Submitted:
11 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ionic Liquids
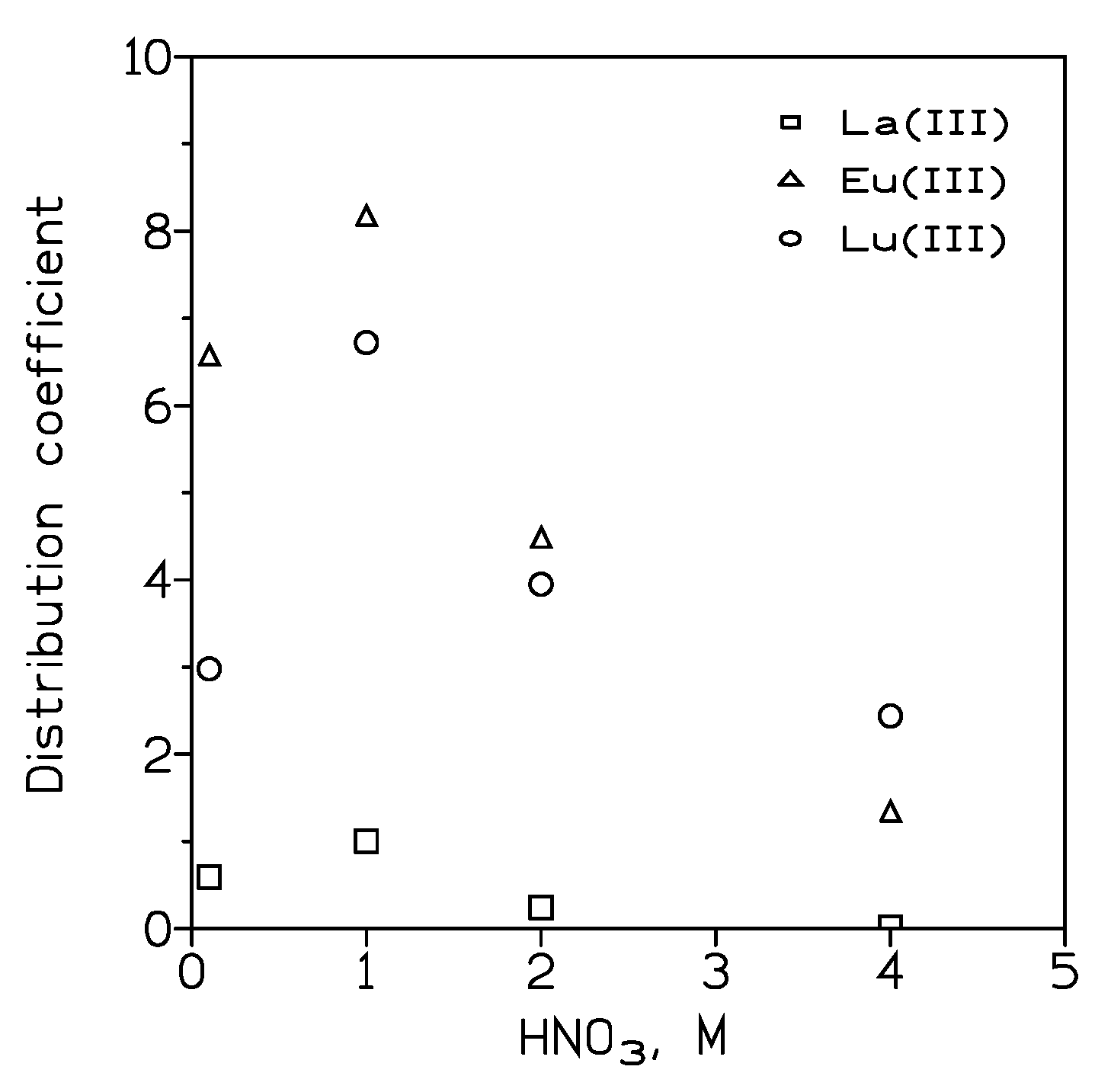
ILs and REEs
3. Deep Eutectic Solvents
Deep Eutectic Solvents and Rare Earth Elements
Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, B. Challenges and opportunities for sustainable valorization of rare earth metals from anthropogenic waste. Rev. Environ. Sci. Biotechnol. 2023, 22, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, P.Y. , Manishkumar D. Recent advances in the approaches to recover rare earths and precious metals from E-waste: a mini-review. Canadian J. Chem. Eng. 2023, 101, 1043–1054. [Google Scholar] [CrossRef]
- Wei, H.-L.; Zheng, W.; Zhang, X.; Suo, H.; Chen, B.; Wang, Y.; Wang, F. Tuning near-infrared-to-ultraviolet upconversion in lanthanide-doped nanoparticles for biomedical applications. Adv. Opt. Mater. 2023, 11, 2201716. [Google Scholar] [CrossRef]
- Zehra, T.; Kaseem, M. Lanthanides: the key to durable and sustainable corrosion protection. ACS Sust. Chem. Eng. 2023, 11, 6776–6800. [Google Scholar] [CrossRef]
- Farooq, M.; Nazir, I.; Rafiq, H.; Rasool, M.H. Exploring technological innovations of doped rare earth materials. ECS J. Solid State Sci. Technol. 2023, 12, 047006. [Google Scholar] [CrossRef]
- Kowsuki, K.; Nirmala, R.; Ra, Y.-H.; Navamathavan, R. Recent advances in cerium oxide-based nanocomposites in synthesis, characterization, and energy storage applications: A comprehensive review. Results in Chemistry 2023, 5, 100877. [Google Scholar] [CrossRef]
- Bahfie, F. Kaban, N.O.Br; Suprihatin S.; Nurjaman F.; Prasetyo E.; Susanti D. Properties of rare-earth element in magnetic material and its processing. Prog. Phys. Metals 2023, 24, 157–172. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Fontes, M.P.F.; Lima, M.T.W.D.C; Cordeiro, S.G.; Wyatt, N.L.P.; Lima, H.N. , Fendorf, S. Human health risk assessment and geochemical mobility of rare earth elements in Amazon soils. Sci. Total Environ. 2022, 806, 151191. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An Emerging Branch of Extractive Metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Dewulf, B.; Riaño, S.; Binnemans, K. Separation of heavy rare-earth elements by non-aqueous solvent extraction: Flowsheet development and mixer-settler tests. Sep. Purif. Technol. 2022, 290, 120882. [Google Scholar] [CrossRef]
- Ehsan, K.; Sajjad, M. Ionic Liquids: properties, application, and synthesis. Fine Chem. Eng. 2021, 2, 22–31. [Google Scholar] [CrossRef]
- Yue, C.; Fang, D.; Liu, L.; Yi, T.-F. Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J. Molec. Liq. 2011, 163, 99–121. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.F.M.; Carreira, A.R.F.; Vargas, S.J.R.; Passos, H.; Schaeffer, N.; Coutinho, J.A.P. Simple gold recovery from e-waste leachate by selective precipitation using a quaternary ammonium salt. Sep. Purif. Technol. 2023, 316, 123797. [Google Scholar] [CrossRef]
- Cao, C.; Xu, X.; Wang, G.; Yang, Z.; Cheng, Z.; Zhang, S.; Li, T.; Pu, Y.; Lv, G.; Xu, C.; Cai, J.; Zhou, W. Characterization of ionic liquids removing heavy metals from electroplating sludge: Influencing factors, optimisation strategies and reaction mechanisms. Chemosphere 2023, 324, 138309. [Google Scholar] [CrossRef]
