Submitted:
11 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Data Table for Analysis
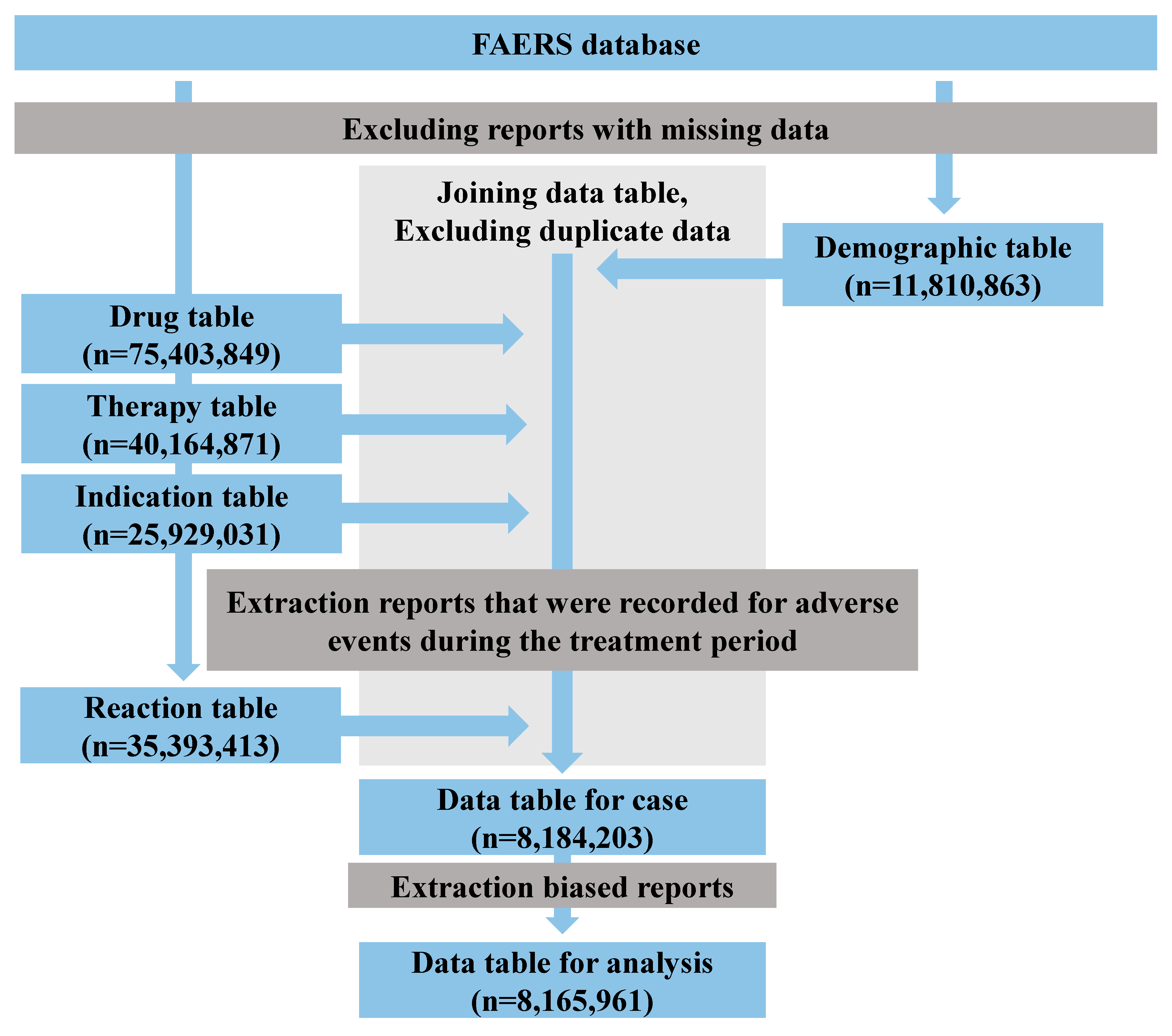
2.2. Relevance of Drug-Induced Pruritus to Patient Characteristics
2.3. Signal Detection of Pruritus-Inducing Drugs
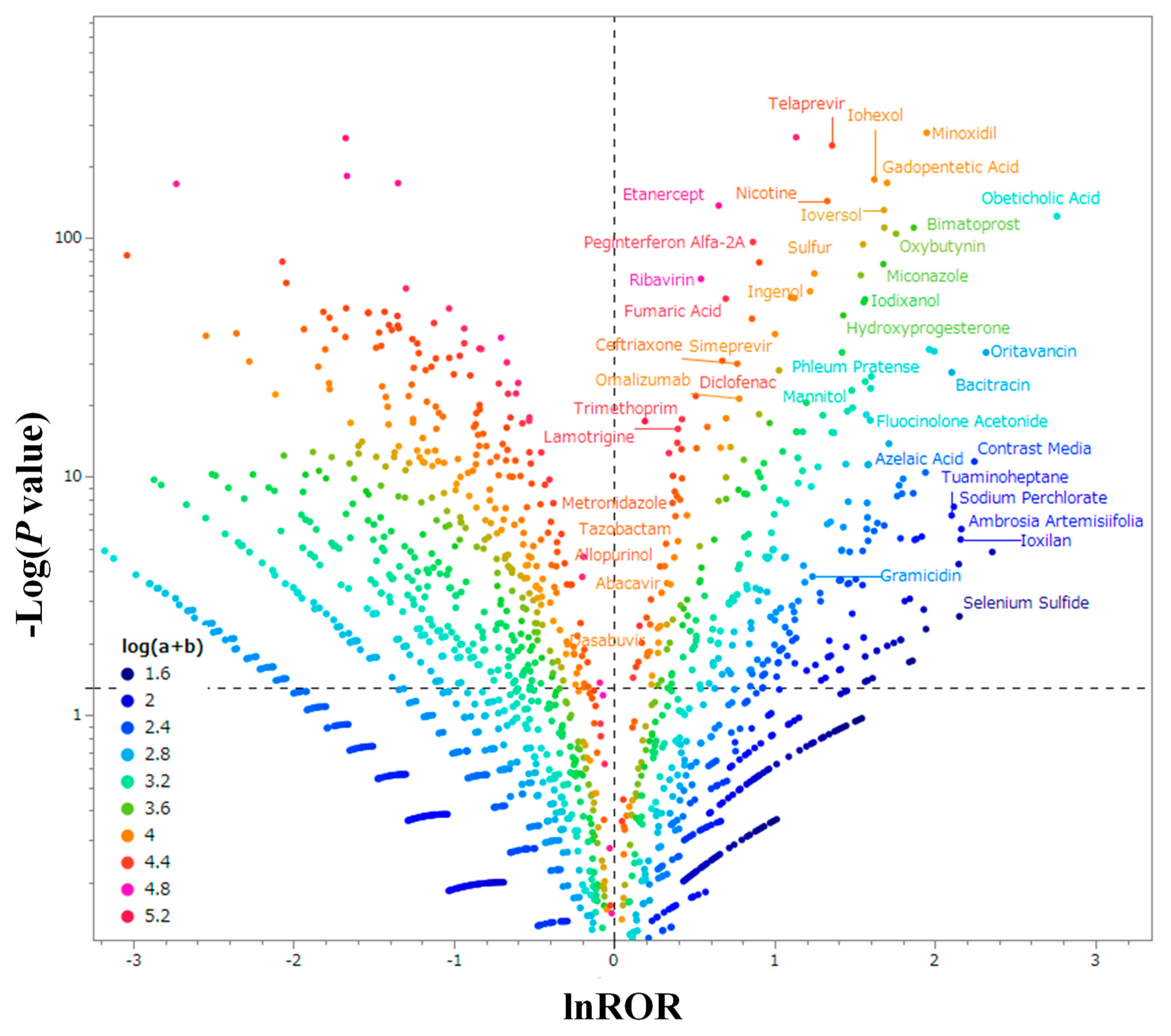
| Therapeutic_correspondence | Drug |
|---|---|
| Ophthalmologicals | Acetylcysteine, Alcaftadine, Azithromycin, Besifloxacin, Bimatoprost, Boric acid, Brimonidine, Brinzolamide, Bromfenac, Carmellose, Cefuroxime, Chlorhexidine, Clonidine, Desonide, Diclofenac, Dorzolamide, Erythromycin, Fluocinolone acetonide, Fluorescein, Fluorometholone, Glycerol, Hypromellose, Ketotifen, Latanoprost, Lifitegrast, Loteprednol, Macrogol 400, Moxifloxacin, Naphazoline, Neomycin, Netarsudil, Olopatadine, Paraffin, Paraffin, Liquid, Polymyxin b, Polysorbate 80, Povidone-iodine, Propylene glycol, Sulfacetamide, Tetracaine, Tetracycline, Tetryzoline, Timolol, Tobramycin, Travoprost, Triamcinolone, Vancomycin |
| Antibacterials for systemic use | Amoxicillin, Azithromycin, Bacitracin, Cefaclor, Cefadroxil, Cefalexin, Cefazolin, Cefdinir, Cefixime, Cefoxitin, Cefprozil, Cefradine, Ceftaroline