Submitted:
10 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
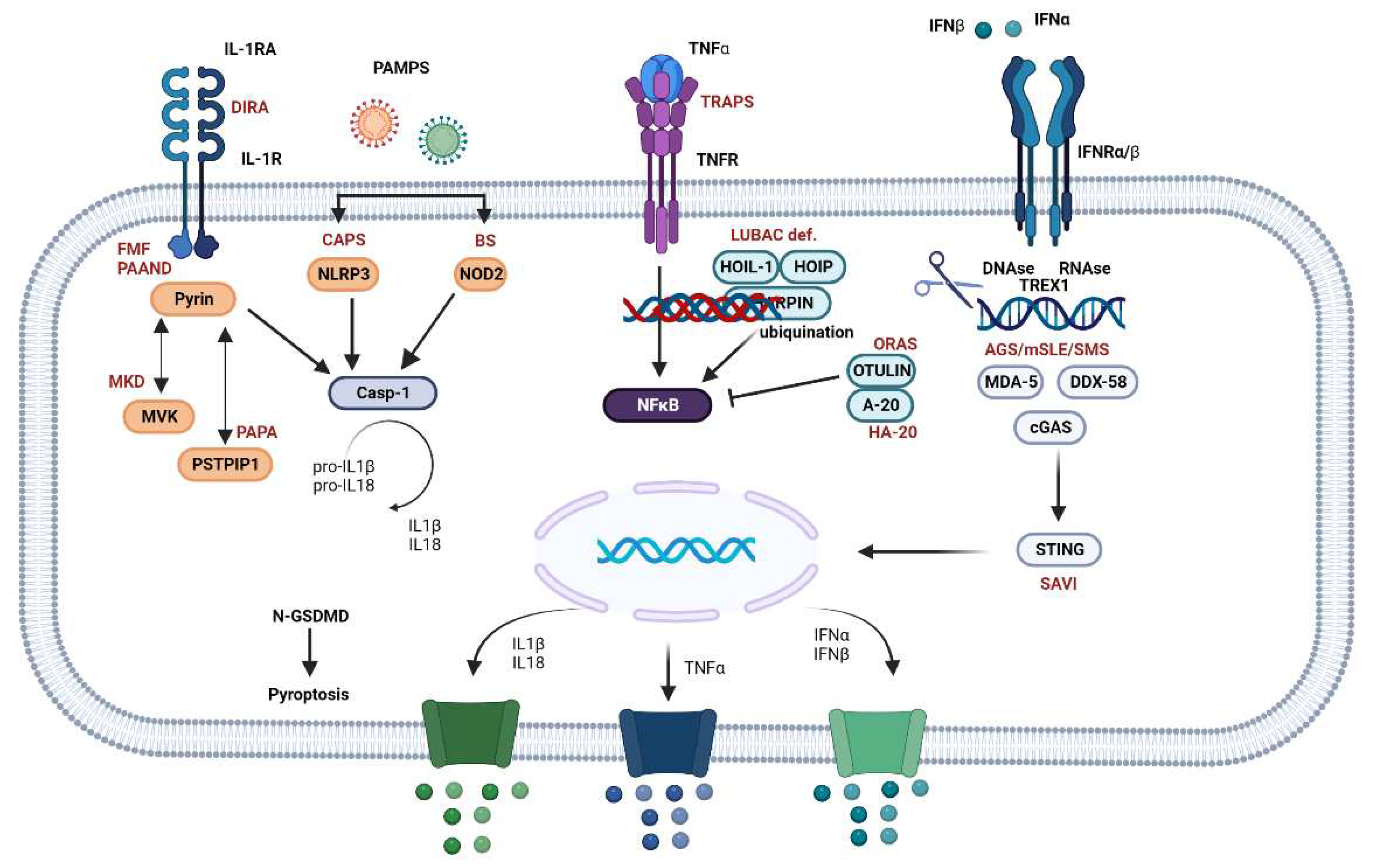
2. Pathophysiological Mechanisms
3. Classification of SAIDs
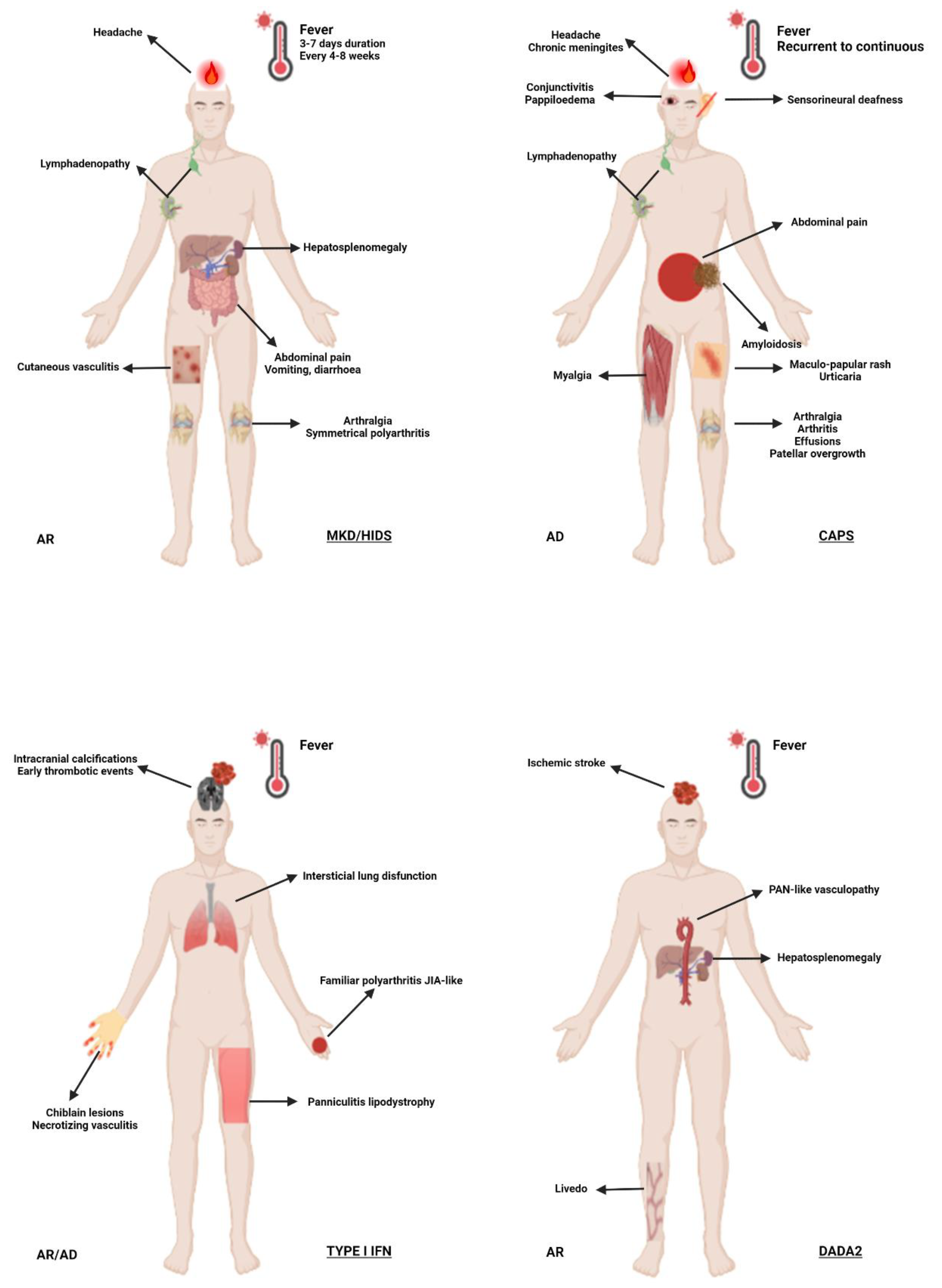
3.1. Cryopirin-Associated Periodic Syndrome (CAPS)
- Cryopirin-Associated Periodic Syndrome comprises a heterogeneous group of diseases caused by NLRP3 (nod-like protein family pyrin domain containing-3) GOF mutations encoded in chromosome 1q44 [15]. Autosomal dominant inheritance with multiple clinical phenotypes has been reported. However, somatic cases have also been described. NLRP3 encodes cryopyrin, which plays an essential role in IL-1β production. Hyperactivation of the inflammasome by NLRP3 GOF mutations induces IL-1β overproduction, leading to uncontrolled and inappropriate systemic inflammation [16,17]. The prevalence of CAPS is around three persons per million and there is no gender or ethnic predilection [16].
- This syndrome includes a continuum of three clinical phenotypes, from the milder familial cold autoinflammatory syndrome (FCAS) to the more severe Neonatal Onset Multisystem Inflammatory Disease (NOMID), also known as Chronic Infantile Neurologic Cutaneous Articular (CINCA) syndrome. Muckle-Wells syndrome (MWS) is an intermediate phenotype. Patients with FCAS present with self-limited (< 24h) episodes of fever, urticaria-like skin lesions, arthralgia and conjunctivitis triggered by cold exposure. Muckle-Wells syndrome is clinically similar but has a chronic course and may progress to sensorineural deafness and AA amyloidosis (30% of cases) characterized by nephrotic syndrome and kidney failure [18]. The most severe presentation is NOMID/CINCA, an early-onset disease (usually before 6 months of age) that leads to death before adulthood unless