- Ali Topçu, M.; Rüşen, A.; Alper Yıldızel, S. High efficiency copper recovery from anode slime with 1-butyl-3-methyl imidazolium chloride by hybrid Taguchi/Box–Behnken optimization method. J. Ind. Eng. Chem. 2023, 120, 261–270. [Google Scholar] [CrossRef]
- Xie, R.; Li, Z.; Qu, G.; Zhang, Y.; Wang, C.; Zeng, Y.; Chen, Y. The selective and sustainable separation of Cd(II) using C6MImT/[C6MIm]PF6 extractant. Ecotox. Environ. Safety 2023, 255, 114792. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A. The Pseudo-Protic Ionic Liquids TOAH+Cl and TODAH+Cl as Carriers for Facilitated Transport of In(III) from HCl Solutions. Membranes 2023, 13, 19. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Robla, J.I. On the Use of Pseudo-Protic Ionic Liquids to Extract Gold(III) from HCl Solutions. Int. J. Mol. Sci. 2023, 24, 6305. [Google Scholar] [CrossRef]
- Kaim, V.; Rintala, J.; He, C. Selective recovery of rare earth elements from e-waste via ionic liquid extraction: A review. Sep. Purif. Technol. 2023, 306, 122699. [Google Scholar] [CrossRef]
- Salehi, H.; Maroufi, S.; Mofarah, S.; Nekouei, R.K.; Sahajwalla, V. Recovery of rare earth metals from Ni-MH batteries: A comprehensive review. Renew. Sust. Energy Rev. 2023, 178, 113248. [Google Scholar] [CrossRef]
- Azhagar, S.; Murugesan, B.; Chinnaalagu, D.K.; Arumugam, M.; Mahalingam, S. [BMIM]-PF6 ionic liquid mediated polyol synthesis of praseodymium (III) oxide nanoparticles: Physicochemical investigation and its interaction with bacterial and cancer cells. Ceram. Int. 2022, 48, 35386–35397. [Google Scholar] [CrossRef]
- Do-Thanh, C.-L.; Luo, H.; Gaugler, J.A.; Dai, S. A task-specific ionic liquid based on hydroxypyridinone for lanthanide separation. Sep. Purif. Technol. 2022, 301, 121939. [Google Scholar] [CrossRef]
- Du, C.; Ma, S.; Xie, M.; Yang, F.; Zhao, Z.; Chen, Y.; Ma, Y. Recovery of high-value rare earth elements from waste NdFeB by the water-soluble ammonium salt [Hbet]Cl. Sep. Purif. Technol. 2023, 308, 122946. [Google Scholar] [CrossRef]
- Emam, Sh.Sh; El-Hefny, N.E. Bi-functional ionic liquid based on Aliquat 336 and Cyanex 572 for effective separation of Nd(III) and Ni(II) from chloride solution and recover Nd2O3 from waste Nd-Ni-Fe magnets. Hydrometallurgy 2023, 219, 106064. [Google Scholar] [CrossRef]
- Larochelle, T.; Noble, A.; Strickland, K.; Ahn, A.; Ziemkiewicz, P.; Constant, J.; Hoffman, D.; Glascock, C. Recovery of Rare Earth Element from Acid Mine Drainage Using Organo-Phosphorus Extractants and Ionic Liquids. Minerals 2022, 12, 1337. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Rudnev, A.V. Electrochemical Codeposition of Sm and Co in a Dicyanamide Ionic Liquid. Russian J. Electrochem. 2022, 58, 1083–1093. [Google Scholar] [CrossRef]
- Pheasey, C.; Angeli, P. Intensified Nd extraction in small channels for NdFeB magnet recycling. Sep. Purif. Technol. 2023, 311, 122958. [Google Scholar] [CrossRef]
