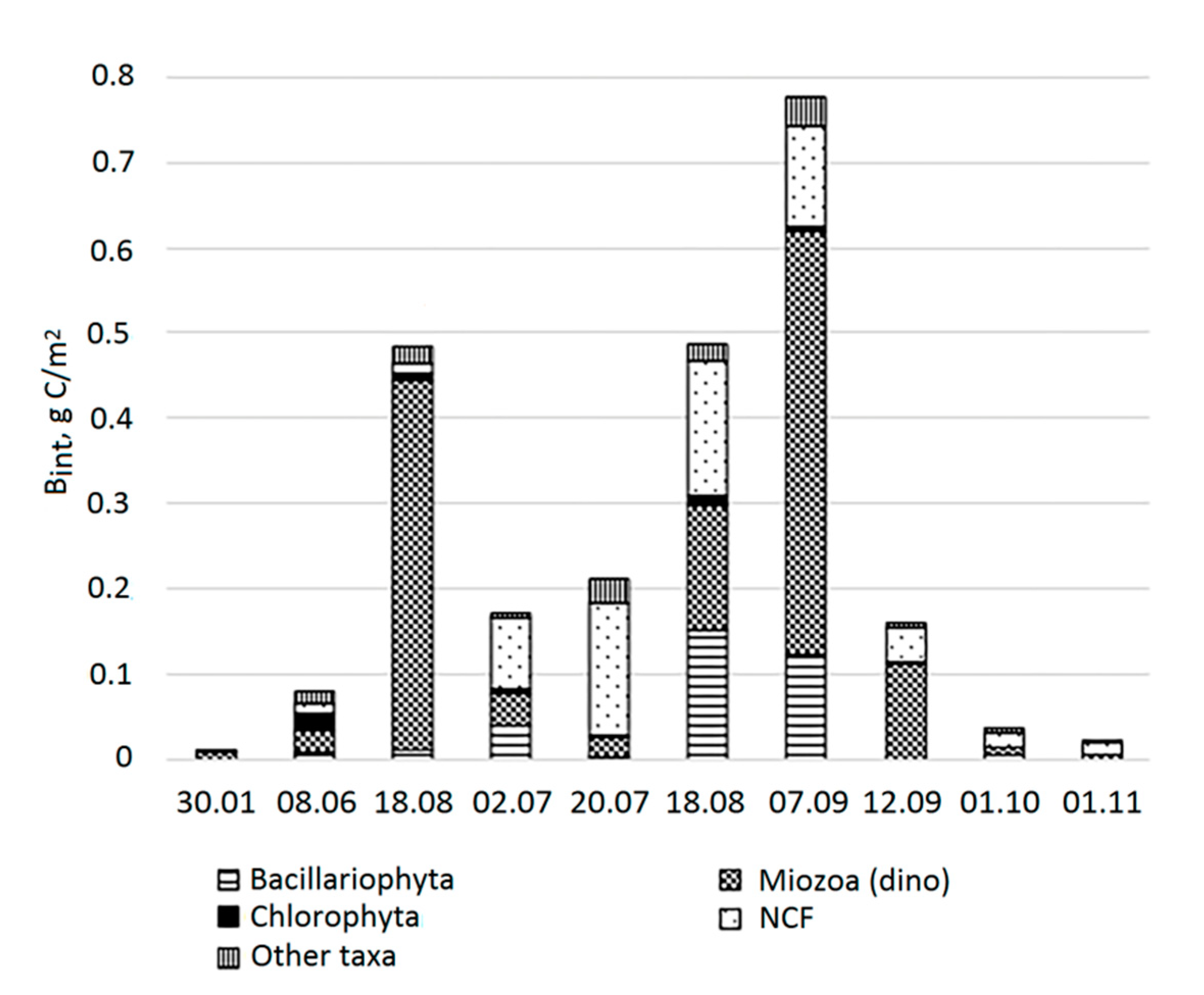
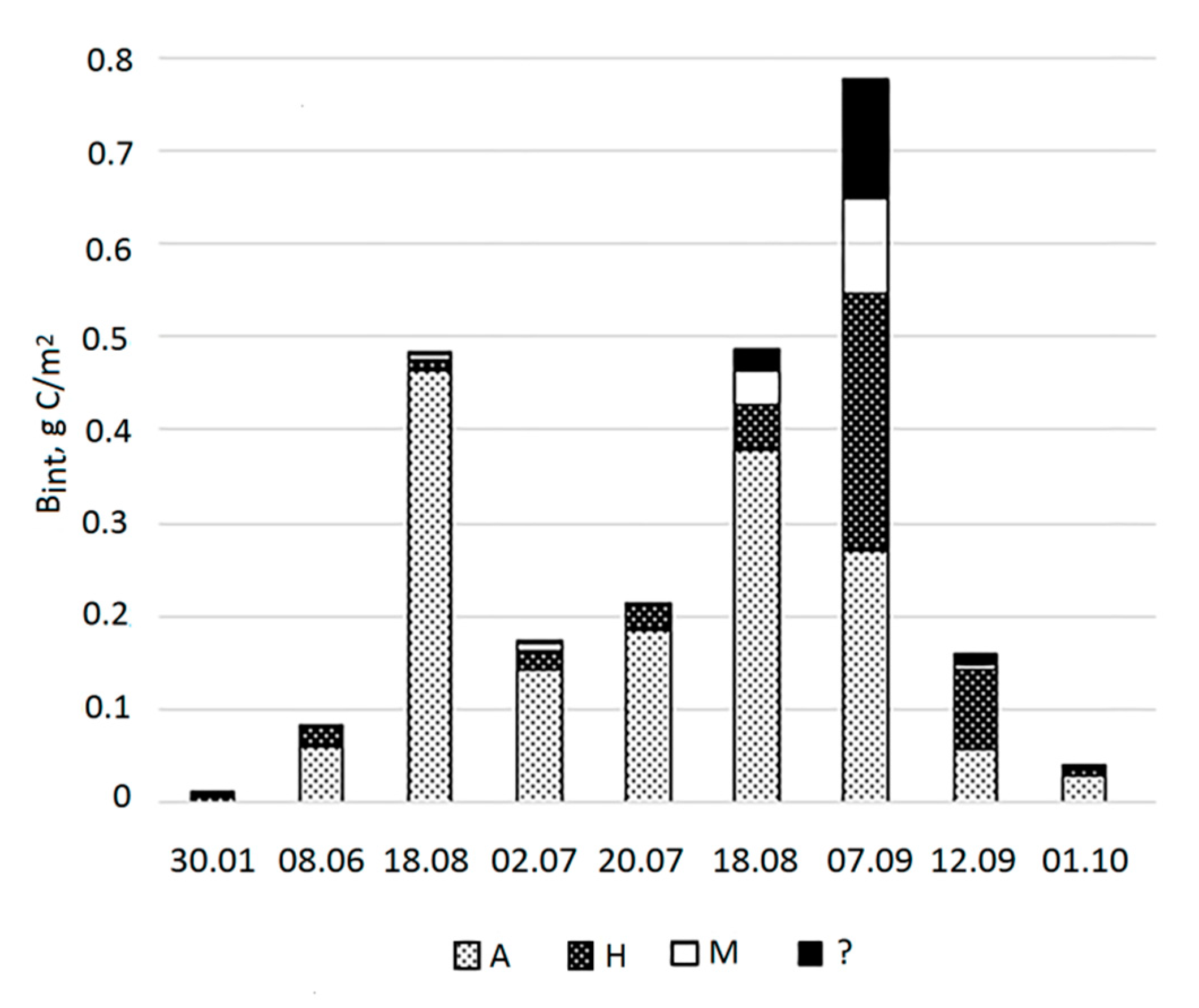
B
int in the water column varied from 0.01 g C/m
2 in January to 0.78 g C/m
2 in early September (
Figure 6).
In winter, PhP in the Lagoon was scarce, a little more concentrated in the chemocline below the redox transition, where oxygen is already absent (table 2). The upper horizons were dominated by autotrophic dinoflagellates and freshwater cyanobacteria. At depths of 2.5–3.5 m, various types of heterotrophic dinoflagellates and autotrophic NCF predominated. In the chemocline, the bulk of algae were heterotrophic dinoflagellates. The similarity of communities growing at different depths was low and was determined by 7 common species (table 3). The most important contributors to the similarity were Gymnodinium wulffii, which dominated the BC at a depth of 2.5-4.5 m, and Paralia sulcata, which was not among the dominants.