controlled by timely intervention [19]. Affected infants fail to thrive and may develop bony overgrowth, joint contractures, destructive arthropathy, dysmorphism, learning disability and progressive neurologic impairment [20]. Diagnostic criteria for CAPS consist of one positive inflammatory marker plus two or more typical symptoms: urticaria-like skin lesions (neutrophilic perivascular infiltrate), cold-induced episodes, sensorineural hearing loss (secondary to chronic cochlear inflammation), musculoskeletal symptoms (arthralgia/arthritis/myalgia), chronic aseptic meningitis and skeletal abnormalities such as epiphyseal overgrowth or frontal bossing [21,22].
- In the largest CAPS cohort (n = 136) investigated to date, 40% of patients had neurological manifestations like headache (70%), papilledema (52%), hearing loss secondary to cochlear inflammation (42%), aseptic meningitis (26%), hydrocephalus (18%), mental retardation (16%) and seizures (4%) [23]. In the severe forms of the disease, permanent central nervous system (CNS) damage may occur in untreated patients, leading to brain atrophy, ventriculomegaly, arachnoid adhesions and leptomeningeal enhancement.
- Ancillary test results are usually non-specific but indicative of an inflammatory state. Chronic anemia and elevation of acute phase reactants during inflammatory episodes, including erythrocyte sedimentation rate (ESR), c-reactive protein (CRP) and serum amyloid A (SAA) protein, are the major laboratory findings [20]. Cerebrospinal fluid (CSF) analysis often reveals elevated intracranial pressure, pleocytosis, high protein levels and normal glucose levels [22]. Predilection for high frequencies (4000-8000Hz) and cochlear enhancement in inner ear MRI studies have been reported in patients with hearing loss.