- Prusty, S.; Pradhan, S.; Mishra, S. Analysis of Extraction behavior of Nd (III), Sm (III) and Eu (III) from EDTA solution using [TOA-D2] in kerosene: Thermophysical properties, Process parameters and Extraction mechanism. Chem. Papers 2022, 76, 7451–7464. [Google Scholar] [CrossRef]
- Xing, L.; Ma, X.; Hu, K.; Yuan, H.; Wei, J.; Gao, H.; Nie, Y. Selective separation of Nd from La/Ce/Pr using phosphate-based ionic liquids: Solvent extraction studies and density functional theory. Miner. Eng. 2023, 191, 107967. [Google Scholar] [CrossRef]
- Xue, W.; Liu, R.; Liu, X.; Wang, Y.; Lv, P.; Yang, Y. Selective extraction of Nd(III) by novel carboxylic acid based ionic liquids without diluent from waste NdFeB magnets. J. Molec. Liq. 2022, 364, 119919. [Google Scholar] [CrossRef]
- Pati, P.; Mishra, S.; Prusty, S. Evaluation of extraction behaviour of techno metal neodymium from nitrate feed using Tri-n-hexylamine di-2-ethyl hexyl phosphate as extractant. Mater. Today: Proc. 2023. [Google Scholar] [CrossRef]
- Panda, P.; Mishra, S. Effect of process variables on the extraction of Y(III) from nitrate medium utilizing ionic liquid [THAH]+[DEHP]-. Mater. Today: Proc. 2023. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Ding, Y. Recovery of rare earths in different media with novel dicarboxylate based ionic liquid and application to recycle SmCo magnets. Hydrometallurgy 2022, 210, 105844. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, X.; Du, Y.; Cao, Y.; Feng, D.; Xie, X. Research Progress on Extraction and Separation of Rare Earth by Solvent Method. Xiyou Jinshu/Chinese J. Rare Metals 2022, 46, 1645–1656. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheedand, R.; Tambyrajah, V. Preparation of novel, moisture-stable, lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 1, 2010–2011. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheedand, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 3, 70–71. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Yong, W.F.; She, Q.; Esteban, J. Status and advances of deep eutectic solvents for metal separation and recovery. Green Chem. 2022, 24, 1895–1929. [Google Scholar] [CrossRef]
- Karzan, A.O.; Sadeghi, R. New chloroacetic acid-based deep eutectic solvents for solubilizing metal oxides. J. Mol. Liq. 2022, 347, 118393. [Google Scholar] [CrossRef]
- Singh, M.B.; Kumar, V.S.; Chaudhary, M.; Singh, P. A mini review on synthesis, properties and applications of deep eutectic solvents. J. Indian Chem. Soc. 2021, 98, 100210. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents. Molecules 2020, 25, 78. [Google Scholar] [CrossRef]
- Thompson, D.L.; Pateli, I.M.; Lei, C.; Jarvis, A.; Abbott, A.P.; Hartley, J.M. Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents. Green Chem. 2022, 24, 4877–4886. [Google Scholar] [CrossRef]
- Tian, G.; Liu, H. Review on the mineral processing in ionic liquids and deep eutectic solvents. Miner. Proc. Extract. Metal. Rev. 2022. [Google Scholar] [CrossRef]
- Chen, T.-W.; Priya, T.S.; Chen, S.-M.; Kokulnathan, T.; Ahmed, F.; Alshahrani, T. Synthesis of praseodymium vanadate in deep eutectic solvent medium for electrochemical detection of furaltadone. Proc. Safety Environ. Protect. 2023, 174, 368–375. [Google Scholar] [CrossRef]