fosamil, Ceftazidime, Ceftibuten, Ceftizoxime, Ceftriaxone, Cefuroxime, Clarithromycin, Clindamycin, Cloxacillin, Dalbavancin, Dicloxacillin, Doxycycline, Erythromycin, Fosfomycin, Gemifloxacin, Metronidazole, Moxifloxacin, Nafcillin, Neomycin, Oritavancin, Phenoxymethylpenicillin, Piperacillin, Polymyxin b, Pristinamycin, Sulfamethoxazole, Tazobactam, Tetracycline, Ticarcillin, Tobramycin, Trimethoprim, Vancomycin |
| Contrast media | Amidotrizoic acid, barium, Gadobenic acid, Gadobutrol, Gadodiamide, Gadofosveset, Gadopentetic acid, Gadoteric acid, Gadoteridol, Gadoxetic acid, Iodixanol, Iohexol, Iomeprol, Iopamidol, Ioversol, Ioxaglic acid, Ioxilan, Perflutren |
| Antifungals for dermatological use | Butenafine, Ciclopirox, Clotrimazole, Eeconazole, Efinaconazole, Fluconazole, Ketoconazole, Miconazole, Nystatin, Naftifine,Selenium sulfide, Tioconazole, Terbinafine, Terbinafine, Tavaborole |
| Other dermatological preparations | Brimonidine, Crisaborole, Diclofenac, Dupilumab, Eflornithine, Hydroquinone, Ivermectin, Minoxidil, Sulfur, Pimecrolimus, Povidone-iodine |
| Corticosteroids, dermatological preparations | Beclometasone, Clobetasol, Desoximetasone, Desonide, Fluorometholone, Fluocinonide, Fluocinolone acetonide, Methylprednisolone, Methylprednisolone, Triamcinolone, Ulobetasol |