- Treatment with IL-1 inhibitors should be promptly started. Nonsteroidal (NSAIDs) and steroidal anti-inflammatory drugs can be prescribed to control symptoms but are not indicated as primary maintenance therapy [9]. Patients should be regularly monitored using complete blood count and ESR/CRP, disease activity scores, audiometry, ophthalmological examination and urine protein test. Periodic cognitive assessment, lumbar puncture, brain MRI and skeletal imaging are also indicated in severe cases [24,25].
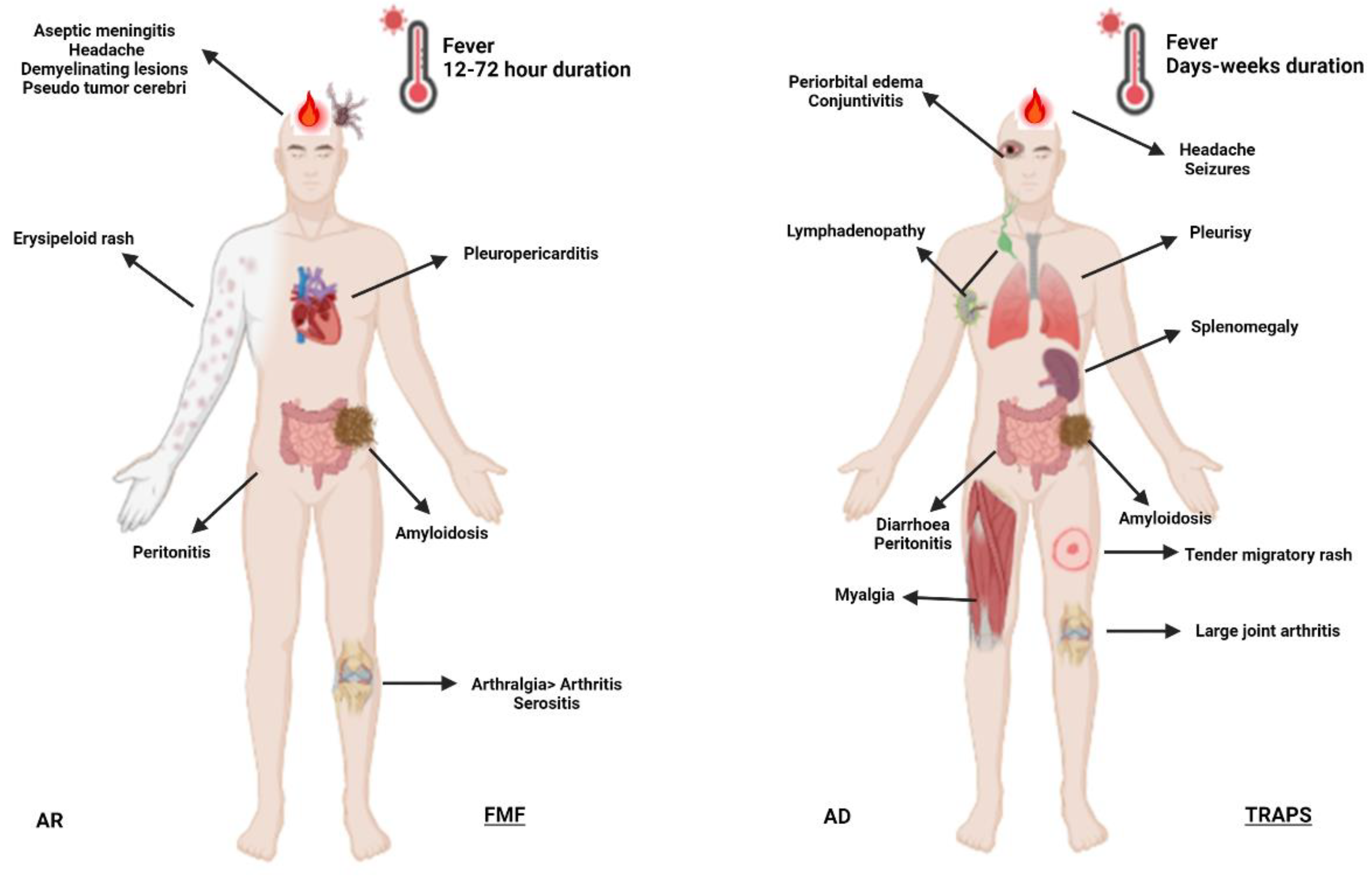
3.2. Familial Mediterranean Fever (FMF)
3.3. Mevalonate Kinase Deficiency (MKD) and Mevalonic Aciduria (MVA)
3.4. Type I Interferonopathies
3.5. Tumor Necrosis Factor Associated Periodic Syndrome (TRAPS)
3.6. A20 Haploinsufficiency (HA20)
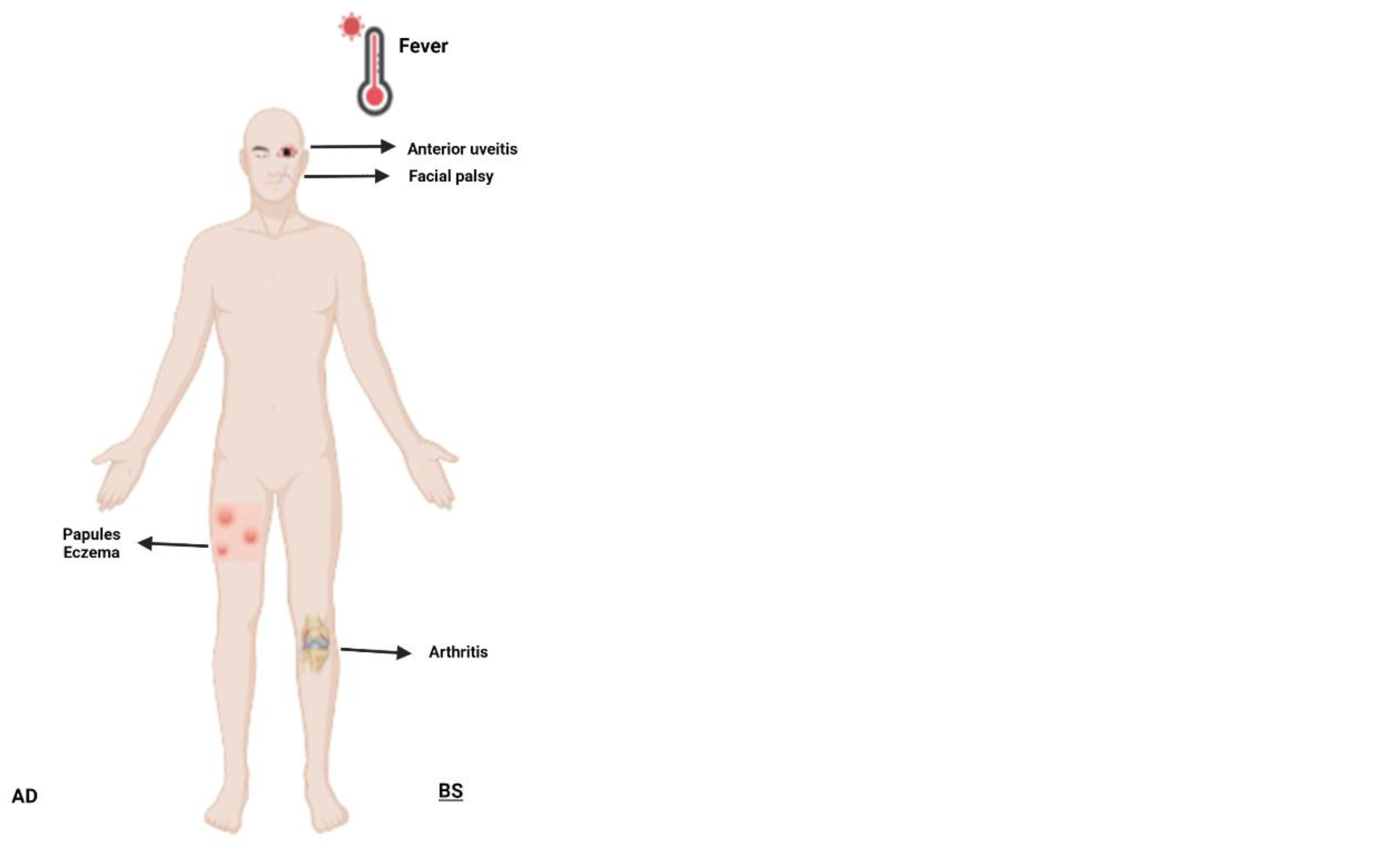
3.7. Blau Syndrome (BS)
3.8. Deficiency of Adenosine Deaminase 2 (DADA-2)
4. Therapeutic Approach to SAIDs
4.1. General Approach
4.2. Therapeutic Agents for SAIDs
4.2.1. Corticosteroids and NSAIDs
4.2.2. Colchicine
4.2.3. IL-1 Inhibitors
4.2.4. Tumor necrosis Factor (TNF)-Alpha Inhibitors
4.2.5. Janus Kinase Inhibitors (JAKi)
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozkurede VU, Franchi L. Immunology in clinic review series; focus on autoinflammatory diseases: role of inflammasomes in autoinflammatory syndromes. Clin Exp Immunol 167:382–390, 2012.
- McDermott MF, Aksentijevich I. The autoinflammatory syndromes. Curr Opin Allergy Clin Immunol 2: 511-516, 2002.
- Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. Journal of Clinical Immunology. 2020 Jan 1;40(1):66–81.
- Gaggiano C, Rigante D, Vitale A et al (2019) Hints for genetic and clinical differentiation of adult-onset monogenic autoinflammatory diseases. Mediators Inflamm 2019.
- Latz, E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013; 13:397-411.
- de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015; 33:823-74.
- Berg, S., Wekell, P., Fasth, A., Hawkins, P. N., & Lachmann, H. (2016). Autoinflammatory Disorders. Primary Immunodeficiency Diseases, 393–435.
- Caso F, Costa L, Nucera V, Barilaro G, Masala IF, Talotta R, Caso P, Scarpa R, Sarzi-Puttini P, Atzeni F (2018) From autoinflammation to autoimmunity: old and recent findings. Clin Rheumatol. 37(9):2305–2321.
- Van Kempen TS, Wenink MH, Leijten EF, Radstake TR, Boes M. Perception of self: distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol. 2015. 11(8):483–492.
- McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoSMed. 2006;3: e297.
- Hausmann J, Dedeoglu, F. Autoinflammatory diseases- Neurorheumatology: A Comprehensive Guide to Immune Mediated Disorders of the Nervous System; Cho, T.A., Bhattacharyya, S., Helfgott, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–133.
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007; 14:10–22.
- Tartey, S, Kanneganti, T-D. Inflammasomes in the pathophysiology of autoinflammatory syndromes. J Leukoc Biol. 2020; 107: 379– 391.
