Submitted:
13 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of g-C3N4 nanosheets
2.2. Synthesis of g-C3N4‒ZnCdS heterojunction
2.3. Photo and electrochemical measurements
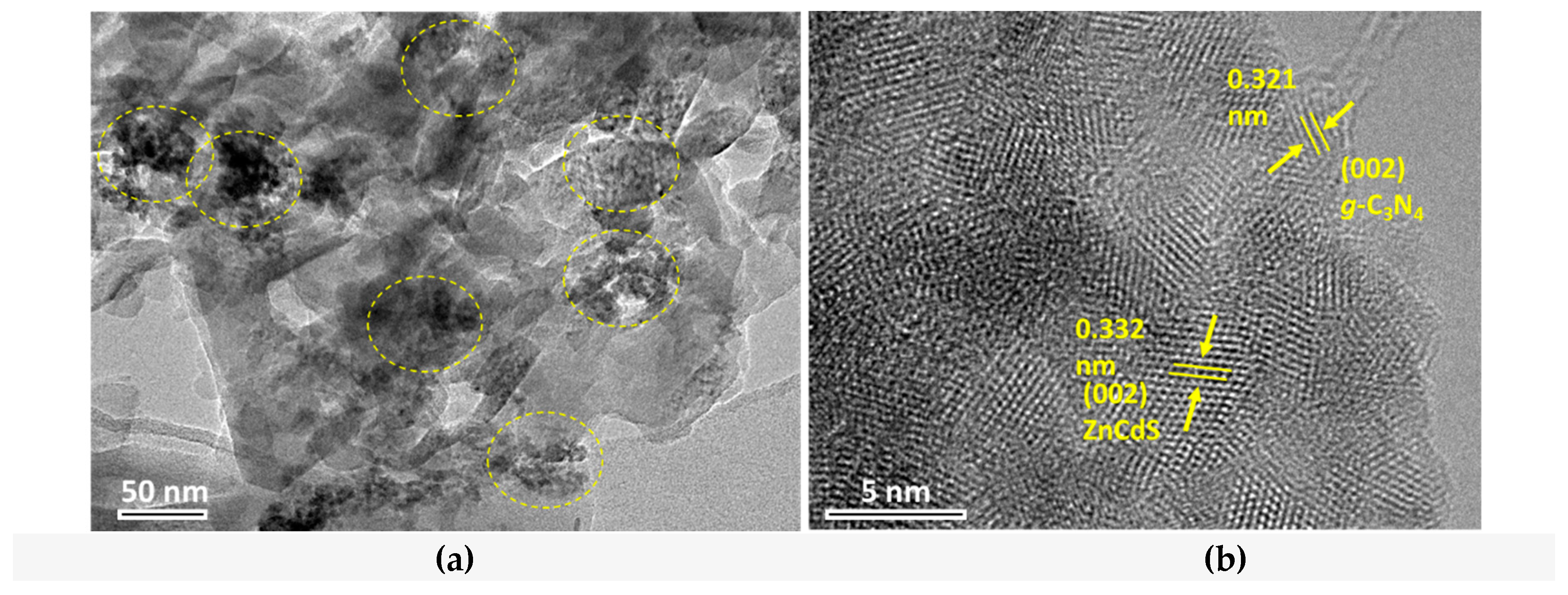
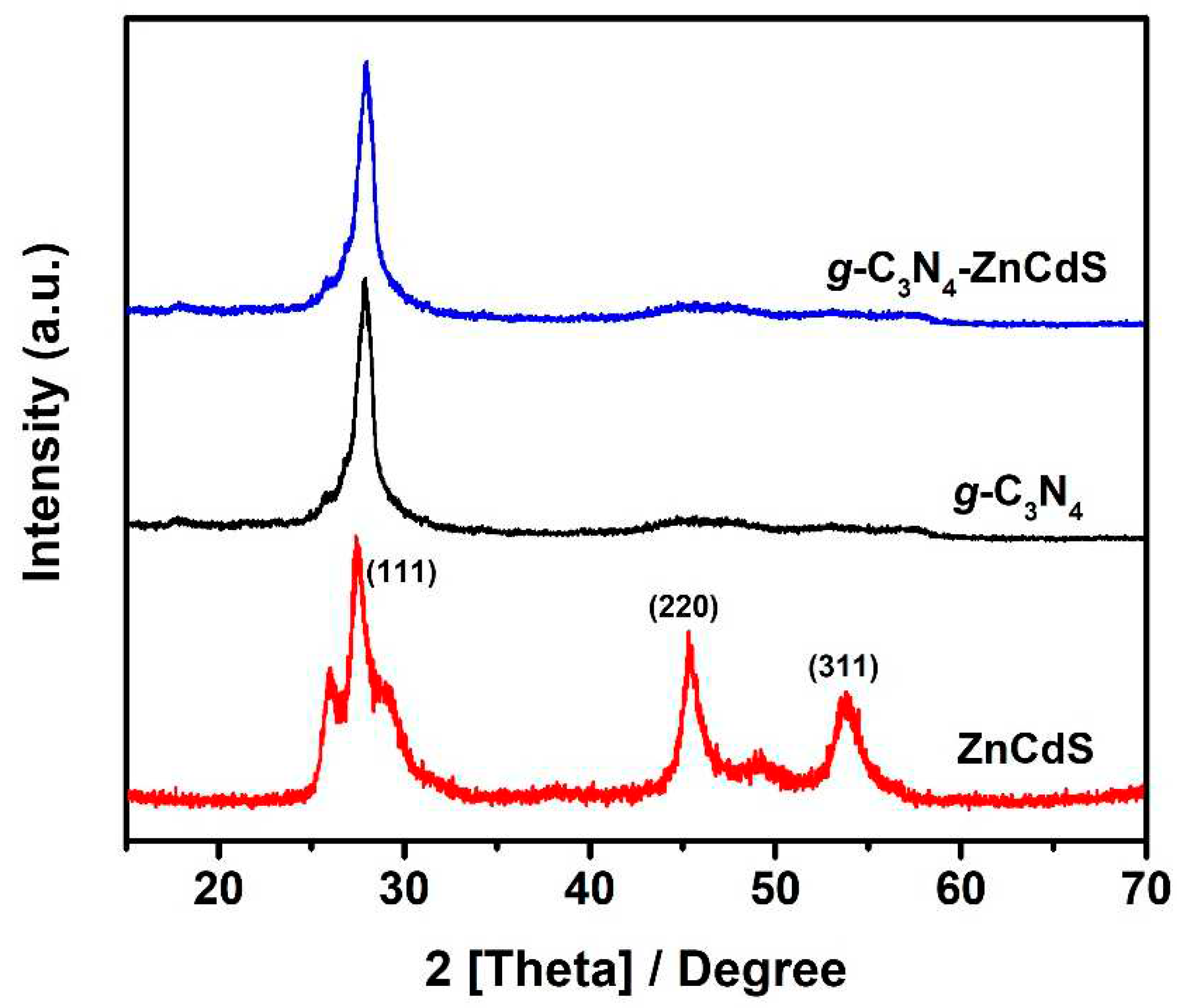
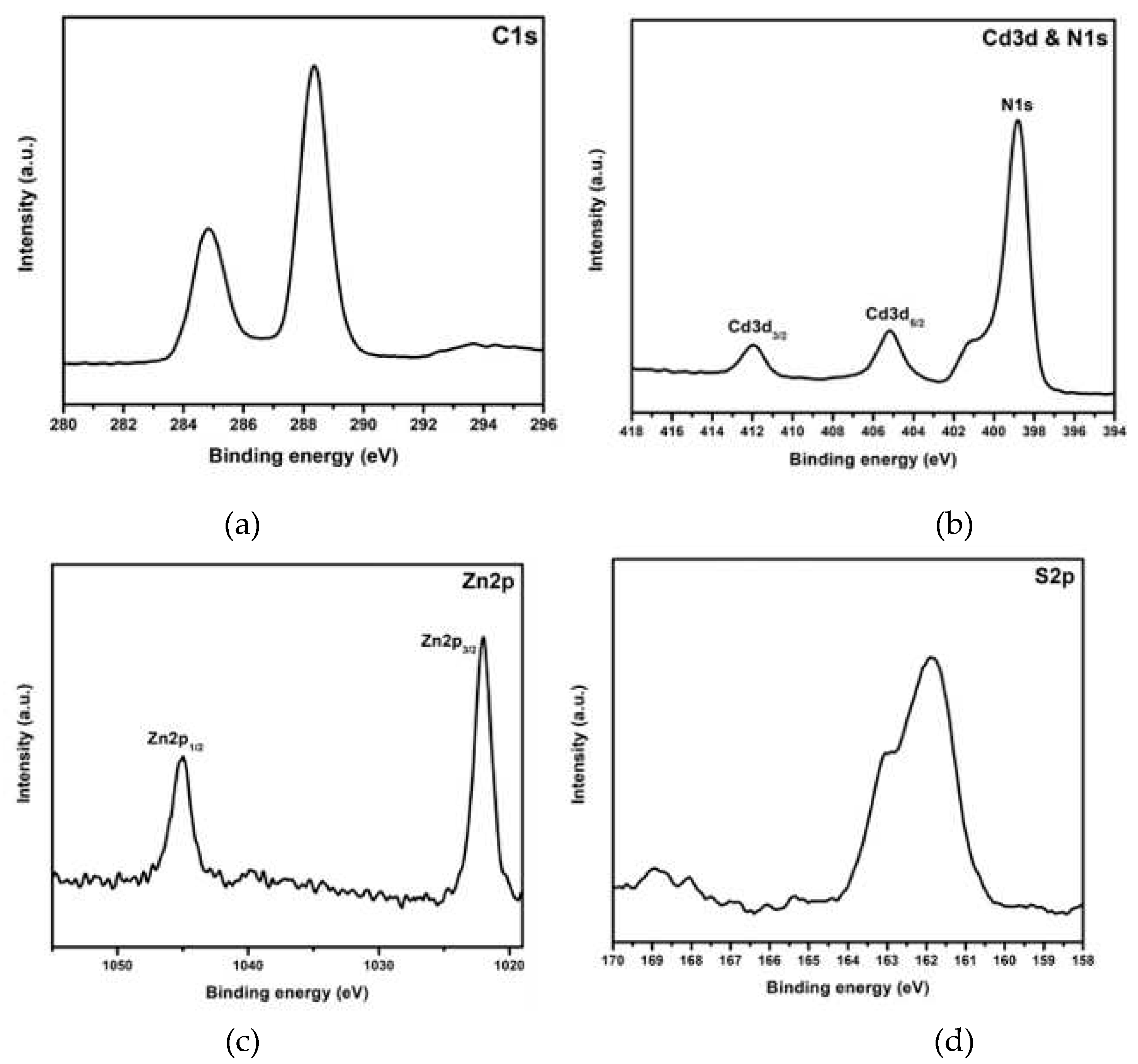
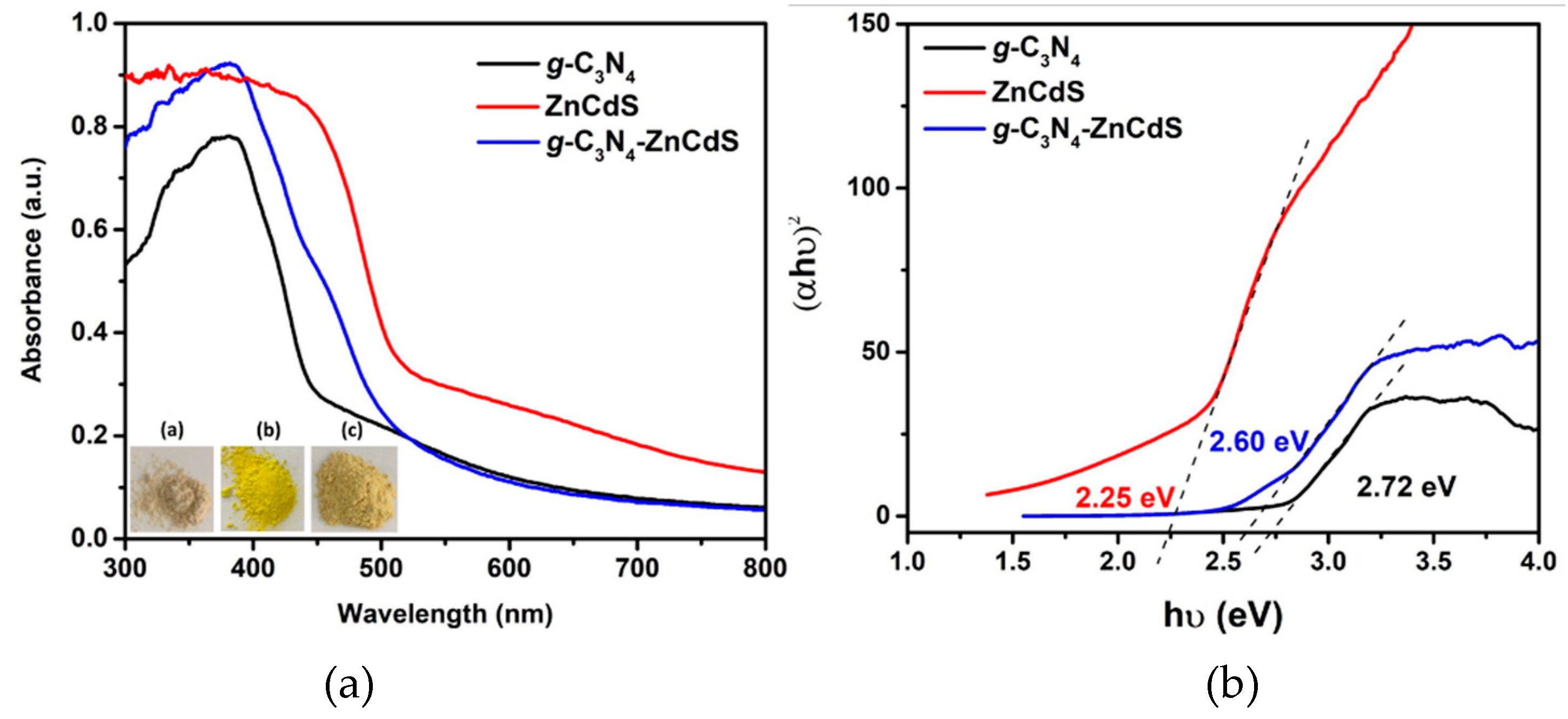
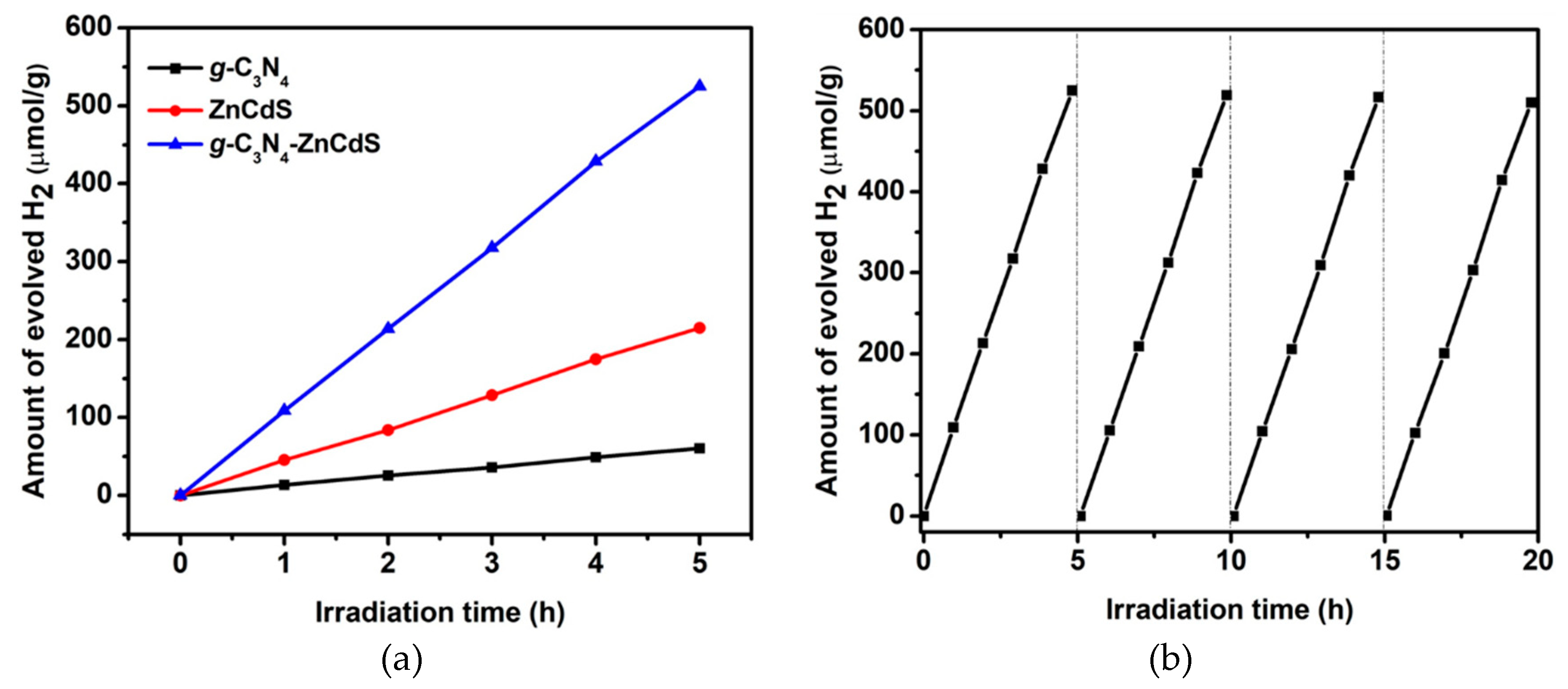
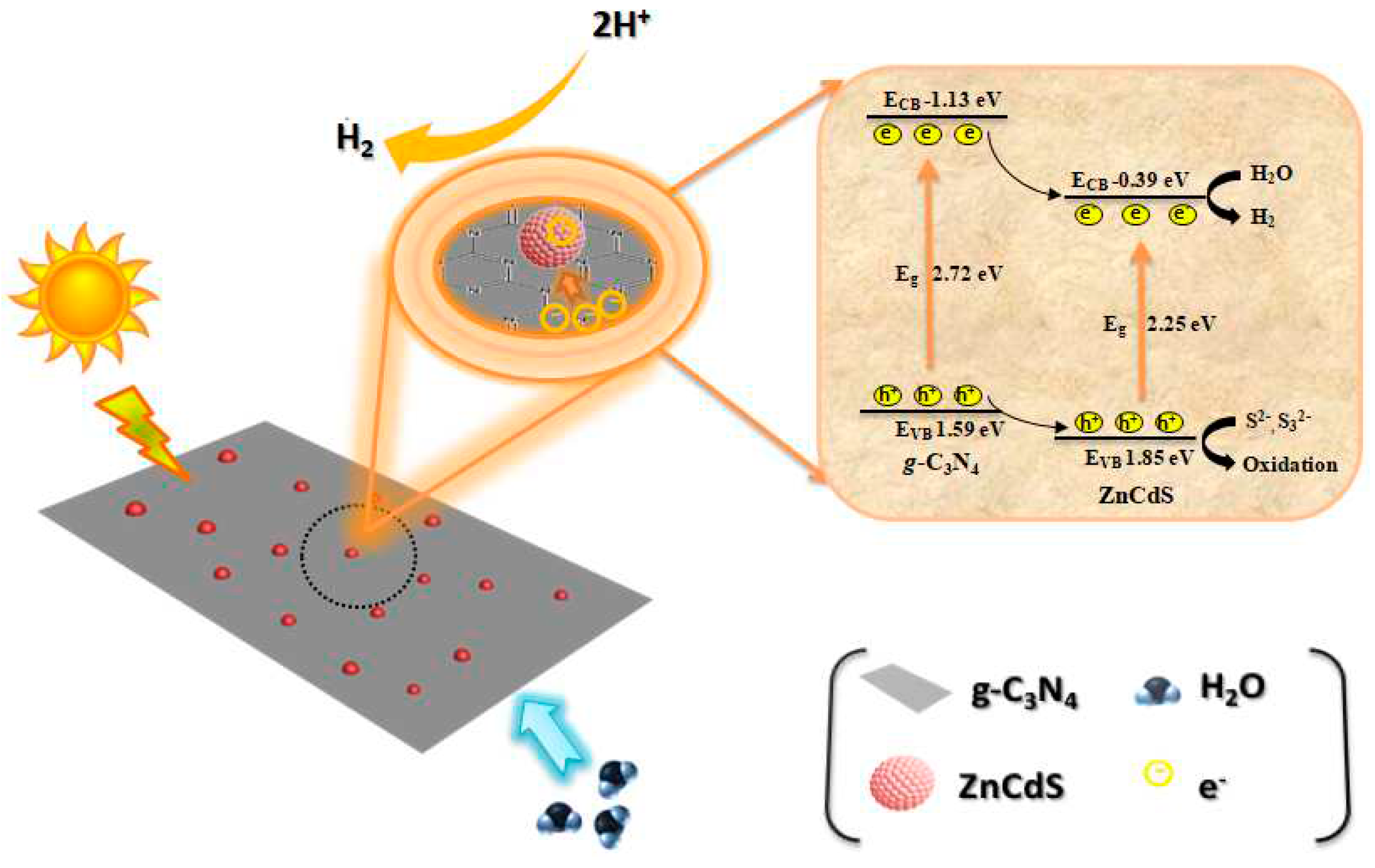
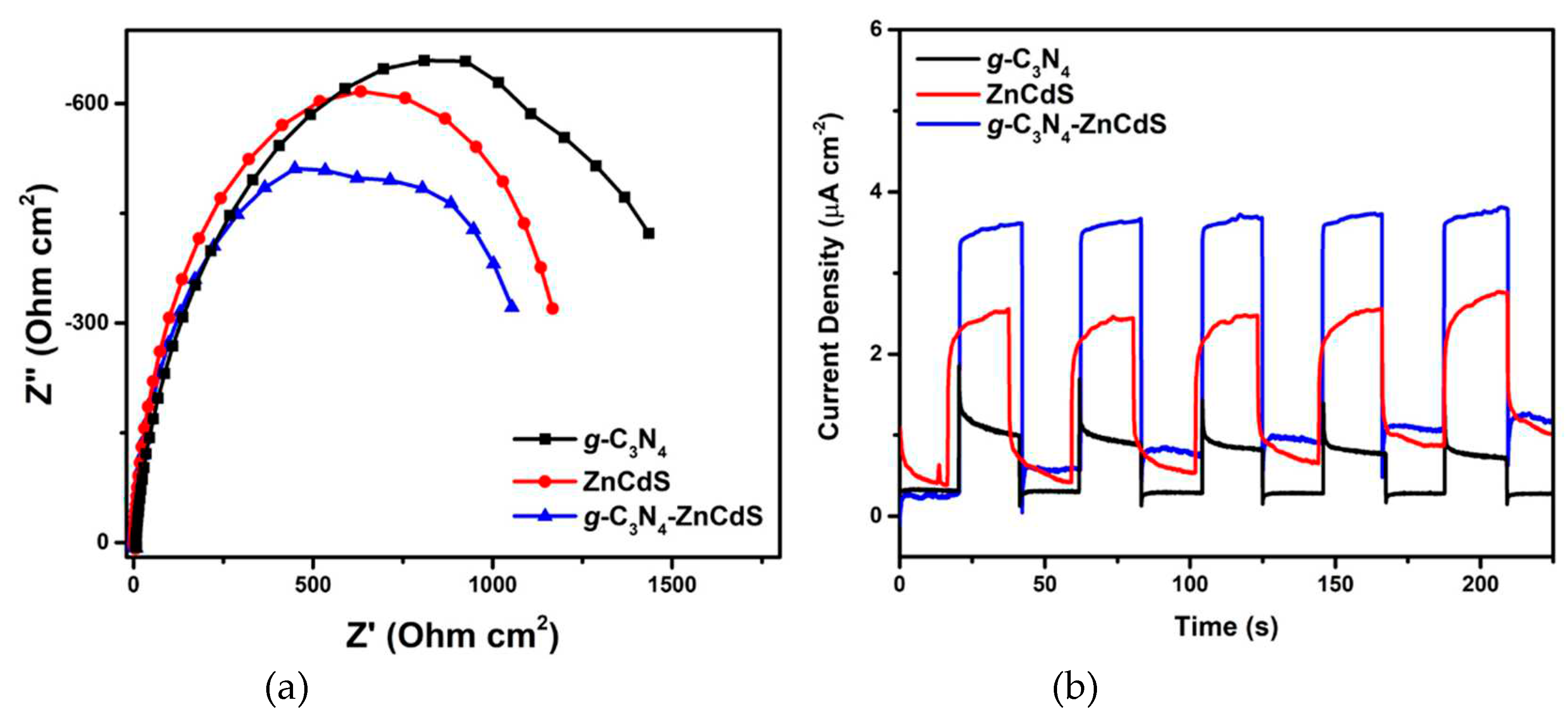
3. Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J Chem Technol Biotechnol 2019, 94, 3049–3063. [Google Scholar] [CrossRef]
- Teets, T.S.; Nocera, D.G. Photocatalytic hydrogen production. Chem. Commun. 2011, 47, 9268–9274. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh banu, J. Techno-economic assessment of various hydrogen production methods – A review. Bioresour. Technol. 2021, 319, 12417. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan. L.L.; Ng. Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J. M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light-responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Synthesis of the g-C3N4/CdS Nanocomposite with a Chemically Bonded Interface for Enhanced Sunlig,ht-Driven CO2Photoreduction. ACS Appl. Energy Mater. 2020, 3, 6422–6433. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Zhang, R.; Cheng, X.; Xia, J.; Xu, Y.; Li, H. Synthesis and characterization of g-C3N4/MoO3 photocatalyst with improved visible-light photoactivity. Appl. Surf. Sci. 2013, 283, 25–32. [Google Scholar] [CrossRef]
- Li, Y.Y.; Qin, T.; Chen, W.; Huang, M.; Xu, J.; Lv, J. Construction of a Switchable g-C3N4/BiVO4Heterojunction from the Z-Scheme to the Type II by Incorporation of Pyromellitic Diimide. Cryst. Growth Des. 2022, 22, 1645–1653. [Google Scholar] [CrossRef]
- Hu, B.; Cai, F.; Chen, T.; Fan, M.; Song, C.; Yan, X.; Shi, W. Hydrothermal Synthesis g-C3N4/Nano-InVO4 Nanocomposites and Enhanced Photocatalytic Activity for Hydrogen Production under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 18247–18256. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Du, Y.; Zheng, X.; Wen, J. High-temperature sulfurized synthesis of MnxCd1-xS composites for enhancing solar-light driven H2 evolution. Int. J. Hydrogen Energy 2022, 47, 9925–9933. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Li, Y. Hollow ZnCdS dodecahedral cages for highly efficient visible-light-driven hydrogen generation. J. Mater. Chem. A 2017, 5, 24116–24125. [Google Scholar] [CrossRef]
- He, J.; Li, B.; Yu, J.; Qiao, L.; Li, S.; Zu, X.; Xiang, X. Ultra-thin CdIn2S4 nanosheets with nanoholes for efficient photocatalytic hydrogen evolution. Opt. Mater. (Amst) 2020, 108, 2–6. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Hu, J.; Zhang, J.; Zheng, L.; et al. Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Meng, H.; Zhou, P.; Zheng, Y.; Wang, J.; Yu, J.; et al. Zn1-xCdxS solid solutions with controlled bandgap and enhanced visible-light photocatalytic H2-production activity. ACS Catal. 2013, 3, 882–889. [Google Scholar] [CrossRef]
- Chen, R.; Li, K.; Zhu, X.S.; Xie, S.L.; Dong, L.Z.; Li, S.L.; et al. In situ synthesis of porous ZnO-embedded Zn1-xCdxS/CdS heterostructures for enhanced photocatalytic activity. CrystEngComm 2016, 18, 1446–52. [Google Scholar] [CrossRef]
- Li, K.; Chen, R.; Li, S.L.; Xie, S.L.; Dong, L.Z.; Kang, Z.H.; et al. Engineering Zn1-xCdxS/CdS Heterostructures with Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 14535–114541. [Google Scholar] [CrossRef]