| Gynecological antiinfectives and antiseptics | Ciclopirox, Clindamycin, Clotrimazole, EconazoleKetoconazole, Metronidazole, Miconazole, Nystatin, Povidone-iodine, Tioconazole |
| Antineoplastic agents | Aminolevulinic acid, Asparaginase, Carboplatin, Celecoxib, Cetuximab, Chlormethine, Oxaliplatin, Pegaspargase, Rituximab, Tretinoin |
| Immunosuppressants | Anakinra, Belimumab, Dalimumab, Etanercept, Fumaric acid, Ixekizumab, Ocrelizumab, Sarilumab, Secukinumab |
| Vaccines | Hpv vaccine, Pneumococcal vaccine, Varicella zoster vaccine |
2.4. Characteristics of the Adverse Event Related to Pruritus Using Principal Component Analysis
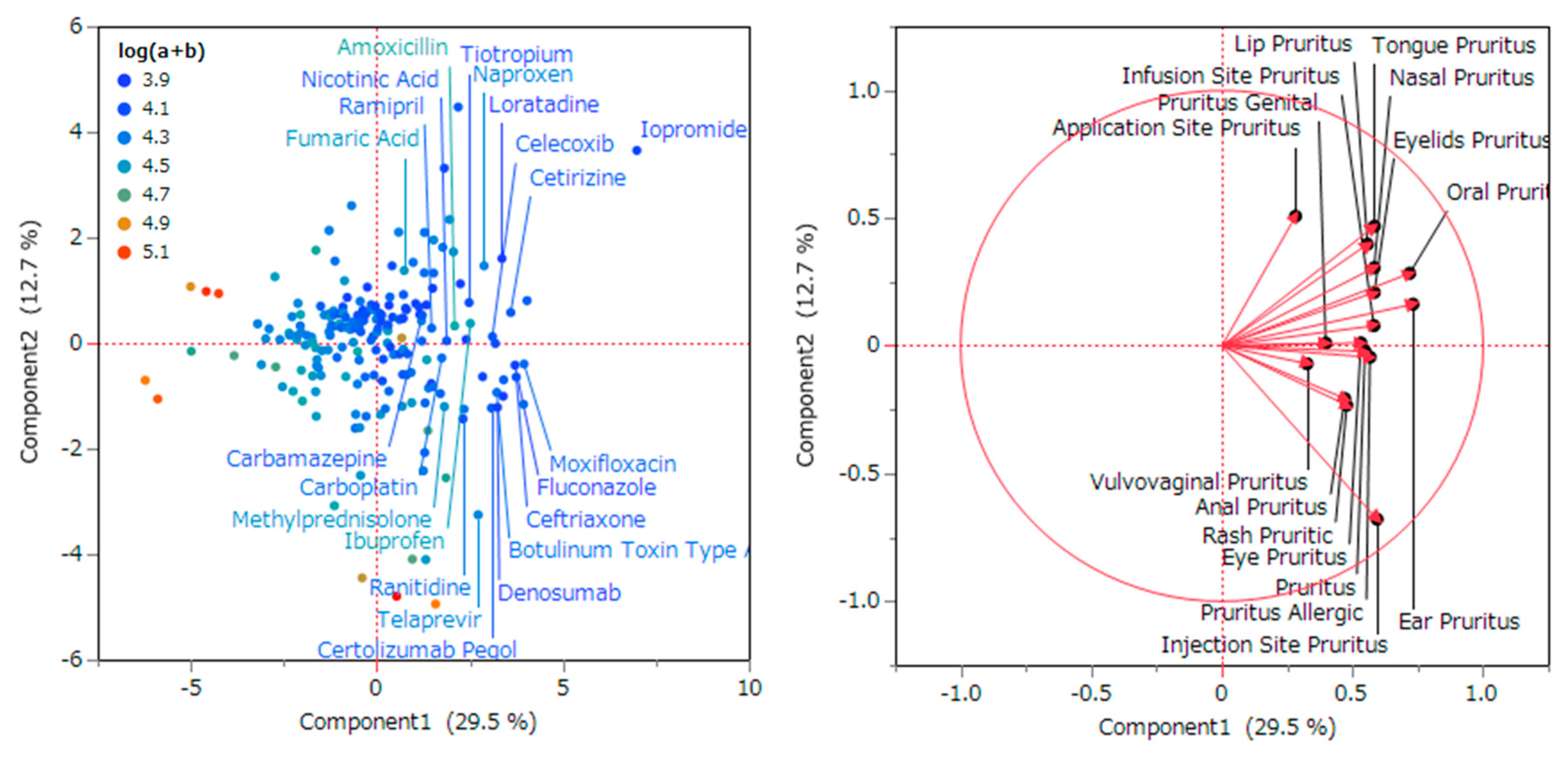
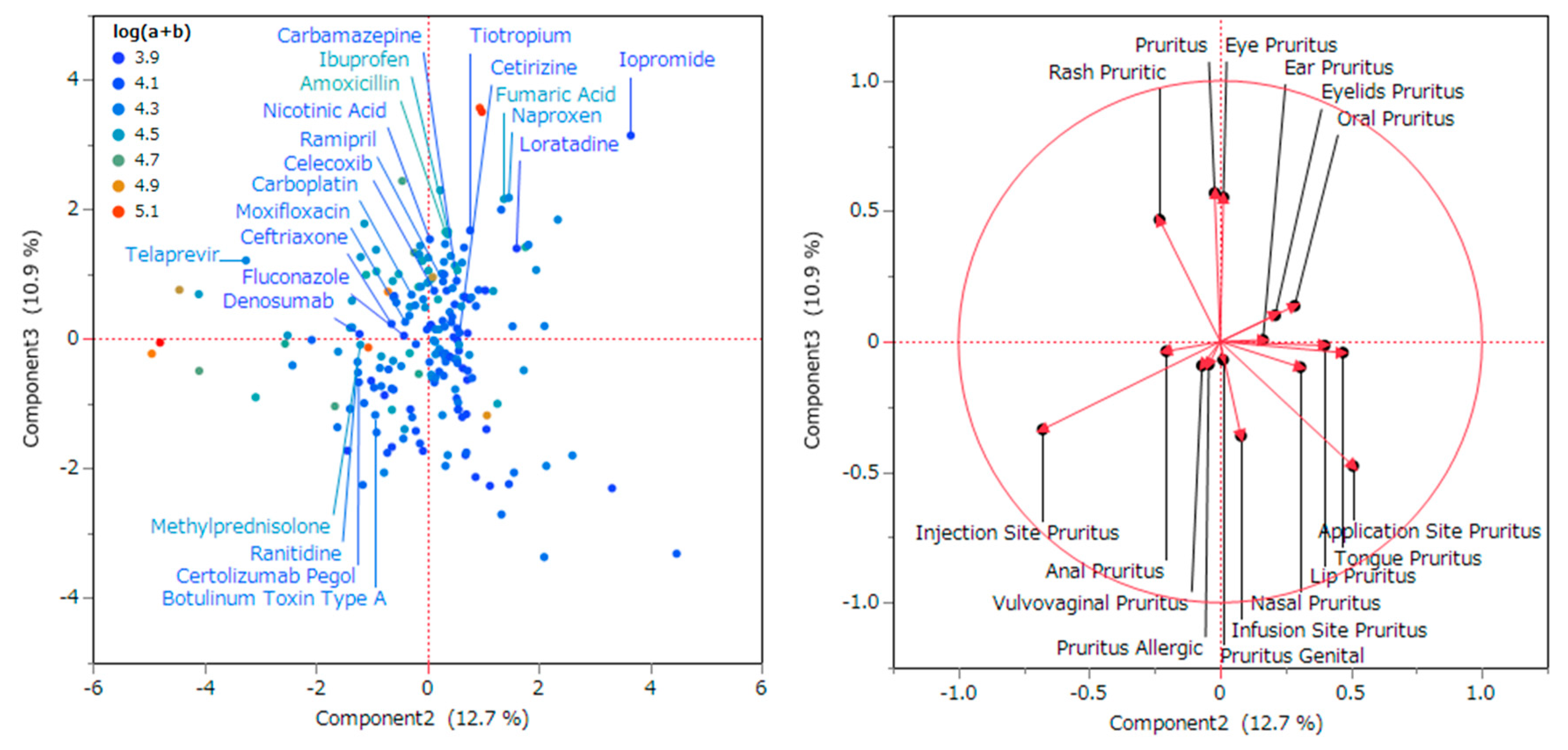
2.5. Characteristics of the Adverse Event Related to Pruritus Using Hierarchical Clustering
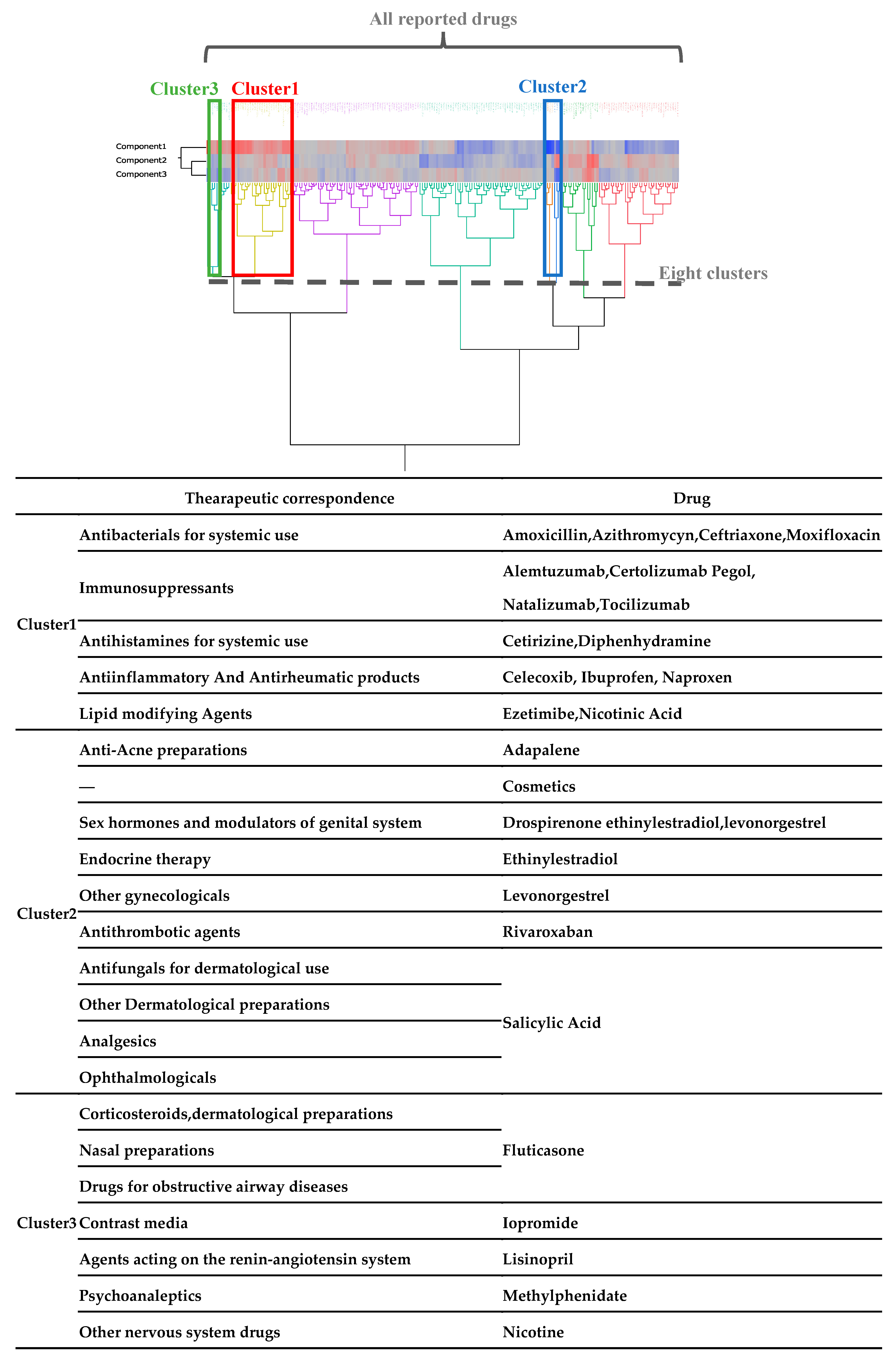
3. Discussion
3.1. Definition of Pruritus
3.2. Data Cleaning
3.3. Drug-Induced Pruritus and Patient Characteristics
3.4. Characteristics of Pruritus-Inducing Drugs
3.5. Principal Component Analysis
3.6. Hierarchical Cluster Analysis