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014; 157:1013-1022.
- Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015; 265:6-21.
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol.2012; 28:137-161.
- Aksentijevich I, Schnappauf O. Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases. Vol. 17, Nature Reviews Rheumatology. Nature Research; 2021. p. 405–25.
- Kone-Paut I. Cryopyrine-associated periodic syndrome: CAPS seen from adulthood. Rev Med Interne. 2015;36(4):277–82.
- Kitley JL, Lachmann HJ, Pinto A, Ginsberg L. Neurologic manifestations of the cryopyrin-associated periodic syndrome. Neurology. 2010 Apr 20;74(16):1267-70.
- Mamoudjy N, Maurey H, Marie I, Koné-Paut I, Deiva K. Neurological outcome of patients with cryopyrin-associated periodic syndrome (CAPS). Orphanet J Rare Dis. 2017 Feb 14;12(1):33.
- Shinkai K, McCalmont TH, Leslie KS. Cryopyrin associated periodic syndromes and autoinflammation. Clin Exp Dermatol 2007; 33:1–9.
- Levy R, Gerard L, Kuemmerle-Deschner J, Lachmann HJ, Kone-Paut I, et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. 2015;74(11):2043–9.
- Parker T, Keddie S, Kidd D, Lane T, Maviki M, Hawkins PN, Lachmann HJ, Ginsberg L. Neurology of the cryopyrin-associated periodic fever syndrome. Eur J Neurol. 2016 Jul;23(7):1145-51.
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017 Jun;76(6):942-947.
- Schnappauf O, Chae JJ, Kastner DL, Aksentijevich I. The Pyrin Inflammasome in Health and Disease. Vol. 10, Frontiers in immunology. NLM (Medline); 2019. p. 1745.
- Heilig R, Broz P. Function and mechanism of the pyrin inflammasome. Vol. 48, European Journal of Immunology. Wiley-VCH Verlag; 2018. p. 230–8.
- Boursier G, Hentgen V, Sarrabay G, Koné-Paut I, Touitou I. The Changing Concepts Regarding the Mediterranean Fever Gene: Toward a Spectrum of Pyrin-Associated Autoinflammatory Diseases with Variable Heredity. Journal of Pediatrics. 2019 Jun 1; 209:12-16. e1.
- Saatci U, Ozen S, Ozdemir S, Bakkaloglu A, Besbas N, Topaloglu R, Arslan S. Familial Mediterranean fever in children: report of a large series and discussion of the risk and prognostic factors of amyloidosis. Eur J Pediatr. 1997; 156:619–23.
- Padeh S. Periodic fever syndromes. Pediatr Clin North Am. 2005;52:577-609.
- Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore). 2005; 84:1–11.
- Salehzadeh F, Azami A, Motezarre M, Nematdoust Haghi R, Ahmadabadi F. Neurological Manifestations in Familial Mediterranean Fever: A Genotype-Phenotype Correlation Study. Open Access Rheumatol. 2020 Jan 15; 12:15-19.
- Kalyoncu U, Eker A, Oguz KK, et al. Familial Mediterranean fever and central nervous system involvement: a case series. Medicine (Baltimore). 2010;89(2):75–84.
- Elhani I, Dumont A, Vergneault H, Ardois S, Le Besnerais M, Levesque H et al. Association between familial Mediterranean fever and multiple sclerosis: a case series from the JIR cohort and systematic literature review. Mult Scler Relat Disord. 2021; 50:102834.
- Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, Migdal A, Padeh S, Pras M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997; 40:1879–85.
- Gattorno M, Hofer M, Federici S, et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis. 2019;78(8):1025-1032.
- Kallinich T, Haffner D, Niehues T, Huss K, Lainka E, et al. Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics. 2007;119: e474–83.
- Hashkes PJ, Spalding SJ, Giannini EH, Huang B, Johnson A, et al. Rilonacept for colchicine resistant or intolerant familial Mediterranean fever: a randomized trial. Ann Intern Med. 2012; 157:533–41.
- Mor A, Pillinger MH, Kishimoto M, Abeles AM, Livneh A. Familial Mediterranean fever successfully treated with etanercept. J Clin Rheumatol. 2007; 13:38–40.
- Mulders-Manders CM, Simon A. Hyper-IgD syndrome/mevalonate kinase deficiency: What is new? Vol. 37, Seminars in Immunopathology. Springer Verlag; 2015. p. 371–6.
- van der Burgh R, ter Haar NM, Boes ML, Frenkel J. Mevalonate kinase deficiency, a metabolic autoinflammatory disease. Vol. 147, Clinical Immunology. Academic Press Inc.; 2013. p. 197–206.
- Haas D, Hoffmann GF. Mevalonate kinase deficiencies: from mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet J Rare Dis. 2006 Apr 26; 1:13.
- Simon A, Kremer HP, Wevers RA, et al. Mevalonate kinase deficiency: evidence for a phenotypic continuum. Neurology. 2004; 62:994-997.
- Brennenstuhl H, Nashawi M, Schröter J, Baronio F, Beedgen L, et al. Unified Registry for Inherited Metabolic Disorders (U-IMD) Consortium and the European Registry for Hereditary Metabolic Disorders (MetabERN). Phenotypic diversity, disease progression, and pathogenicity of MVK missense variants in mevalonic aciduria. J Inherit Metab Dis. 2021 Sep;44(5):1272-1287.
- Hoffmann GF, Charpentier C, Mayatepek E, et al. Clinical and biochemical phenotype in 11 patients with mevalonic aciduria. Pediatrics. 1993; 91:915-921.