In early June, the B
int was also low. It was represented by different phyla, among which dinoflagellates and green algae predominated, and to a lesser extent, diatoms and NCF. Autotrophs accounted for the largest share (0.75) in B
int (
Figure 7). They dominated mostly in the surface layer of the Lagoon and in the chemocline, as well as in the surface horizon of the sea. The highest B
C was noted in the chemocline of Lagoon. The similarity of communities in different horizons was 38% determined by 19 species with the major contribution of dinoflagellate
Heterocapsa rotundata and prasinophyte flagellate
Pyramimonas cf
. diskoicola. In mid-June B
int increased due to the growth of dinoflagellates, mostly autotrophic. Autotrophs dominated in the surface horizon, but also in the chemocline. The highest B
C was observed in the chemocline, where
Gymnodinium arcticum dominated (0.97). The similarity of communities at different horizons was 37% determined by 16 species. TSs were diatom
Cyclotella choctawhatcheeana and marine heterotrophic Cercozoa
Ebria tripartita, brackish-water cyanobacterium
Synechocystis salina, and dinoflagellate
G. arcticum.
In early July, Bint decreased by more than 2 times compared to that in mid-June, mainly due to a decrease in the Bint of dinoflagellates by more than 11 times. Same time the Bint of diatoms and NCF increased. As well as in June, the PhP was mostly autotrophic in the Lagoon with the dominance of autotrophs in all horizons except for 1.5 m. The highest BC, as before, was observed in the chemocline, but this time due to the growth of small-cell NCF. The similarity of communities of different horizons was 40% and determined by 10 species. The TSs for all horizons were C. choctawhatcheeana and chrysophyte Ollicola vangoorii.
In mid-July, Bint was close to that in early July. The Bint of diatoms and dinoflagellates continued decrease, the abundance of NCF and other taxa (Ochrophyta, Cercozoa, and Cryptophyta) increased. The PhP continued to be mostly autotrophic with the dominance of autotrophs in the surface and two horizons close to chemocline. The highest BC was also observed in the chemocline due to the growth of small unidentified cells. The similarity between communities of different horizons was 42% determined by 19 species. C. choctawhatcheeana, as well as cryptomonads and E. tripartita, remained TS for all layers of the Lagoon.
In mid-August, Bint increased again to the level of mid-June due to an abundance of diatoms and dinoflagellates. PhP continued to be mostly autotrophic, but the Bint of hetero- and mixotrophs increased. Autotrophs dominated from the surface down to a depth of 3.5, while in the chemocline, unlike in previous periods, both autotrophs and heterotrophs dominated. The highest BC, as previously, was observed in the chemocline due to the massive development of unidentifiedgreen oval cells, as well as heterotrophic dinoflagellate O. marina. The similarity of communities from different horizons was 40% and was determined by 19 species with C. choctawhatcheeana as a most TS throughout lagoon community.
In early September Bint increased to maximum values mainly due to increase in the abundance of dinoflagellates. Trophic statue of the PhP shifted to mostly heterotrophic and mixotrophic. Autotrophs continued dominate form the surface to a depth of 3.5 m, and in the chemocline, while heterotrophs dominated above the chemocline. Mass proliferation of two species (Kryptoperidinium triquetrum and C. choctawhatcheeana) led to a significant increase in BC at the horizons of 1.5 and 3.5 m, nevertheless, the highest BC, as previously, was observed in the chemocline where dinoflagellates O. marina and Lebouridinium glaucum, Gymnodinium sp. and unidentified small green oval cells dominated. The similarity of communities from different horizons was 45% determined by 25 species. C. choctawhatcheeana remained TS for the lagoon community, as well as dinoflagellate K. triquetrum spores.
In mid-September, 5 days after the previous sampling, the values of Bint decreased by almost 5 times, along with decrease of the abundance of all taxa. PhP became mostly heterotrophic. Heterotrophs dominated in all horizons except of the surface layer. The highest BC, as before, was observed in the chemocline, where the main contribution of the heterotrophic dinoflagellate O. marina, and to a lesser extent of NCF. The similarity of communities of different horizons was 46% determined by 21 species, none of which contributed more than 10% to the similarity. K. triquetrum was the TS.
By the beginning of October, Bint decreased by more than 4 times due to a decrease in the abundance of all taxa. Autotrophs dominated again. BC was the highest in the chemocline. O. marina constituted the bulk of the PhP in the chemocline. The similarity of communities from different horizons was 41% and was determined by 9 species. TSs for the lagoon PhP community were C. choctawhatcheeana, cryptomonads, and K. triquetrum.
By the beginning of November, the abundance of all taxa continued to decrease, and Bint decreased by another 1.5 times. PhP remained mostly autotrophic. It is not possible to assess the difference in trophic status between the horizons, since many horizons were dominated by unidentified species. The highest BC registered at a depth of 1.5 m with a large amount of NCF with a cell diameter of 10 µm. O. marina was absent in the chemocline, replaced by cryptomonads, few species of Gymnodinium, and the heterotrophic dinoflagellate Micracanthodinium claytonii as a dominant. The similarity of communities from different horizons was 32% determined by 13 species. The TSs for the lagoon PhP community were species of the genus Gymnodinium and the freshwater algae Monoraphidium contortum (Chlorophyceae).
3.4. Phytoplankton Communities on the Lagoon and in the Sea
One-way ANOSIM revealed significant differences only between samples collected on different dates (R=0.442, p=0.1%) and insignificant differences in PhP communities from different horizons (R=0.07, p=2.7).
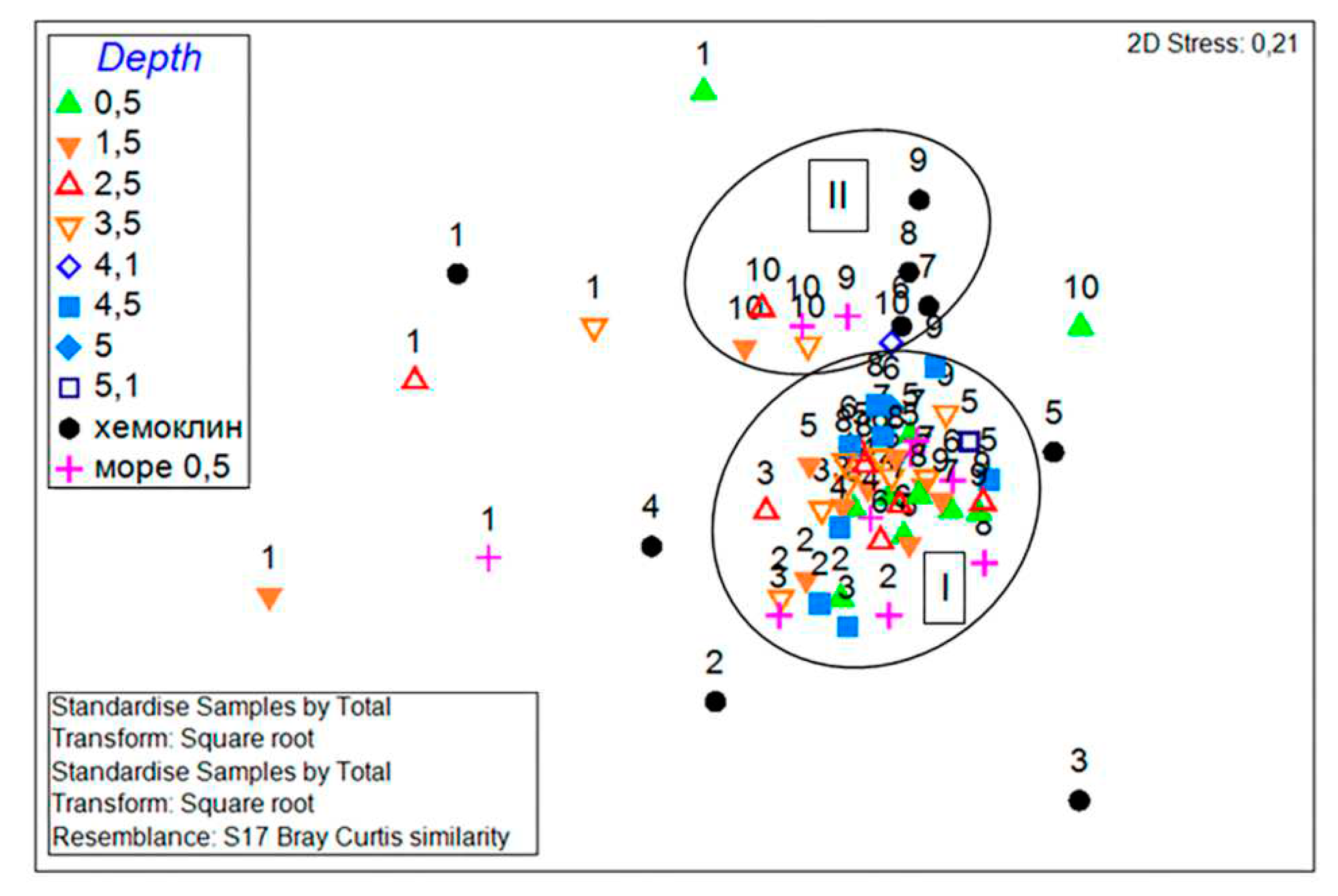
Non-metric multidimensional scaling (nMDS) of biomass-based (B
C) PhP samples grouped the samples into 2 similarity groups (
Figure 8). Group I includes samples from water column above the chemocline from June to October, and marine samples from June to September. Group II includes almost all November samples throughout the water column in the Lagoon, most of the seasonal samples from the chemocline, and marine samples in October–November. None of the similarity groups include samples taken in January; June and July samples from the chemocline are located apart, as well as the November surface sample.