3.7. Limitations
4. Materials and Methods
4.1. Data Source
4.2. Definitions of Pruritus-Related Adverse Events
4.3. Preparation of Data Table for Analysis
4.4. Patient Information on Drug-Induced Pruritus
4.5. Extraction of Suspect Drugs for Pruritus Using Data Mining Methods
4.6. Analysis of Drugs Associated with Pruritus Using Principal Component Analysis and Hierarchical Cluster Analysis
4.7. Statistical Analysis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, A.; Ständer, S.; Szepietowski, J. Drug-induced Pruritus: A Review. Acta Dermato-Venereologica 2009, 89, 236–244. [Google Scholar] [CrossRef]
- Ensslin, C.J.; Rosen, A.C.; Wu, S.; Lacouture, M.E. Pruritus in patients treated with targeted cancer therapies: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 2013, 69, 708–720. [Google Scholar] [CrossRef]
- Ebata, T. Drug-Induced Itch Management. Curr. Probl. Dermatol. 2016, 50, 155–163. [Google Scholar] [CrossRef]
- Huang, A.H.; Kaffenberger, B.H.; Reich, A.; Szepietowski, J.C.; Ständer, S.; Kwatra, S.G. Pruritus Associated with Commonly Prescribed Medications in a Tertiary Care Center. Medicines 2019, 6, 84. [Google Scholar] [CrossRef]