- Prietsch V, Mayatepek E, Krastel H, et al. Mevalonate kinase deficiency: enlarging the clinical and biochemical spectrum. Pediatrics. 2003; 111:258-261.
- Arkwright PD, Abinun M, Cant AJ. Mevalonic aciduria cured by bone marrow transplantation. N Engl J Med. 2007 Sep 27;357(13):1350.
- Ter Haar NM, Jeyaratnam J, Lachmann HJ, Simon A, Brogan PA et al. Paediatric Rheumatology International Trials Organisation and Eurofever Project. The Phenotype and Genotype of Mevalonate Kinase Deficiency: A Series of 114 Cases from the Eurofever Registry. Arthritis Rheumatol. 2016 Nov;68(11):2795-2805.
- Crow YJ, Black DN, Ali M, Bond J, Jackson AP, Lefson M, et al. Cree encephalitis is allelic with Aicardi-Goutières syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet. (2003) 40:183–7.
- Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J Exp Med. (2016) 213:2527–38.
- Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. (2011) 1238:91–8.
- Davidson S, Steiner A, Harapas CR, Masters SL. An update on autoinflammatory diseases: interferonopathies. Curr Rheumatol Rep. (2018) 20:38.
- Negishi H, Taniguchi T, Yanai H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb Perspect Biol. (2018) 10: a028423.
- Trinchieri G. Type I interferon: friend or foe? J Exp Med. (2010) 207:2053– 63.
- Lee-Kirsch MA, Wolf C, Kretschmer S, Roers A. Type I interferonopathies – an expanding disease spectrum of immunodysregulation. Semin Immunopathol. 2015; 37:349–57.
- Crow YJ, Stetson DB. The type I interferonopathies: 10 years on. Nature Reviews Immunology. Nature Research; 2021.
- Anderson SR, Vetter ML. Developmental roles of microglia: a window into mechanisms of disease. Dev Dyn. (2019) 248:98–117.
- McDonough A, Lee RV, Weinstein JR. Microglial interferon signaling and white matter. Neurochem Res. (2017) 42:2625– 38.
- Goldmann T, Blank T, Prinz M. Fine-tuning of type I IFN signaling in microglia-implications for homeostasis, CNS autoimmunity and interferonopathies. Curr Opin Neurobiol. (2016) 36:38–42.
- Crow YJ, Rehwinkel J. Aicardi-Goutières syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum Mol Genet. (2009) 18: R130–6.
- Benjamin P, Sudhakar S, D'Arco F, Löbel U, Carney O, et al. Spectrum of Neuroradiologic Findings Associated with Monogenic Interferonopathies. AJNR Am J Neuroradiol. 2022 Jan;43(1):2-10.
- Wilms AE, de Boer I, Terwindt GM. Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic manifestations (RVCL-S): An update on basic science and clinical perspectives. Cerebral Circulation - Cognition and Behavior. 2022 Jan 1;3.
- Stam AH, Kothari PH, Shaikh A, Gschwendtner A, Jen JC et al. Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Brain. 2016 Nov 1;139(11):2909-2922.
- N. Pelzer, E.S. Hoogeveen, J. Haan, et al., Systemic features of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: a monogenic small vessel disease, J. Intern. Med. 285 (3) (2019) 317–33.
- Lindahl H, Bryceson YT. Neuroinflammation Associated with Inborn Errors of Immunity. Front Immunol. 2022 Jan 19; 12:827815.
- Cetin Gedik K, Lamot L, Romano M, Demirkaya E, Piskin D et al. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology Points to Consider for Diagnosis and Management of Autoinflammatory Type I Interferonopathies: CANDLE/PRAAS, SAVI, and AGS. Arthritis Rheumatol. 2022 May;74(5):735-751.
- F. Magnotti1 AVDROML. The most recent advances in pathophysiology and management of tumour necrosis factor receptor-associated periodic syndrome (TRAPS): personal experience and literature review. Clinical Experimental Rheumatology. 2013;(31 (Suppl. 77): S141-S149).
- Rigante D, Lopalco G, Vitale A, Lucherini OM, de Clemente C, Caso F, et al. Key facts and hot spots on tumor necrosis factor receptor-associated periodic syndrome. Vol. 33, Clinical Rheumatology. Springer-Verlag London Ltd; 2014. p. 1197–207.
- Lachmann HJ, Papa R, Gerhold K, et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: a series of 158 cases form the Eurofever/ EUROTRAPS international registry. Ann Rheum Dis 2014; 73:2160–7.
- Caminero A, Comabella M, Montalban X. Role of tumour necrosis factor (TNF)-α and TNFRSF1A R92Q mutation in the pathogenesis of TNF receptor-associated periodic syndrome and multiple sclerosis. Clin Exp Immunol 2011; 166:338–45.
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci 2001; 2:734–44.
- Kirresh, Ali; Everitt, Alex; Kon, Onn Min; DasGupta, Ranan; Pickering, Matthew C; Lachmann, Helen J (2016). Trapped without a diagnosis: Tumour necrosis factor receptor-associated periodic syndrome (TRAPS). Practical Neurology, 16(4), 304–307.
- Catrysse L, Vereecke L, Beyaert R, et al. A20 in inflammation and autoimmunity. Trends Immunol 2014; 35:22–31.
- Aeschlimann FA, Batu ED, Canna SW, Go E, Gül A, Hoffmann P et al.A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognized NF-kB-mediated autoinflammatory disease. Ann Rheum Dis. 2018 May;77(5):728-735.