One-level ANOSIM revealed significant differences between the two similarity groups (R=0.677, p=0.1%). According to the results of the SIMPER analysis, the groups differ by 79%, differentiating species are O. marina, Gymnodinium spp., H. rotundata and P. cf. diskoicola. The similarity of the PhP structure within group I was 35%, TSs of the group are C. choctawhatcheeana, P. cf. diskoicola, and H. rotundata. The similarity within group II was 32%, TSs of the group were Gymnodinium spp., O. marina and C. choctawhatcheeana. The last species was TSs in both groups, but its abundance differed, being higher in group I.
3.5. The Structure of the PhP in the Surface Layer of the Lagoon and the Sea
In winter, BC in the sea was several times higher than the in the Lagoon. The composition of dominants in the surface layer of the Lagoon and in the sea differed. Autotrophic dinoflagellates predominated in the Lagoon, and mixo- and heterotrophic forms vegetated in the sea (table 2). Two species of Dinophysiales (Dinophysis acuminata and Phalacroma rotundatum) dominated in the sea, and BC was low. The similarity of the marine and lagoon communities was 10% due to Protoperidinium brevipes vegetating in both areas (table 3).
In early June, BC was the same in the sea and in the Lagoon. Marine PhP was partly similar with those in the Lagoon: two dominating species were same (Heterocapsa rotundata, P. cf. diskoicola), but co-dominants were different. In the lagoon there were NCF cells (3-5 µm), and in the sea Skeletonema costatum and cryptomonads. The first was present in all horizons of the Lagoon except for the surface layer; cryptomonads appeared everywhere but in smaller quantities. Due to 10 common species, the similarity between PhP in the sea and in the Lagoon (42%)was higher than the similarity between communities from different horizons of the Lagoon. Dominants H. rotundata, P. cf. diskoicola, along with cryptomonads and golden algae O. vangoorii contributed the most to the similarity between the marine and lagoon communities.
In mid-June, BC in the sea was several times higher than that in the Lagoon, and the dominants were different: in the sea, the filamentous diatom S. costatum, but in the Lagoon, C. choctawhatcheeana. The similarity between the PhP of the sea and Lagoon was only 23% due to nine TSs. E. tripartita and euglenoids influenced the most.
In early July, the BC in the sea was also several times higher than in the Lagoon. In marine plankton, S. costatum continued blooming accompanied by dinoflagellate H. rotundata. In the Lagoon, C. choctawhatcheeana still remained among the dominant species, the dinoflagellates H. rotundata common to the Lagoon and the sea, and the green algae P. cf. diskoicola, but the most abundant were unidentified flagellates. The similarity between the sea and lagoon communities was quite high and amounted to 42% due to 9 characteristic species. H. rotundata and P. cf. diskoicola were the main contributors.
In mid-July, BC in the sea slightly exceeded that in the lagoon surface water layer. In the sea, the dominants have changed. Vegetative cells and spores of dinoflagellates K. triquetrum appeared, cryptomonad cells and NCF appeared in high numbers. Whereas in the Lagoon the dominants remained the same: H. rotundata and P. cf. diskoicola. The similarity between communities of the sea and the Lagoon was high (47%) due to 14 TSs. H. rotundata and Gymnodinium spp. had the greatest influence on the similarity.
Starting from mid-August, the BC in the sea began to lag behind the Lagoon, where it was several times higher. The sea was dominated by H. rotundata and P. cf. diskoicola; in the Lagoon, H. rotundata and Triposarcticus dominated. The similarity between the surface PhP community in the sea and in the Lagoon was 47% due to 9 TSs. H. rotundata and P. cf. diskoicola made the greatest contribution to the similarity.
In early September, BC in the sea was slightly lower than that in the Lagoon. In the marine area, P. cf. diskoicola remained among the dominants and cryptomonads were added. In the Lagoon H. rotundata which dominated here in the previous period reduced its contribution to BC, while K. triquetrum, Lepidodinium chlorophorum, and C. choctawhatcheeana came to the forefront. The similarity between communities of the sea and the Lagoon decreased to 40% determined by 9 TSs. The greatest contribution to the similarity, as in the previous period, made H. rotundata and P. cf. diskoicola, as well as by C. choctawhatcheeana and cryptomonads.
By mid-September, BCin the sea became somewhat higher than in the Lagoon. Marine PhP was represented mainly by cryptomonads, while K. triquetrum, H. rotundata. Cryptomonads continued dominating in the Lagoon. The similarity between the sea and lagoon PhP communities increased to 43% and was determined by 6 TSs. Same with the previous period, H. rotundata, cryptomonads, P. diskoicola, as well as C. choctawhatcheeana contributed to the similarity the most.
In October and November, BC in the sea decreased faster than in the Lagoon. In early October, BC in the sea was more than 8 times less compared with the marine one. Marine PhP was dominated by cryptomonad cells and NCF; in the Lagoon, cryptomonads, C. choctawhatcheeana, and species of the genus Gyrodinium were dominating. The similarity between the PhP communities of the sea and the lagoon was 32% and was determined by 4 TSs with the largest contribution of cryptomonads, C. choctawhatcheeana and K. triquetrum.
In early November, BC in the sea was more than 5 times less than that in the Lagoon. At the same time, only NCF (10 μm) dominated in the sea, while E. tripartita and the diatom Melosira sp. dominated in the Lagoon. The similarity of PhP was 25% and was determined by 7 TSs with the largest contribution of K. triquetrum, Cocconeis costata, M. contortum, and Thalassionema nitzschioides.