- Imran, M.; Yousaf, A Bin. ; Kasak, P.; Zeb, A.; Zaidi, S.J. Highly efficient sustainable photocatalytic Z-scheme hydrogen production from an A-Fe2O3 engineered ZnCdS heterostructure. J. Catal. 2017, 353, 81–88. [Google Scholar] [CrossRef]
- Vijayan, M.; Manikandan, V.; Rajkumar, C.; Hatamleh, A.A.; Alnafisi, B.K.; Easwaran, G.; et al. Constructing Z-scheme g-C3N4/TiO2 heterostructure for promoting degradation of the hazardous dye pollutants. Chemosphere 2023, 311, c136928. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, X.; Wang, Q.; Li, D.S.; Li, X.; Li, X.; et al. Plasma synthesis of Pt/g-C3N4 photocatalysts with enhanced photocatalytic hydrogen generation. J. Alloys Compd. 2021, 873, 159871. [Google Scholar] [CrossRef]
- Tan, L.; Xu, J.; Zhang, X.; Hang, Z.; Jia, Y.; Wang, S. Synthesis of g-C3N4/CeO 2 nanocomposites with improved catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Hao, X.; Xiang, D.; Jin, Z. Zn-Vacancy Engineered S-Scheme ZnCdS/ZnS Photocatalyst for Highly Efficient Photocatalytic H2 Evolution. ChemCatChem 2021, 13, 4738–4750. [Google Scholar] [CrossRef]
- Yang, Q, Yu, L. ; Zhao, X.; Wang, Y.; Zhu, H.; Zhang, Y. Highly stable γ-NiOOH/ZnCdS photocatalyst for efficient hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 27516–27526. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Ge, F.; Li, X.; Wu, M.; Ding, H.; Li, X. A type II heterojunction α-Fe2O3/g-C3N4 for the heterogeneous photo-Fenton degradation of phenol. RSC Adv. 2022, 12, 8300–8309. [Google Scholar] [CrossRef]
- Wang, Y.; Fiaz, M.; Kim, J.; Carl, N.; Kim, Y.K. Kinetic Evidence for Type-II Heterojunction and Z-Scheme Interactions in g-C3N4/TiO2Nanotube-Based Photocatalysts in Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2023, 6, 5197–206. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Sreńscek-Nazzal, J.; Llorca, J. Photocatalytic hydrogen production from alcohol aqueous solutions over TiO2-activated carbon composites decorated with Au and Pt. J.Photochem. Photobiol. A Chem. 2022, 425, 113726. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, Y.; Thirugnanam, N.; Li, G. Double Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H 2 production. Appl. Surf. Sci. 2018, 430, 293–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