- Wouters CH, Maes A, Foley KP, Bertin J, Rose CD. Blau Syndrome, the prototypic auto-inflammatory granulomatous disease. Vol. 12, Pediatric Rheumatology. BioMed Central Ltd.; 2014.
- Sfriso P, Caso F, Tognon S, Galozzi P, Gava A, Punzi L. Blau syndrome, clinical and genetic aspects. Vol. 12, Autoimmunity Reviews. 2012. p. 44–51.
- Negroni A, Pierdomenico M, Cucchiara S, Stronati L. NOD2 and inflammation: Current insights. Vol. 11, Journal of Inflammation Research. Dove Medical Press Ltd; 2018. p. 49–60.
- Rose CD, Arostegui JI, Martin TM, Espada G, Scalzi L et al. NOD2-associated pediatric granulomatous arthritis, an expanding phenotype: study of an international registry and a national cohort in Spain. Arthritis Rheum. 2009; 60:1797–803.
- Barron KS, Aksentijevich I, Deuitch NT, Stone DL, Hoffmann P et al. The Spectrum of the Deficiency of Adenosine Deaminase 2: An Observational Analysis of a 60 Patient Cohort. Front Immunol. 2022 Jan 10; 12:811473.
- Zhou O, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-Onset Stroke and Vasculopathy Associated with Mutations in ADA2. N Engl J Med (2014) 370(10):911–20.
- Sanchez GAM, Hashkes PJ. Neurological manifestations of the Mendelian- inherited autoinflammatory syndromes. Vol. 51, Developmental Medicine and Child Neurology. 2009. p. 420–8.
- Diprose WK, Jordan A, Anderson NE. Autoinflammatory syndromes in neurology: when our first line of defence misbehaves. Vol. 22, Practical Neurology. NLM (Medline); 2022. p. 145–53.
- Soriano A, Soriano M, Espinosa G, Manna R, Emmi G, Cantarini L, et al. Current Therapeutic Options for the Main Monogenic Autoinflammatory Diseases and PFAPA Syndrome: Evidence-Based Approach and Proposal of a Practical Guide. Vol. 11, Frontiers in Immunology. Frontiers Media S.A.; 2020.
- Welzel T, Benseler SM, Kuemmerle-Deschner JB. Management of Monogenic IL-1 Mediated Autoinflammatory Diseases in Childhood. Vol. 12, Frontiers in Immunology. Frontiers Media S.A.; 2021.
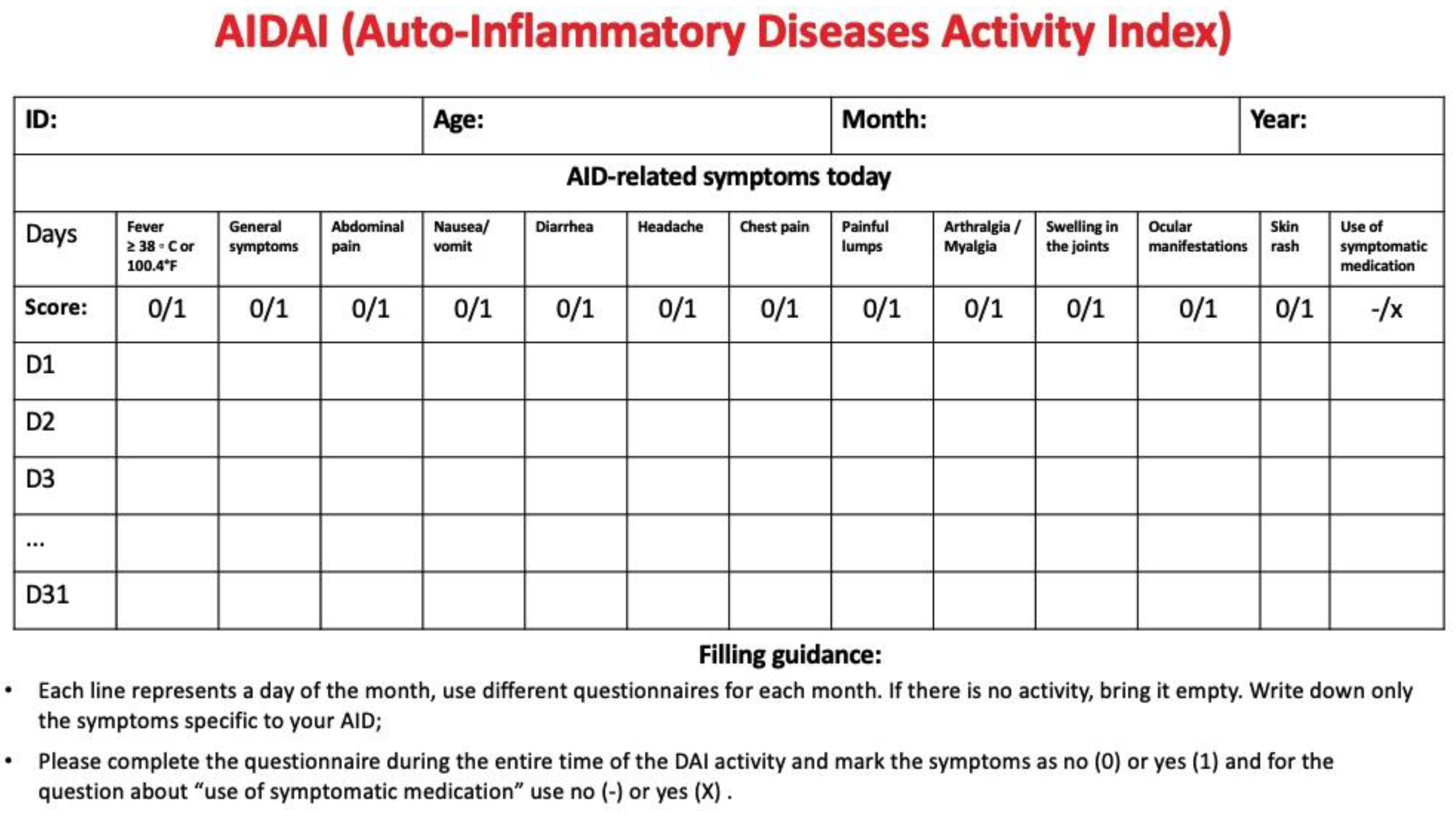
- Piram M, Koné-Paut I, Lachmann HJ, Frenkel J, Ozen S, Kuemmerle-Deschner J, et al. Validation of the Auto-Inflammatory Diseases Activity Index (AIDAI) for hereditary recurrent fever syndromes. Annals of the Rheumatic Diseases. 2014 Dec 1;73(12):2168–73.