3.6. Environmental Factors that Determine the Dynamics and Structure of Phytoplankton
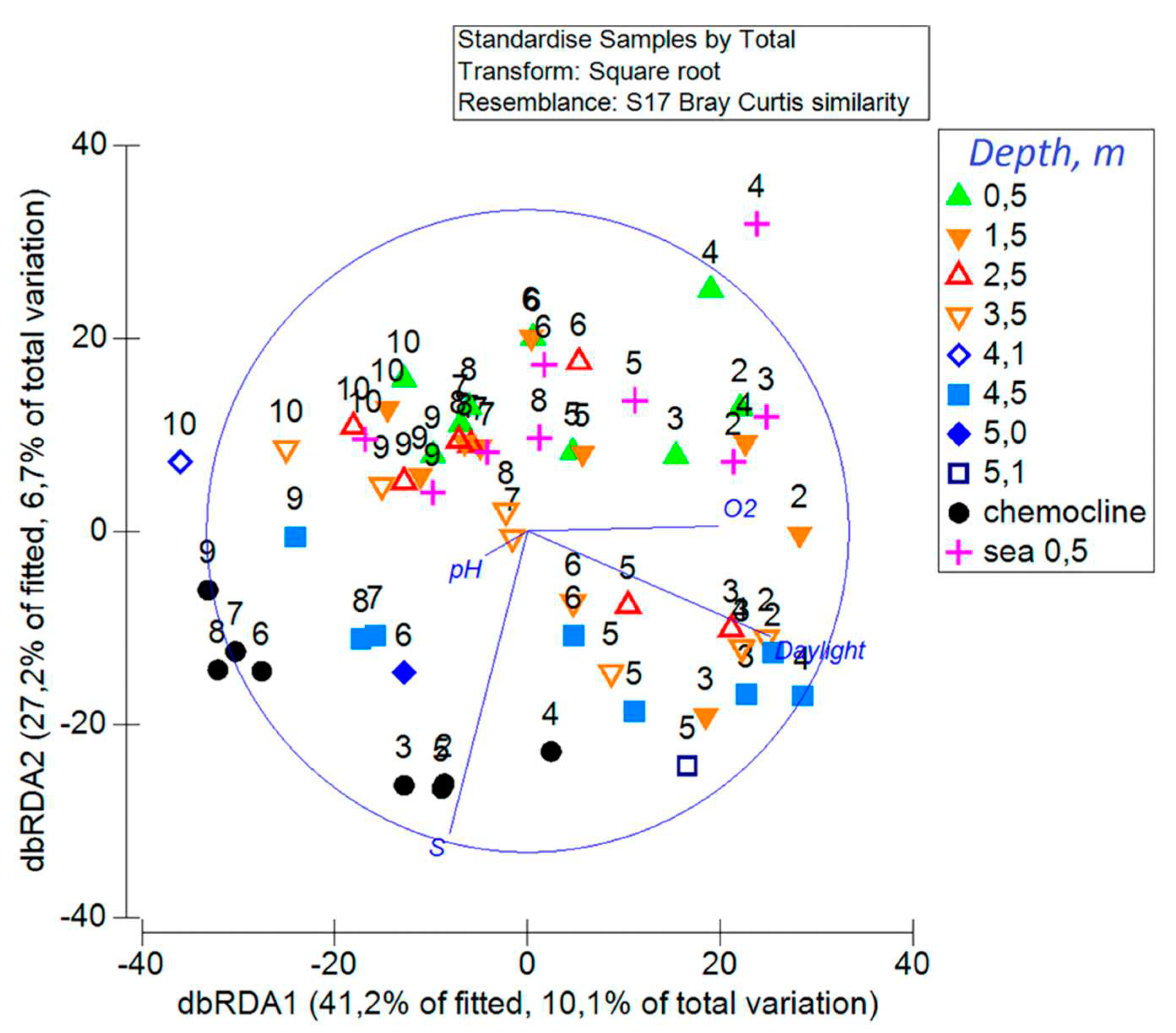
In the marginal tests DistLM, which determines the relationship of each factor with the structure of the community, regardless of other environmental factors, 7 of the 8 studied parameters (salinity, air temperature, concentration of dissolved O
2, pH, ORP, daylight, depth), determined or were determined by the dynamics of PhP statistically significantly (p < 0.0003 in all cases). Only the effect of water temperature was found to be unreliable. In sequential tests, only 4 out of 7 studied parameters showed a statistically significant relationship with the dynamics of the PhP structure (p<0.0004 in all cases): daylight, salinity, dissolved oxygen and pH. They accounted for 24.5% of the variability in the PhP structure. Distance-based redundancy analysis (dbRDA) showed that during the year the PhP structure in the sea and in the entire water column of the Lagoon changes chronologically along the gradient of the daylight (
Figure 9).
The dependence of the PhP structure on the water salinity is also clearly expressed. Moreover, the division of samples into two groups is clearly visible: while one is confined to the upper part of the water column, the other – to the lower part. The boundary between the two groups of samples in early June was located at a depth of between 0.5 and 1.5 m, from mid-June to early September – between 1.5 and 2.5 m, from mid-September to November – between 2.5 and 3.5 m. Despite the distinct division of the Lagoon samples into groups, ANOSIM did not reveal significant differences between the communities of these groups (R=0.08, p=0.4%). The vector of oxygen content in water is close to the daylight vector and reflects its chronological decrease. BC and O2 were negatively correlated (-0.42), while the correlation of BC of autotrophs and BC of heterotrophs with O2 were also negative (-0.31 and 0.39, respectively). In summer, the pH values are more significantly associated with the development of PhP in the chemocline region, where the pH is shifted to the alkaline side, which reflects a decrease in the concentration of carbonates as a result of their use in photosynthesis.
Table 1.
Phytoplankton biomass (BC), dominant species, their contribution to BC and mode of nutrition (P – phototrophs, H – heterotrophs, M – mixotrophs, “?”– protists with an undetermined mode of nutrition) in the Lagoon on the Cape Zelenyat different depths, and in the adjacent area of the White Sea in 2020.In parentheses the depth of the chemocline (Zchem) and the boundary of the euphotic zone (Zeu) in the lagoon are indicated.
Table 1.
Phytoplankton biomass (BC), dominant species, their contribution to BC and mode of nutrition (P – phototrophs, H – heterotrophs, M – mixotrophs, “?”– protists with an undetermined mode of nutrition) in the Lagoon on the Cape Zelenyat different depths, and in the adjacent area of the White Sea in 2020.In parentheses the depth of the chemocline (Zchem) and the boundary of the euphotic zone (Zeu) in the lagoon are indicated.
| Biotope (depth, m) |
BC, mg С/м3
|
Taxon |
Mode of nutrition |
| 30.01.20 |
| 0.5 |
0.06 |
Protoperidinium brevipes (0.58) |
P |
| 1.5 (Zeu) |
0.23 |
cf. Planktolyngbya limnetica (0.78) |
P |
| 2.5 |
0.16 |
Oscillatoria sp.3 (0.19) |
P |
|
Gymnodinium wulffii (0.15) |
H |
| NCF 10 μm (0.15) |
P |
| 3.5 |
0.92 |
Gymnodinium wulffii (0.33) |
H |
| NCF 9-14 μm (0.21) |
P |
|
Dinophyta spp. (0.18) |
? |
4.5
(Zchem) |
16.60 |
Gymnodinium wulffii (0.65) |
H |
|
Gyrodinium spirale (0.35) |
H |
| Sea |
0.47 |
Dinophysis acuminata (0.57) |
М |
|
Phalacroma rotundatum (0.21) |
H |
| 08.06.20 |
| 0.5 |
10.63 |
NCF 3-5 μm (0.21) |
P |
|
Pyramimonas cf.diskoicola (0.20) |
P |
|
Heterocapsa rotundata (0.16) |
P |
| 1.5 |
15.82 |
Ebria tripartita (0.26) |
H |
|
Heterocapsa rotundata (0.12) |
P |
|
Diplopsalis lenticula (0.11) |
H |
| 3.5 |
10.30 |
Pyramimonas cf.diskoicola (0.38) |
P |
|
Heterocapsa rotundata (0.11) |
P |
| 4.5 |
8.87 |
Gyrodinium spirale (0.21) |
H |
| NCF 6-8 μm (0.17) |
P |
|
Heterocapsa rotundata (0.12) |
P |
5.2
(Zchem;Zeu) |
80.37 |
Tetraselmis cordiformis (0.35) |
P |
|
Gymnodinium arcticum (0.18) |
P |
|
Skeletonema costatum (0.11) |
P |
| Sea |
10.22 |
Heterocapsa rotundata (0.16) |
P |
|
Pyramimonas cf. diskoicola (0.11) |
P |
|
Skeletonema costatum (0.11) |
P |
| Unidentified cryptomonad cells 10-20 μm (0.11) |
P |
| 18.06.20 |
| 0.5 |
25.46 |
Cyclotella choctawhatcheeana (0.55) |
P |
| 1.5 |
23.55 |
Ebria tripartita (0.30) |
H |
|
Cyclotella choctawhatcheeana (0.21) |
P |
| 2.5 |
3.17 |
Protoperidinium pellucidum (0.23) |
H |
| 3.5 |
7.56 |
Dinophysis norvegica (0.22) |
М |
|
Ebria tripartita (0.17) |
H |
|
Dinophysis acuminata (0.12) |
М |
| 4.5 |
7.26 |
Tripos arcticus (0.31) |
P |
| Bunch of unidentified green cells 3 μm (0.29) |
P |
5
(Zchem;Zeu) |
1730.91 |
Gymnodinium arcticum (0.97) |
P |
| Sea |
98.09 |
Skeletonema costatum (0.75) |
P |
| 02.07.20 |
| 0.5 |
12.44 |
Flagellate non det. 10-14 μm (0.30) |
P |
|
Heterocapsa rotundata (0.14) |
P |
|
Pyramimonas cf.diskoicola (0.14) |
P |
|
Cyclotella choctawhatcheeana (0.12) |
P |
| 1.5 |
21.10 |
Diplopsalis lenticula (0.37) |
H |
| 3.5 |
13.90 |
Cyclotella choctawhatcheeana (0.63) |