- Kuraishi, Y. Methods for Preclinical Assessment of Antipruritic Agents and Itch Mechanisms Independent of Mast-Cell Histamine. Biol. Pharm. Bull. 2015, 38, 635–644. [Google Scholar] [CrossRef]
- Sanjel, B.; Shim, W.-S. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: A review. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2020, 1866, 165958. [Google Scholar] [CrossRef]
- Harpaz, R.; DuMouchel, W.; LePendu, P.; Bauer-Mehren, A.; Ryan, P.; Shah, N.H. Performance of Pharmacovigilance Signal-Detection Algorithms for the FDA Adverse Event Reporting System. Clin. Pharmacol. Ther. 2013, 93, 539–546. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Golpanian, R. S., Yosipovitch, G., & Dodiuk-Gad, R. P. (2022). Drug-induced pruritus without primary rash. In Drug Eruptions (pp. 211–226). Cham: Springer International Publishing.
- Bahali, A.G.; Onsun, N.; Su, O.; Ozkaya, D.B.; Dizman, D.; Topukcu, B.; Uysal, O. The relationship between pruritus and clinical variables in patients with psoriasis. An. Bras. de Dermatol. 2017, 92, 470–473. [Google Scholar] [CrossRef]
- Leunig, A.; Szeimies, R.-M.; Wilmes, E.; Gutmann, R.; Stolz, W.; Feyh, J. Klinische und elektronenmikroskopische Untersuchung zur Hörsturztherapie mit der Kombination 10% HES 200/0,5 und Pentoxifyllin [Clinical and electron microscopy study of sudden deafness treatment with the 10% HES 200/0.5 and pentoxifylline combination]. Laryngo-Rhino-Otologie 1995, 74, 135–140. [Google Scholar] [CrossRef]
- Huang, A. S., & Meyer, J. J. (2022). Bimatoprost Ophthalmic Solution. In StatPearls. StatPearls Publishing.
- Lupia, T.; De Benedetto, I.; Bosio, R.; Shbaklo, N.; De Rosa, F.G.; Corcione, S. Role of Oritavancin in the Treatment of Infective Endocarditis, Catheter- or Device-Related Infections, Bloodstream Infections, and Bone and Prosthetic Joint Infections in Humans: Narrative Review and Possible Developments. Life 2023, 13, 959. [Google Scholar] [CrossRef]
- Messina, J.A.; Fowler, V.G.; Corey, G.R. Oritavancin for acute bacterial skin and skin structure infections. Expert Opin. Pharmacother. 2015, 16, 1091–8. [Google Scholar] [CrossRef]
- The MICAD Research Team. (2006). N-(2,3-Dihydroxypropyl)-N´-(2-hydroxyethyl)-5-[N-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodoisophthalamide. In Molecular Imaging and Contrast Agent Database (MICAD). National Center for Biotechnology Information (US).