- ter Haar NM, Annink K v., Al-Mayouf SM, Amaryan G, Anton J, Barron KS, et al. Development of the autoinflammatory disease damage index (ADDI). Annals of the Rheumatic Diseases. 2017 May 1;76(5):821–30.
- ter Haar NM, van Delft ALJ, Annink KV, van Stel H, Al-Mayouf SM, Amaryan G, et al. In silico validation of the autoinflammatory disease damage index. Annals of the Rheumatic Diseases. 2018 Nov 1;77(11):1599–605.
- Cetin Gedik K, Lamot L, Romano M, Demirkaya E, Piskin D et al. The 2021 Eurpean Alliance of Associations for Rheumatology/American College of Rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI and AGS. Annals of the Rheumatic Diseases. 2022.
- Ozen S, Demirkaya E, Erer B, Livneh A, Ben-Chetrit E, Giancane G, et al. EULAR recommendations for the management of familial Mediterranean fever. Annals of the Rheumatic Diseases. 2016 Apr 1;75(4):644–51.
- Pamuk ON, Pamuk GE, Hamuryudan V. Colchicine neuromyopathy: A report of six cases [Internet]. 2014.
- Romano M, Arici ZS, Piskin D, Alehashemi S, Aletaha D, Barron K et al. The 2021 EULAR/American College of Rheumatology Points to Consider for Diagnosis, Management and Monitoring of the Interleukin-1 Mediated Autoinflammatory Diseases: Cryopyrin-Associated Periodic Syndromes, Tumor Necrosis Factor Receptor-Associated Periodic Syndrome, Mevalonate Kinase Deficiency, and Deficiency of the Interleukin-1 Receptor Antagonist. Arthritis and Rheumatology. 2022 Jul 1.
- Deuitch NT, Yang D, Lee PY, Yu X, Moura NS, Schnappauf O, et al. TNF inhibition in vasculitis management in adenosine deaminase 2 deficiency (DADA2). Journal of Allergy and Clinical Immunology. 2022 May 1;149(5):1812-1816.e6.
- Cooray S, Omyinmi E, Hong Y, Papadopoulou C, Harper L, Al-Abadi E, et al. Anti-tumor necrosis factor treatment for the prevention of ischaemic events in patients with deficiency of adenosine deaminase 2 (DADA2). Rheumatology. 2021 Sep 1;60(9):4373–8.
- Manna R, Rigante D. The everchanging framework of autoinflammation. Intern Emerg Med. 2021 Oct;16(7):1759-1770.
- Gómez-Arias PJ, Gómez-García F, Hernández-Parada J, Montilla-López AM, Ruano J, et al. Efficacy and Safety of Janus Kinase Inhibitors in Type I Interferon-Mediated Monogenic Autoinflammatory Disorders: A Scoping Review. Dermatol Ther (Heidelb). 2021 Jun;11(3):733-750.
- Boyadzhieva Z, Ruffer N, Burmester G, Pankow A, Krusche M. Effectiveness and Safety of JAK Inhibitors in Autoinflammatory Diseases: A Systematic Review. Front Med (Lausanne). 2022 Jun 27;9:930071.

| Treatments | ||
|---|---|---|
| IL-1β-Mediated Autoinflammatory Disorders | Cryopyrin-Associated Periodic Syndrome (CAPS) | IL-1 antagonists, steroids |
| Familial Mediterranean Fever (FMF) | Colchicine, steroids, TNF antagonists, IL-6 antagonists, and IL-1 antagonists | |
| Mevalonate kinase deficiency (MKD) and mevalonic aciduria (MVA) | IL-1 antagonists, steroids, colchicine, IL-6 antagonists, and TNF antagonists | |
| Relopathies | A20 Haploinsufficiency | anti-TNF, anti-IL-1, and hematopoietic stem cell transplant (severe and refractory disease) |
| Dysregulation of TNF activity | Blau syndrome | Steroids, TNF antagonist |
| Deficiency of adenosine deaminase 2 (DADA2) | anti-TNF, and hematopoietic stem cell transplant | |
| Type I interferonopathies | Aicardi-Goutières syndrome Proteasome-associated autoinflammatory syndromes (PRAAS) ISG15 (interferon-stimulated gene 15) deficiency Singleton–Merten syndrome (SMS) COPA (coatomer protein subunit alpha) syndrome STING-associated vasculopathy with onset in infancy (SAVI) |
JAK inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