P |
| 4.5 |
88.42 |
Cyclotella choctawhatcheeana (0.33) |
P |
| Bunch of unidentified green cells 3 μm (0.29) |
P |
| NCF 3-5 μm (0.11) |
P |
5
(Zchem) |
197.19 |
Flagellate non det. 5μm (0.54) |
P |
| NCF 3-5 μm (0.29) |
P |
| Sea |
35.25 |
Heterocapsa rotundata (0.47) |
P |
|
Skeletonema costatum (0.14) |
P |
| 20.07.20 |
| 0.5 |
10.82 |
Heterocapsa rotundata (0.24) |
P |
|
Pyramimonas cf.diskoicola (0.14) |
P |
| 1.5 |
12.93 |
Diplopsalis lenticula (0.19) |
H |
| NCF 13 μm (0.11) |
P |
| 2.5 |
25.75 |
Gonyaulax spinifera (0.30) |
P |
|
Ebria tripartita (0.11) |
H |
| 3.5 |
5.43 |
NCF 3-5 μm (0.23) |
P |
|
Ebria tripartita (0.20) |
H |
|
Akashiwo sanguinea (0.15) |
P |
| NCF 6-8 μm (0.13) |
P |
| 4.5 |
24.90 |
Ebria tripartita (0.36) |
H |
| NCF 3-5 μm (0.23) |
P |
| NCF 6-8 μm (0.18) |
P |
| 5.1 |
261.85 |
Unidentified green oval cells 5-6 μm (0.73) |
P |
| NCF 3-5 μm (0.12) |
P |
5.4
(Zchem;Zeu) |
182.94 |
Unidentified green oval cells 5-6 μm (0.82) |
P |
| Sea |
14.08 |
Kryptoperidinium triquetrumvegetative cells and spores (0.18) |
P |
| Unidentified cryptomonad cells 10-20 μm (0.14) |
? |
| NCF 6-8 μm (0.14) |
P |
| NCF 3-5 μm (0.11) |
P |
| 18.08.20 |
| 0.5 |
124.77 |
Heterocapsa rotundata (0.42) |
P |
|
Tripos arcticus (0.16) |
P |
| 1.5 |
15.18 |
Heterocapsa rotundata (0.24) |
P |
|
Kryptoperidinium triquetrum vegetative cells and spores (0.22) |
P |
| NCF 6-8 μm (0.12) |
P |
| NCF 3-5 μm (0.12) |
P |
| 2.5 |
46.49 |
Kryptoperidinium triquetrum (0.18) |
P |
|
Kryptoperidinium triquetrumspores (0.17) |
P |
|
Heterocapsa rotundata (0.11) |
P |
| 3.5 |
151.06 |
Cyclotella choctawhatcheeana (0.89) |
P |
4.5
|
36.15
|
Unidentified green oval cells 5-6 μm (0.22) |
P |
| NCF 3-5 μm (0.20) |
P |
|
Ebria tripartita (0.11) |
H |
5
|
280.03
|
Unidentified green oval cells 5-6 μm (0.39) |
P |
| NCF 3-5 μm (0.33) |
P |
5.4
(Zchem;Zeu) |
303.40
|
Unidentified green oval cells 5-6 μm (0.53) |
P |
|
Oxyrrhis marina (0.32) |
H |
Sea
|
20.22
|
Heterocapsa rotundata (0.36) |
P |
|
Pyramimonas cf.diskoicola (0.12) |
P |
| 07.09.20 |
0.5
|
21.70
|
Kryptoperidinium triquetrumspores (0.20) |
P |
|
Lepidodinium chlorophorum (0.19) |
P |
|
Cyclotella choctawhatcheeana (0.14) |
P |
|
Heterocapsa rotundata (0.11) |
P |
1.5
|
120.56
|
Cyclotella choctawhatcheeana (0.31) |
P |
|
Kryptoperidinium triquetrumspores (0.28) |
P |
|
Kryptoperidinium triquetrum (0.16) |
P |
2.5
|
31.38
|
Cyclotella choctawhatcheeana (0.40) |
P |
|
Kryptoperidinium triquetrum vegetative cells and spores (0.29) |
P |
3.5
|
130.55
|
Cyclotella choctawhatcheeana (0.41) |
P |
|
Kryptoperidinium triquetrumvegetative cells and spores (0.25) |
P |
|
Ebria tripartita (0.12) |
H |
4.5
|
45.75
|
Ebria tripartita (0.28) |
H |
|
Micracanthodinium claytonia (0.25) |
H |
5.1
(Zchem;Zeu)
|
1492.99 |
Oxyrrhis marina/Lebouridinium glaucum (0.44) |
H |
|
Gymnodinium spp. 8-15 μm (0.25) |
? |
| Unidentified green oval cells 5-6 μm (0.21) |
P |
Sea
|
17.23
|
Pyramimonas cf.diskoicola (0.18) |
P |
| Unidentified cryptomonad cells 10-20 μm (0.11) |
? |
| 12.09.20 |
0.5
|
15.54 |
Kryptoperidinium triquetrum vegetative cells and spores (0.44) |
P |
|
Heterocapsa rotundata (0.14) |
P |
| Unidentified cryptomonad cells 6-10 μm (0.12) |
? |
1.5
|
5.79 |
Protoperidinium bipes (0.14) |
H |
|
Tripos fusus (0.11) |
P |
|
Gonyaulax spinifera (0.11) |
P |
| 2.5 |
8.58 |
Gonyaulax spinifera (0.13) |
P |
3.5
|
19.89 |
Micracanthodinium claytonii (0.33) |
H |
| NCF 6-8 μm (0.13) |
P |
|
Gonyaulax spinifera (0.11) |
P |
4.5
|
32.36 |
NCF 6-8 μm (0.57) |
P |
| NCF 3-5 μm (0.25) |
P |
5.1
(Zchem;Zeu) |
310.55 |
Oxyrrhis marina (0.75) |
H |
| NCF 3-5μm (0.11) |
P |
Sea
|
18.77
|
Unidentified cryptomonad cells 10-20 μm (0.36) |
? |
| Unidentified cryptomonad cells 6-10 μm (0.25) |
? |
| 01.10.20 |
0.5
|
12.16
|
Unidentified cryptomonad cells 10-20 μm (0.34) |
? |
| Unidentified cryptomonad cells 6-10 μm (0.18) |
? |
|
Cyclotella choctawhatcheeana (0.18) |
P |
|
Gyrodinium spp. 21-32 μm (0.11) |
? |
1.5
|
13.78
|
NCF 6-8 μm (0.60) |
P |
|
Cyclotella choctawhatcheeana (0.16) |
P |
2.5
|
5.97
|
NCF 6-8 μm (0.31) |
P |
|
Cyclotella choctawhatcheeana (0.20) |
P |
| NCF10 μm(0.13) |
P |
|
Heterocapsa rotundata (0,11) |
P |
3.5
|
2.91
|
Cyclotella choctawhatcheeana (0,31) |
P |
| NCF 6-8 μm (0.21) |
P |
| NCF 3-5μm (0.17) |
P |
4.5
|
3,84
|
NCF 6-8 μm (0.41) |
P |
| NCF 3-5μm (0.22) |
P |
| Unidentified cryptomonad cells 10-20 μm (0.12) |
? |
4.9
(Zchem) |
35.98 |
Oxyrrhis marina (0.94) |
H |
Sea
|
1.45
|
Unidentified cryptomonad cells 10-20 μm (0.19) |
? |
| NCF 6-8 μm (0.16) |
P |
| NCF11 μm (0.11) |
P |
| 01.11.20 |
0.5
|
3.84
|
Ebria tripartia (0.31) |
H |
|
Melosira sp. (0.15) |
P |
| 1.5 |
19.76 |
NCF 10 μm (0.59) |
P |
2.5
|
0.92
|
Melosira arctica (0.26) |
P |
| Unidentified cryptomonad cells 6-10 μm (0.19) |
? |
|
Protoperidinium brevipes (0.13) |
H |
|
Odontella aurita (0.11) |
P |
3.5
|
1.04
|
Gymnodinium spp. 11-15μm (0.22) |
? |
| Unidentified cryptomonad cells 10-20 μm (0.19) |
? |
| Unidentified cryptomonad cells 6-10 μm (0.15) |
? |
4.1
|
6.48
|
Gymnodinium spp. 11-15 μm(0.27) |
? |
| Unidentified cryptomonad cells 10-20 μm (0.19) |
? |
|
Micracanthodinium claytonii (0.18) |
H |
| Unidentified cryptomonad cells 6-10 μm (0.13) |
? |
| Sea |
0.69 |
NCF10 μm (0.13) |
P |
Table 2.
