Submitted:
13 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Search strategy
2.2. Study selection
2.3. Inclusion criteria
2.4. Exclusion criteria
2.5. Data extraction
2.6. Data summary
2.7. Ethics and distribution
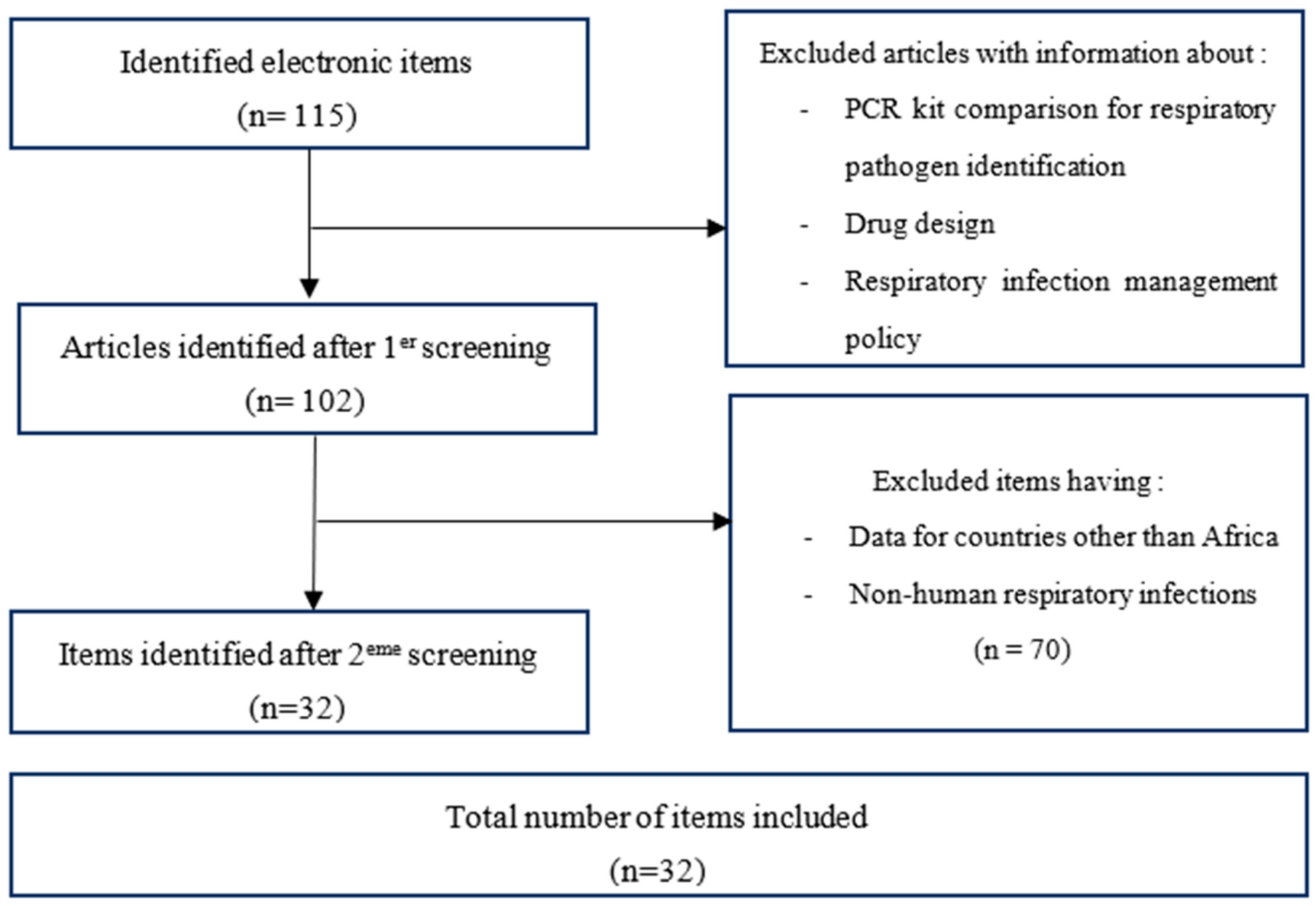
3. Results
3.1. Literature review
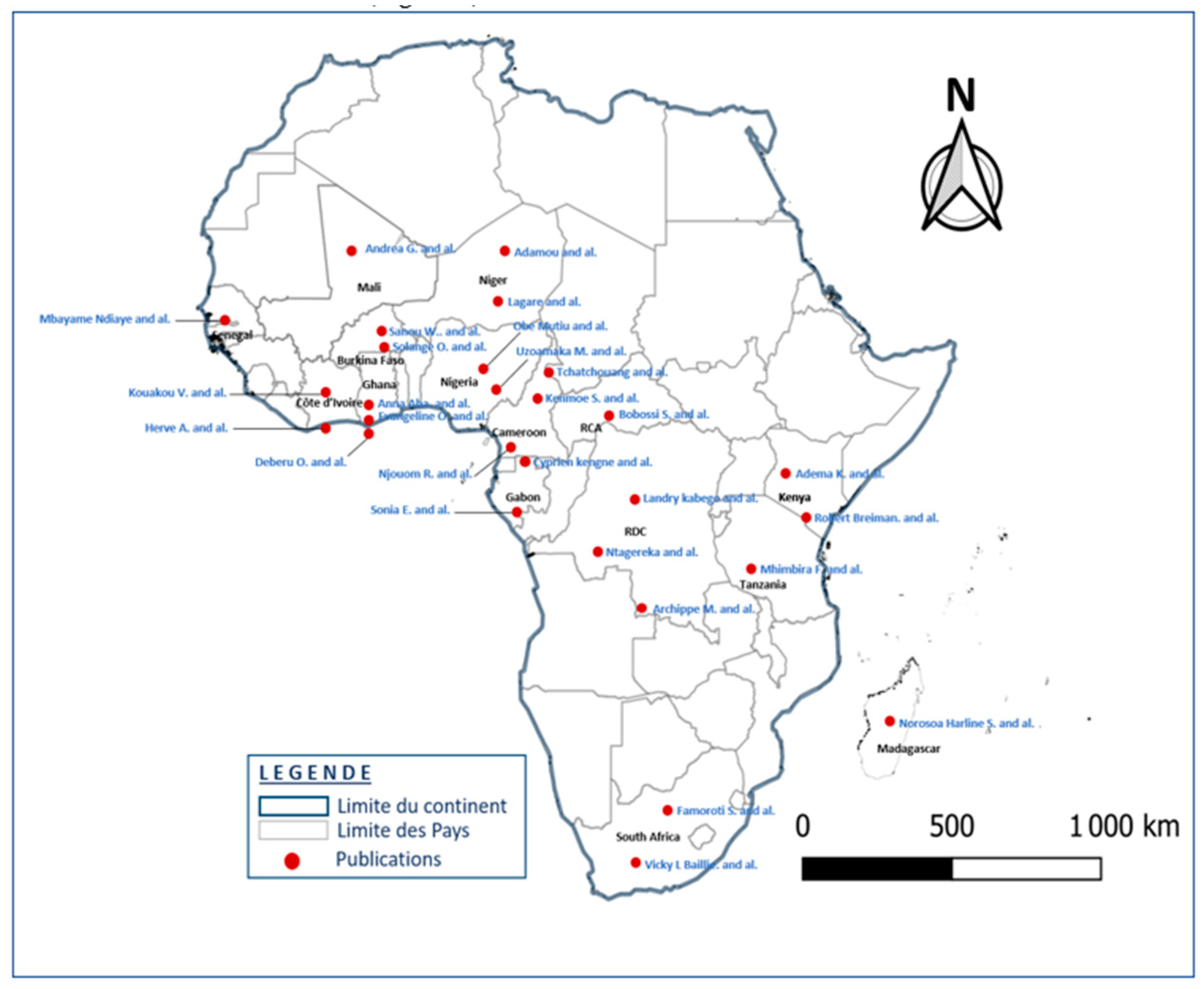
3.2. Features of included items
3.3. Etiology of pathogens detected
4. Discussion
5. Conclusion
6. Study limits
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Zhang, W.; Tang, Y.W. Molecular diagnosis of viral respiratory infections. Current Infectious Disease Reports 2011, 13, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, S.; Traoré, B.; Nene Bi, Z.A.B.; Yonli, F.T.; Kima, D.; Bonané, P.; Gueudin, M. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso). PloS one 2014, 9, e110435. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.M.; Cissé, A.; Millogo, T.; Sagna, T.; Tialla, D.; Williams, T. Systematic review of articles on etiologies of acute respiratory infections in children aged less than five years in sub-Saharan Africa, 2000-2015. EC Microbiology 2016, 6, 556–71. [Google Scholar]
- Sonego, M.; Pellegrin, M.C.; Becker, G.; Lazzerini, M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies. PloS one 2015, 10, e0116380. [Google Scholar] [CrossRef]