- Thomsen, H.; Morcos, S. Radiographic contrast media. BJU Int. 2002, 86, 1–10. [Google Scholar] [CrossRef]
- Massiot, P.; Clavaud, C.; Thomas, M.; Ott, A.; Guéniche, A.; Panhard, S.; Muller, B.; Michelin, C.; Kerob, D.; Bouloc, A.; et al. Continuous clinical improvement of mild-to-moderate seborrheic dermatitis and rebalancing of the scalp microbiome using a selenium disulfide–based shampoo after an initial treatment with ketoconazole. J. Cosmet. Dermatol. 2021, 21, 2215–2225. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M.; Venkataraman, M.; Bamimore, M.A. Minoxidil: a comprehensive review. J. Dermatol. Treat. 2022, 33, 1896–1906. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Luketic, V.; Chapman, R.; Hirschfield, G.M.; Poupon, R.; Schramm, C.; Vincent, C.; Rust, C.; Parés, A.; Mason, A.; et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 2018, 67, 1890–1902. [Google Scholar] [CrossRef]
- Bergasa, N.V. The pruritus of cholestasis: From bile acids to opiate agonists: Relevant after all these years. Med Hypotheses 2018, 110, 86–89. [Google Scholar] [CrossRef]
- Alemi, F.; Kwon, E.; Poole, D.P.; Lieu, T.; Lyo, V.; Cattaruzza, F.; Cevikbas, F.; Steinhoff, M.; Nassini, R.; Materazzi, S.; et al. The TGR5 receptor mediates bile acid–induced itch and analgesia. J. Clin. Investig. 2013, 123, 1513–1530. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, J.-F.; Zhu, L.-L.; Chen, M.; Xu, H.-M.; Wang, H.-F.; Feng, X.-Q.; Zhu, X.-P. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Preference Adherence 2015, 9, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y., Hauri, A., Chiarello, L., Catlin, M., Stilwell, B., Ghebrehiwet, T., Garner, J., & Injection Safety Best Practices Development Group (2003). Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bulletin of the World Health Organization, 81(7), 491–500.
- Seirafianpour, F.; Pourriyahi, H.; Mesgarha, M.G.; Pour Mohammad, A.; Shaka, Z.; Goodarzi, A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol. Ther. 2022, 35, e15461. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19, 31–39. [Google Scholar] [CrossRef]
- Stern, R.S. Exanthematous Drug Eruptions. New Engl. J. Med. 2012, 366, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Adkinsonjr, N. Risk factors for drug allergy. J. Allergy Clin. Immunol. 1984, 74, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Hasford, J.; Goettler, M.; Munter, K.-H.; Müller-Oerlinghausen, B. Physicians' knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J. Clin. Epidemiology 2002, 55, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.; Tawfiq, A.M.; Hassan, N.; Palaian, S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther. Adv. Drug Saf. 2020, 11. [Google Scholar] [CrossRef]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data Mining of the Public Version of the FDA Adverse Event Reporting System. Int. J. Med Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef]
- Gravel, C. A., Douros, A. (2023). Considerations on the use of different comparators in pharmacovigilance: A methodological review. British journal of clinical pharmacology, Advance online publicationpublication. [CrossRef]
- Poleksic, A.; Xie, L. Database of adverse events associated with drugs and drug combinations. Sci. Rep. 2019, 9, 20025. [Google Scholar] [CrossRef]
- Yu, R.J.; Krantz, M.S.; Phillips, E.J.; Stone, C.A. Emerging Causes of Drug-Induced Anaphylaxis: A Review of Anaphylaxis-Associated Reports in the FDA Adverse Event Reporting System (FAERS). J. Allergy Clin. Immunol. Pr. 2021, 9, 819–829. [Google Scholar] [CrossRef]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data Mining of the Public Version of the FDA Adverse Event Reporting System. Int. J. Med Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef] [PubMed]
- FDA Adverse Event Reporting System (FAERS): Latest quarterly data. Available online: fileshttps://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files (accessed on 4 August 2023).
- Introductory Guide for Standardized MedDRA Queries (SMQs) Version 23.0 March 2020. Available online: https://admin.meddra.org/sites/default/files/guidance/file/SMQ_intguide_23_0_English.pdf.