Average similarity between phytoplankton communities integrated throughout a water column, number of taxa with cumulative contribution 90-92%, and taxa contributing at least 10% (9% in one case) to similarity (in brackets –percentage contribution to the similarity)in the Lagoon on the Cape Zeleny in 2020.
Table 2.
Average similarity between phytoplankton communities integrated throughout a water column, number of taxa with cumulative contribution 90-92%, and taxa contributing at least 10% (9% in one case) to similarity (in brackets –percentage contribution to the similarity)in the Lagoon on the Cape Zeleny in 2020.
Date |
Average similarity(%) |
Number of taxa cumulatively contributing 90-92% |
Taxa contributing at least9% to similarity, andtheir percentage contribution |
| 30.01.20 |
7 |
7 |
Gymnodinium wulffii (46),
Paralia sulcata (17) |
08.06.2020
|
38 |
19 |
Heterocapsa rotundata (14),
Pyramimonas cf.diskoicola (10) |
18.06.2020
|
37 |
16 |
Cyclotella choctawhatcheeana (14),
Ebria tripartita (12),
Synechocystis salina (11),
Gymnodinium arcticum (11) |
02.07.2020
|
40 |
10 |
Cyclotella choctawhatcheeana (30),
Ollicola vangoorii (11) |
20.07.2020
|
42 |
19 |
Unidentified cryptomonad cells 6-10 μm (11),
Ebria tripartita (11),
Cyclotella choctawhatcheeana (10) |
| 18.08.2020 |
40 |
19 |
Cyclotella choctawhatcheeana (13) |
07.09.2020
|
45 |
25 |
Cyclotella choctawhatcheeana (13), Kryptoperidinium triquetrum spore (11) |
| 12.09.2020 |
46 |
21 |
Kryptoperidinium triquetrum (9) |
01.10.2020
|
41 |
9 |
Cyclotella choctawhatcheeana (27)
Unidentified cryptomonad cells 10-20 μm (17)
Unidentified cryptomonad cells 6-10 μm (17)
Kryptoperidinium triquetrum (11) |
01.11.2020
|
32 |
13 |
Gymnodinium spp. 11-15 μm (18),
cf. Peridinielladanica (12),Monoraphidium contortum (11) |
Table 3.
Average similarity betweensurface phytoplankton communities in the Lagoon and adjacent marine area in 2020. Number of taxa cumulatively contributed 90-92% and taxa contributing at least 10% (9% in two cases) to similarity, and the typical species (in brackets- individual percentage contribution to the similarity).
Table 3.
Average similarity betweensurface phytoplankton communities in the Lagoon and adjacent marine area in 2020. Number of taxa cumulatively contributed 90-92% and taxa contributing at least 10% (9% in two cases) to similarity, and the typical species (in brackets- individual percentage contribution to the similarity).
| Average similarity (%) |
Number of taxa cumulatively contributing
90-92% |
Taxa contributing at least9% to similarity,
and their percentage contribution |
| 30.01.20 |
| 10 |
1 |
Protoperidinium brevipes (100) |
| 08.06.20 |
| 42 |
10 |
Heterocapsa rotundata (21), Pyramimonas cf.diskoicola (13), Unidentified cryptomonad cells 10-20 μm (13), Ollicola vangoorii (11) |
| 18.06.20 |
| 23 |
9 |
Ebria tripartita (18), Euglenozoa cells 21-40 μm (14), Unidentified cryptomonad cells 10-20 μm (11), Kryptoperidinium triquetrum (11) |
| 02.07.20 |
| 42 |
9 |
Heterocapsa rotundata (21), Pyramimonas cf.diskoicola (13) |
| 20.07.20 |
| 47 |
14 |
Heterocapsa rotundata (9), Gymnodinium spp. 16-20 μm (9) |
| 18.08.20 |
| 47 |
9 |
Heterocapsa rotundata (18), Pyramimonas cf.diskoicola (11) |
| 07.09.20 |
| 40 |
9 |
Heterocapsa rotundata (16), Cyclotella choctawhatcheeana (14),
Unidentified cryptomonad cells 6-10 μm (13), Pyramimonas cf. diskoicola (13) |
| 12.09.20 |
| 43 |
6 |
Heterocapsa rotundata (23), Unidentified cryptomonad cells 6-10 μm (22), Unidentified cryptomonad cells 10-20 μm (17), Pyramimonas cf. diskoicola (14), Cyclotella choctawhatcheeana (11) |
| 01.10.20 |
| 32 |
4 |
Unidentified cryptomonad cells 10-20 μm (34), Unidentified cryptomonad cells 6-10 μm (26), Cyclotella choctawhatcheeana (18), Kryptoperidinium triquetrum (17) |
| 01.11.20 |
| 25 |
7 |
Kryptoperidinium triquetrum (19)Cocconeis costata (18)
Monoraphidium contortum (16), Thalassionema nitzschioides (15), Paulinella ovalis (11), Pterosperma sp.1 (11), Cyclotella choctawhatcheeana (11) |
4. Discussion
The Lagoon on the Cape Zeleny is the second coastal stratified body of water, after the lake Kislo-Sladkoe, in which similar studies have been carried out. Unlike the lake Kislo-Sladkoe, the Lagoon is at an earlier stage of separation from the sea [
1].
Several hydrological phenomena typical of coastal meromictic water bodies are observed in the Lagoon, and at the same time there is a number of features [
1].The Lagoon is connected to the sea and receives daily injections of sea water during the ice-free period; however, this does not impair stable stratification, which was confirmed in this study. During the annual cycle the layers with different water densities persisted: the upper water mass with a salinity approximately the same as in the sea, the underlying layer with a higher salinity, and the bottom layer with a significantly salinity exceeding that of the sea. We explain the latter by the release of brine during the freezing of salt water and its accumulation in the bottom depression. This Lagoon does not have a desalinated layer, despite the fact that the surface layer of water receives runoff from the catchment and precipitation. The reason is that the Lagoon has a very small catchment area, only 7 times the area of the water table [
1].
According to the totality of abiotic parameters, the water column of the Lagoon can be divided into several zones with different habitats and corresponding PhP communities.
The surface layer 0−1 m is a zone of wind mixing, heat exchange with the atmosphere, maximum solar insolation, influenced by an fresh runoff in summer and partially frozen in winter, therefore, wide daily and seasonal fluctuations in temperature and salinity of water are observed here; 2) a layer at a depth of 1−2 m is a halocline, 3) at a depth of 2−4.5 m is a layer with a higher salinity compared to the top layer, where due to solar heating in the absence of convection a mid-depth temperature maximum as well as high dissolved oxygen concentration exceeding saturation sometimes appears in summer. The deep oxygen maximum is apparently associated with the photosynthetic activity of PhP, which is also evidenced by a local increase in pH, indicating the consumption of the bicarbonate ion. The thermal and salinity regime is steadier here. In autumn, heat is retained in this layer of water longer than in the overlying surface layer; 4) in the depth range of 4.5−5.5 m the boundary of the anaerobic zone (chemocline) is observed. Chemocline is characterized by steep gradients in a variety of hydrological parameters, including salinity, temperature, oxygen concentration, ORP, pH, and, according to studies [
38], the concentration of biogenic substances. It also serves as the boundary of the photic zone because of dense population of microorganisms in chemocline including eukaryotes and bacteria, among which are anoxygenic phototrophic purple sulfur bacteria and brown-colored green sulfur bacteria characteristic of the chemocline of this Lagoon [
41,
44,
51]; 5) from a horizon of 5.5 m and below, there is a euxinic zone unsuitable for the vast majority of PhP representatives, with even higher salinity (more than 28‰), exceeding salinity in adjacent marine area, stagnant habitat.