- Favero, U.G.; Schaeffer, N.; Passos, H.; Cruz, K.A.M.L.; Ananias, D.; Dourdain, S.; Hespanhol, M.C. Solvent extraction in non-ideal eutectic solvents – Application towards lanthanide separation. Sep. Purif. Technol. 2023, 314, 123592. [Google Scholar] [CrossRef]
- Karan, R.; Sreenivas, T.; Kumar, M.A.; Singh, D.K. Recovery of rare earth elements from coal flyash using deep eutectic solvents as leachants and precipitating as oxalate or fluoride. Hydrometallurgy 2022, 214, 105952. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Gao, Y.; Tian, G. Effect of Mechanical Activation on the Leaching Process of Rare Earth Metal Yttrium in Deep Eutectic Solvents. Appl. Sci. 2022, 12, 8253. [Google Scholar] [CrossRef]
- Ni, S.; Gao, Y.; Yu, G.; Zhang, S.; Zeng, Z.; Sun, X. Tailored ternary hydrophobic deep eutectic solvents for synergistic separation of yttrium from heavy rare earth elements. Green Chem. 2022, 24, 7148–7161. [Google Scholar] [CrossRef]
- Pires, C.M.G.; Ribeiro, A.B.; Mateus, E.P.; Ponte, H.A.; Ponte, M.J.J.S. Extraction of rare earth elements via electric field assisted mining applying deep eutectic solvents. Sust. Chem. Phar. 2022, 26, 100638. [Google Scholar] [CrossRef]
- Priya, T.S.; Chen, T.-W.; Chen, S.-M.; Kokulnathan, T.; Lou, B.-S.; Al-Onazi, W.A.; Al-Mohaimeed, A.M.; Elshikh, M.S.; Yu, J. Synthesis of perovskite-type potassium niobate using deep eutectic solvents: A promising electrode material for detection of bisphenol A. Chemosphere 2023, 318, 137948. [Google Scholar] [CrossRef]
- Sanchez-Segado, S.; Stodd, S.; Chipakwe, V.; Loye, E.; Smith, M.; Wall, F.; Abbott, A.P.; Jha, A. Influence of the alkali-promoted phase transformation in monazite for selective recovery of rare-oxides using deep eutectic solvents. Miner. Eng. 2022, 182, 107564. [Google Scholar] [CrossRef]
- Ushizaki, S.; Kanemaru, S.; Sugamoto, K.; Baba, Y. Selective extraction equilibria of Sc(III), Y(III), Fe(III) and Al(III) from acidic media with toluene mixture of deep eutectic solvent (DES) composed of TOPO and isostearic acid. Anal. Sci. 2023, 39, 473–481. [Google Scholar] [CrossRef]
| Extractant | Acronysm |
|---|---|
| di-2-ethylhexyl phosphoric acid tri(hexyltetradecyl phosphonium chloride 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester Mixture of phosphonic acid and phosphinic acids |
D2EHPA C101 EHEHPA C572 |
| Ionic liquids | |
| trihexyltetradecylphosphonium and di-2-ethylhexyl phosphate trihexyltetradecylphosphonium and 2-ethylhexyl phosphate mono-2-ethylhexyl ester trihexyltretadecylphosphonium and mixture of phosphate and phosphinate |
[C101]+[D2EHP]- [C101]+[HEHP]- [C101]+[C572]- |
| REE | pH 1 | pH 2 | pH 3 |
|---|---|---|---|
| Nd(III) Sm(III) Eu(III) |
33 31 24 |
69 69 51 |
69 60 55 |
| Aqueous medium | Species |
|---|---|
| Acetate Nitrate Chloride |
[N4,4,4,Bm]3Nd[SA]1.5(OAc)3 [N4,4,4,Bm]3Nd[SA]1.5(NO3)3 [N4,4,4,Bm]4Nd[SA]2Cl3 |
| Electrolyte | Ce(IV) | La(III) | Nd(III) |
|---|---|---|---|
| Acetic acid ChCl:acetic acid Citric acid ChCl:citric acid Oxalic acid ChCl:oxalic acid |
70 nil 40 32 18 nil |
45 nil 63 51 22 2 |
37 nil 35 30 12 nil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