- Khan, M.A.A.; Hamid, S.; Babar, Z.-U. Pharmacovigilance in High-Income Countries: Current Developments and a Review of Literature. Pharmacy 2023, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, K.; Uesawa, Y. Molecular Initiating Events Associated with Drug-Induced Liver Malignant Tumors: An Integrated Study of the FDA Adverse Event Reporting System and Toxicity Predictions. Biomolecules 2021, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Neha, R.; Beulah, E.; Anusha, B.; Vasista, S.; Stephy, C.; Subeesh, V. Aromatase inhibitors associated osteonecrosis of jaw: signal refining to identify pseudo safety signals. Int. J. Clin. Pharm. 2020, 42, 721–727. [Google Scholar] [CrossRef]
- Weber, F.; Knapp, G.; Ickstadt, K.; Kundt, G.; Glass, Ä. Zero-cell corrections in random-effects meta-analyses. Res. Synth. Methods 2020, 11, 913–919. [Google Scholar] [CrossRef]
- Cui, X.; A Churchill, G. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 2003, 4, 210. [Google Scholar] [CrossRef]
- Zhang, Z.; Castelló, A. Principal components analysis in clinical studies. Ann. Transl. Med. 2017, 5, 351–351. [Google Scholar] [CrossRef]
| Pruritus | Non-pruritus | Sum | ||||||
|---|---|---|---|---|---|---|---|---|
| Average±SD | Number of cases | Number of patients | Average±SD | Number of cases | Number of patients | Number of cases | ||
| All data table | Both gender | - | 86,989 | 57,559 | - | 7,309,045 | 1,326,056 | 7,396,343 |
| Age | 50.30±20.24 | 81,230 | 53,512 | 53.17±21.09 | 6,760,759 | 1,219,276 | 6,841,989 | |
| Weight | 88.26±44.93 | 39,102 | 26,865 | 83.44±44.55 | 3,821,673 | 612,533 | 3,860,775 | |
| Male data table | Age | 52.13±20.65 | 26,081 | 16,403 | 55.57±21.11 | 2,726,069 | 491,447 | 2,752,150 |
| Weight | 91.27±44.77 | 13,804 | 8,843 | 86.22±45.34 | 1,566,742 | 255,369 | 1,580,546 | |
| Female data table | Age | 49.44±19.99 | 55,149 | 37,109 | 55.15±20.92 | 4,034,690 | 727,829 | 4,089,839 |
| Weight | 86.63±44.94 | 25,298 | 18,022 | 81.87±43.90 | 2,254,931 | 357,164 | 2,280,229 | |
| Therapeutic_correspondence | Drug |
|---|---|
| Antibacterials for systemic use | Amoxicillin, Azithromycin, Ceftriaxone, Ciprofloxacin, Clarithromycin, Levofloxacin, Linezolid, Metronidazole, Moxifloxacin, Piperacillin, sulfamethoxazole, tazobactam, trimethoprim, vancomycin |
| Antineoplastic agents | Carboplatin, Celecoxib, Gemcitabine, Imatinib, Nilotinib, Nivolumab, Oxaliplatin, Pazopanib, Regorafenib, Rituximab |
| Ophthalmologicals | Aciclovir, Azithromycin, Ciclosporin, Ciprofloxacin, Diclofenac, Levofloxacin, Lidocaine, Moxifloxacin, Vancomycin |
| Immunosuppressants | Alemtuzumab, Certolizumab pegol, Ciclosporin, Fumaric acid, Infliximab, Natalizumab, Teriflunomide, Tocilizumab, Tofacitinib |
| Antivirals for systemic use | Aciclovir, Dasabuvir, Emtricitabine, Lamivudine, Sofosbuvir, Telaprevir, Valaciclovir |
| Preferred Term | Number of patients | |
|---|---|---|
| 1 | Pruritus | 40618 |
| 2 | Rash Pruritic | 6287 |
| 3 | Injection Site Pruritus | 3034 |
| 4 | Application Site Pruritus | 2401 |
| 5 | Eye Pruritus | 2278 |
| 6 | Vulvovaginal Pruritus | 647 |
| 7 | Oral Pruritus | 400 |
| 8 | Ear Pruritus | 294 |
| 9 | Eyelids Pruritus | 263 |
| 10 | Anal Pruritus | 253 |
| 11 | Pruritus Genital | 195 |
| 12 | Pruritus Allergic | 127 |
| 13 | Tongue Pruritus | 124 |
| 14 | Infusion Site Pruritus | 122 |
| 15 | Implant Site Pruritus | 97 |
| 16 | Nasal Pruritus | 89 |
| 17 | Lip Pruritus | 80 |
| 18 | Instillation Site Pruritus | 46 |
| 19 | Catheter Site Pruritus | 23 |
| 20 | Gingival Pruritus | 14 |
| 21 | Itching Scar | 7 |
| 22 | Administration Site Pruritus | 6 |
| 23 | Incision Site Pruritus | 6 |
| 24 | Vaccination Site Pruritus | 6 |
| 25 | Aquagenic Pruritus | 5 |
| 26 | Cholestatic Pruritus | 4 |
| 27 | Stoma Site Pruritus | 2 |
| 28 | Vessel Puncture Site Pruritus | 2 |
| 29 | Medical Device Site Pruritus | 1 |
| 30 | Papular Pruritic Eruption Of Hiv | 0 |
| 31 | Vascular Access Site Pruritus | 0 |
| 32 | Tumor Pruritus | 0 |
| 33 | Post Procedural Pruritus | 0 |
| 34 | Puncture Site Pruritus | 0 |
| 35 | Uraemic Pruritus | 0 |
| 36 | Senile Pruritus | 0 |
| 37 | Brachioradial Pruritus | 0 |
| Pruritus | Non-Pruritus | |
| Suspected drug | a | b |
| Other drugs | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