The daily influx of a small amount of sea water at high tide could serve as a factor in the unification of plankton in the sea and in the Lagoon. There are indeed many common species in the surface layer of the sea and in the water column above the chemocline of the Lagoon. In particular, from June to October in the Lagoon (with the exception of the layer above the chemocline), and in the sea from June to September there was a PhP community C. choctawhatcheeana, P. cf. diskoicola and H. rotundata as TSs. Interestingly, in October–November, the structure of PhP near the sea surface was more consistent with its structure in the lagoon chemocline.
However, as shown by this study, the PhP of the surface layer in the Lagoon and in the sea differs significantly in composition, quantitative parameters, and seasonal dynamics. Not only dominants differed, but also the ratio of taxa with different nourishment. Some forms that dominated the Lagoon we were not met at sea, such as
L. chlorophorum which was not found in the White Sea before, and
T.
arcticus previously sometimes dominated in Velikaya Salma Straight [
52]. Three mass species that dominated the Lagoon,
P. brevipes,
C. choctawhatcheeana and
E. tripartita, never achieved a high biomass in the sea. These species are common in the White Sea [
53], but rare listed among the dominants.
C. choctawhatcheeana was among
mass species in the under-ice water in the estuarine zone of the Severnaya Dvina River (Dvina bay of the White Sea
) in March [
54]
. The dominance of
C. choctawhatcheeana in nano-micro-phytoplankton was also noted in autumn samples from the Velikaya Salma Strait and the Rugozerskaya Bay of the Kandalaksha Bay of the White Sea [
55].
C. choctawhatcheeana was also among the dominants in the Siberian saline meromictic lake Shira [
14]. In coastal lake Kislo-Sladkoe in 2019,
C. choctawhatcheeana was also present in plankton, although it was not among the dominant and typical species; but
E. tripartita was among the dominants almost all summer [
22]. The last species was also among the dominants in
Dvina bay of the White Sea [
53]; dominating of
P. brevipes was also registered earlier under ice in Kandalaksha Bay [
52]. Characteristic species of both the Lagoon and the lake were
P. cf.
diskoicola,
H. rotundata and
K. triquetrum; they all are typical of the White Sea [
52,
56].
H. rotundata sometimes dominated in Kandalaksha Bay in July – August [
52], or in July and October [
57];
K. triquetrumas under the name of its synonym
Gymnodinium triquetrum dominated a vast area of
Dvina and Onega bays and in central part of the White Sea in the beginning of July [
58]. Species of the genus
Pyramimonas have also been found in the White Sea PF in recent years [56−58], but the species identity is not always clear, and it is probably
P. cf.
diskoicola was one of the PhP dominants in the Chupa Bay in summer [
57].
Vice versa, all the species that dominated the sea were also present in the lagoon. Some of usual dominants in PhP of the White Sea, such as the filamentous diatom
S.costatum and dinoflagellate
D. acuminata [
55], or dinoflagellate
P. rotundatum found in the White Sea by [
56], have never been abundant in the Lagoon.
Autotrophs always dominated in the surface water layer in the Lagoon, except for the moment of the onset of freeze-up (late autumn period, October 1 and November 1), while at the same depth in the sea, the contribution of cryptomonads potentially capable of mixotrophy was often high [
59]. The similarity of the communities in the sea and the Lagoon never exceeded 50%, neither during the ice-free period (no more than 42%), nor in summer (less than 47%). The largest contribution to the similarity belonged, as a rule, to the dinoflagellates
H. rotundata,
K. triquetrum, diatom
C. choctawhatcheeana, and green prasinophyte flagellate
P. cf.
diskoicola.
The seasonal trend of BC in the sea and in the Lagoon was also different. The mass development of PhP in the Lagoon began later than in the sea; in early summer, it lagged behind the sea in terms of biomass. But in August, as a result of an outbreak of H. rotundata, BC in the surface layer of the Lagoon exceeded that in the sea by an order of magnitude. In September, it decreased, but, nevertheless, remained quite high, although the sea experienced a depression of PhP. Even in November, BC in the surface water layer in the Lagoon was more than 5 times greater than in the sea.
But even more were the differences in the structure of PhP within the Lagoon itself, on its different horizons. The most plentiful throughout the year was the chemocline layer, where the BC was 1–2 orders of magnitude higher than that in the overlying horizons. The dominant species in it were usually different from those in the overlying water column. Typical of the chemocline in different seasons were different species of the genus Gymnodinium, Gyrodinium spirale Tetraselmis cordiformis, S. costatum, O. marina, L. glaucum, and small unidentified cocci and flagellates.
In addition, a regular change in the predominant type of nutrition was observed in the chemocline. In January, heterotrophs dominated in the chemocline, in the first half of the growing season, autotrophs, in mid-August, the community changed from autotrophic to mixed, and by early October, heterotrophic due to the actively growing heterotrophic species
O. marina. In the lake Kislo-Sladkoe, heterotrophic flagellates, including
O. marina, appeared in the Lagoon a month earlier, already in July [
22]. Compared to a lake, the Lagoon is characterized by higher water transparency, thicker photic zone, which creates favorable conditions for autotrophic PhP, including in the chemocline. Good light conditions last longer there. Light conditions worsen only in autumn, as a result mixotrophs and heterotrophs get an advantage in the chemocline where they have enough food such as bacteria, including phototrophic sulfur bacteria that form a high-density population there, and their metabolic products. In autumn heterotrophic dinoflagellates
O. marina develop in mass actively eating cells of small autotrophic algae, so the PhP in the chemocline is reduced. A similar effect was observed in the lake Kislo-Sladkoe in 2018, where this predatory protist together with
E. tripartita significantly reduced the abundance of PhP in the chemocline [
60].
Not only the chemocline, but also other layers differing in abiotic parameters also differ in the structure of PhP. The similarity between communities in different horizons in different months was 32-46% in summer and only 7% in winter, which is significantly lower than the similarity of the PhP structure of different layers noted in the White Sea during the full period from hydrological winter (end of March) to the beginning of hydrological summer (mid-June): 18-49% during the ice cover period from March to April, and 45-75% in May-mid-June [
56]. The gradual increase in the role of heterotrophs in the PhP community during the growing season, the high B
C in the chemocline, the autumn dominance of
O. marina in the chemocline - the same patterns were noted in the lake Kislo-Sladkoe [
22].
Among the important differences, one should mention the different seasonal biomass dynamics, its higher values in the Lagoon as a whole, as well as differences in the species composition and dominants. Two summer and one autumn increases in Bint were noted in the seasonal PhP dynamics in the Lagoon, while there was no summer peak in the lake Kislo-Sladkoe, PhP bloom was noted in spring, and an increase in autumn.
The maximum values of B
int in the Lagoon were noted in mid-June (0.49 g C/m
2) and mid-August (0.49 g C/m
2), which were almost 3 times higher than the values of B
int in the same months in the lake [
22]. In autumn, B
C peak in the Lagoon started earlier: it was noted in early September, while in the lake it occurred in early October, and B
int in the Lagoon (0.78 g C/m2) was more than 2 times higher than that in the lake. Studies of the PhP in the White Sea show a difference in the dynamics of the abundance of PhP depending on local conditions and in different years [
22]. The values of B
int and its dynamics in the Lagoon were close to those in the Chupa Bay of the Kandalaksha Bay of the White Sea [
58], where B
int increased to 0.61 g C/m
2 in early June and was 0.55 g C/m
2 in mid-July, 0.62 g C/m
2at the end of August, and reached maximum in early September (1.08 g C/m
2). The difference in the dynamics and abundance of PhP was also shown for the lakes separated from the Sea of Okhotsk on Sakhalin Island [
31,
32,
33,
36].
Not only the abundance of algae, but also their taxonomic composition changed in the Lagoon during the year. Dinoflagellates dominated in winter and early June, NCF in July, diatoms, dinoflagellates and NCF in August, dinoflagellates in September, and NCF in October–November. In the lake Kislo-Sladkoe
E. tripartita and dinoflagellates contributed mainly to B
C in the summer-autumn, the contribution of NCF to B
int was insignificant, and diatoms dominated only in spring [
22].
The most populated water layer in the Lagoon is the chemocline. Throughout the study period maximum algal B
C in the water column was above the chemocline, except for November 1st, when maximum B
C appeared at a depth of 1.5 m due to the development of NCF. The deep maximum of PhP biomass is quite common in lakes during the stratification period [
23,
61], etc.. In the relict meromictic Lake Mogilnoye, in August 2018, a maximum of B
C up to 6 g/m
3was noted above the chemocline due to the development of the heterotrophic algae
O. marina [
62]. In our studies, the maximum B
C of PhP with the dominance of
O. marina (along with other dinoflagellates and NCF) in the chemocline was recorded in early September. At that time, the B
C reached 1.5 g C/m
3, which, in approximate conversion to wet biomass, was 25 g/m
3, and several times exceeded the biomass in Lake Mogilnoye. Even higher B
C value (1.73 g C/m
3) was noted in the chemocline of the Lagoon in mid-June with the dominance of autotrophic dinoflagellates
G. arcticum. In water bodies with an anaerobic zone, biogenic elements accumulate in it [
61]. If the photic zone reaches the chemocline, then conditions for the active growth of PhP are created above it due to the upward diffusion of nutrients [
22].
One of the features of the chemocline is the concentration of heterotrophic and mixotrophic algae there, since this zone contains a larger number of their food objects, as well as suspended and dissolved organic matter [
63]. In the water bodies separating from the White Sea cryptophyte flagellates
Rhodomonas bloom often appears at early stages of isolation causing a coloration of water in the chemocline [
27,
44]. In the water bodies more advanced on the path of isolation with a fresh mixolimnion colored layer sometimes is formed by
Cryptomonas algae, or
Euglena sp. [
44]. They all are all mixotrophs. In the Lagoon, in previous summer seasons, there was a red layer with
Rhodomonas sp. [
27,
44]. In 2020 when this study was carried out, these flagellates were not abundant; they didn’t form a colored layer.
In November, BC maximum shifted upward, into the subsurface horizon, which is apparently associated with water cooling and increased vertical circulation, partially eroding the chemocline, so the algae appear in overlying layers.
On the contrary, in the lake Kislo-Sladkoe algae were concentrated not only in the chemocline zone, but also in the middle part of the water column in the summer-autumn period. A possible reason is a release of biogenic elements from the anaerobic zone as a result of the previous complete mixing of water [
22].
The PhP structure and dynamics in the Lagoon and the sea by 24.5% was related to the daylight, water salinity, oxygen content and pH in it, as well as in the lake Kislo-Sladkoe, but did not depend on water T, illuminance in water, and the depth, was noted for that lake. In Lake Kislo-Sladkoe, complex temperature profiles were observed with the appearance of a “greenhouse effect” from May to September; illumination, when low transparency intermittently limited the photic layer; and salinity with significantly desalinated surface water early in the season, causing a freshwater PhP to develop there in June. Of the three factors that were important in Lake Kislo-Sladkoe (temperature, salinity and illumination), only salinity determines the structure of the PhP in the Lagoon. The layered hydrological structure in coastal meromictic water bodies is associated with different water densities, which are determined by the concentration of substances dissolved in water and temperature. At the same time, the contribution of temperature to variations in water density is much less than the contribution of its mineralization. Thus, the salinity profile better than other parameters reflects the vertical sequence of biotopes, not only as an ecological factor in itself, but as a vector of environmental variability.
Underwater illuminance did not limit the development of autotrophic PhP in the Lagoon, since its water is transparent, and most of the year light reaches the chemocline, below which PhP doesn’t inhabit due to the presence of toxic hydrogen sulfide. It's interesting that BC usually reaches its highest values at the lower boundary of the photic zone, despite shaded conditions.
Dissolved oxygen concentration and pH depend on the activity of PhP. In summer, at a depth of 1.5-4.5 m, a layer appeared supersaturated with oxygen due to the accumulation of oxygen, realized as a result of photosynthesis. During the growing season the oxygen content in water declined with increase in the BC, both auto- and heterotrophic. Shift of proportion between these groups towards the heterotrophs explains this phenomenon only partially. Additionally, an increase in the total PhP biomass contributes to an increase in the intensity of the microbial loop and an increase in oxygen consumption for bacterial respiration and organic oxidation. The relationship between the structure of PhP and the pH factor above the chemocline reflects the shift of the carbonate equilibrium to the alkaline side due to the consumption of carbon dioxide during PhP photosynthesis.
The day length is important for phototrophic organisms and determines the seasonal dynamics of the PhP structure as a whole. In the Lagoon, this manifests itself in an increase in B
int in summer, a successive change in dominant forms, an increase in the role of mixotrophs and heterotrophs in the community with a decrease in daylight hours, and a biomass reduction in autumn. In the lake Kislo-Sladkoe, a seasonal change in the dominant abiotic factors was observed [
22]. In the Lagoon, seasonality manifested itself in differences in the structure of the PhP and in the summer-autumn period (from June to October), and in November. At the same time, the chemocline community and the community of the overlying water mass are differently affected by the combination of the above mentioned factors.
5. Conclusions
293 species and supraspecific taxa of algae and cyanobacteria were found in a saline semi-isolated Lagoon on the Cape Zeleny on the coast of the White Sea.
Most of the identified species are marine, and 38 species are freshwater.
Taxonomic composition changed in the Lagoon during the year. Dinoflagellates dominated in winter and early June, NCF in July, diatoms, dinoflagellates and NCF in August, dinoflagellates in September, and NCF in October–November. The abundance of algae also changed in the Lagoon during the year. Bint in the water column varied from 0.01 g C/m2 in January to 0.78 g C/m2 in early September.
According to the totality of abiotic parameters, the water column of the Lagoon can be divided into several vertical zones with different habitats and corresponding PhP communities.
Although the lagoon has not lost its connection with the sea and receives some portion of sea water daily at high tide, it is characterized by stable vertical density stratification.
The structure of PhP in the surface water layer in the Lagoon and in the sea differs significantly in composition, quantitative parameters, and seasonal dynamics. In terms of biomass, the PhP development in the Lagoon lags behind the sea until mid-summer, but, starting from August, it surpasses it, and lasts longer, than in the sea, until late autumn.
The similarity of the communities in the sea and the Lagoon never exceeded 50%. The largest contribution to the similarity belonged, as a rule, to the dinoflagellates H. rotundata, K. triquetrum, diatom C. choctawhatcheeana and green prasinophyte flagellate P. cf. diskoicola.
The vertical heterogeneity of PhP in the Lagoon appears in a different structure of PhP in different water layers with a similarity between the communities of individual horizons of 32-46% in summer and only 7% in winter. Non-metric multidimensional scaling (nMDS) divided the samples collected in summer-autumn period from different depth into two groups: one matches the community of water column above the chemocline, and the second corresponds to the community of the chemocline, the latter is closer to PhP of the adjacent marine area. Marine PhP in June-September were closer to the first community, and in October-November – to the chemocline.
The most plentiful throughout the year was the chemocline layer. Here, for almost the entire year, there is a maximum BC, which is 1-2 orders of magnitude higher than that in the overlying horizons. Typical of the chemocline in different seasons were some species of the genus Gymnodinium, Gyrodinium spirale, T.cordiformis, S. costatum, O. marina, L. glaucum, as well as small unidentified cocci and flagellates. Starting from the second half of summer, mixo- and heterotrophic species of algae predominate.
The PhP structure and dynamics in the Lagoon and the sea by 24.5% was related to the daylight, water salinity, oxygen content and pH in it, but did not depend on water T, illumination in water, and depth. Oxygen content and pH are defined by PhP activity.
According to the marginal tests DistLM 7 of the 8 studied parameters (salinity, air temperature, concentration of dissolved O2, pH, ORP, daylight, depth) determined (or were determined by) the dynamics of PhP in the Lagoon as well as in the sea statistically significantly; the effect of water temperature was found to be unreliable. In sequential tests 4 out of these 7 parameters showed a statistically significant relationship with the dynamics of the PhP structure in the Lagoon, as well as in the sea: daylight, salinity, dissolved oxygen and pH. They describe 24.5% of the PhP variability. Two last factors are defined by PhP activity.
Salinity serves as a vector of the vertical sequence of ecological niches in the Lagoon. The daylight seems to be the crucial factor of the seasonal PhP dynamics in the semi-isolated coastal stratified lakes and lagoons.