- Gadsby, N.J.; McHugh, M.P.; Russell, C.D.; Mark, H.; Morris, A.C.; Laurenson, I.F.; Templeton, K.E. Development of two real-time multiplex PCR assays for the detection and quantification of eight key bacterial pathogens in lower respiratory tract infections. Clinical microbiology and infection 2015, 21, 788–e1. [Google Scholar] [CrossRef]
- Ayar, G.; Sahin, S.; Yazici, M.U.; Parlakay A, Ö.; Tezer, H. RSV pneumonia in the pediatric intensive care unit. J Pediatr Inf 2014, 8, 12–7. [Google Scholar] [CrossRef]
- Dube, F.S.; Kaba, M.; Robberts, F.J.; Ah Tow, L.; Lubbe, S.; Zar, H.J.; Nicol, M.P. Respiratory microbes present in the nasopharynx of children hospitalized with suspected pulmonary tuberculosis in Cape Town, South Africa. BMC infectious diseases 2016, 16, 597. [Google Scholar] [CrossRef]
- Deberu, O.; Nkrumah, B.; Sylverken, A.A.; Sambian, D.; Acheampong, G.; Amuasi, J.; Owusu, M. Common bacteria in sputum or gastric lavage of patients presenting with signs and symptoms of lower respiratory tract infections. The Pan African Medical Journal 2021, 38. [Google Scholar] [CrossRef]
- Lagare, A.; Maïnassara, H.B.; Issaka, B.; Sidiki, A.; Tempia, S. Viral and bacterial etiology of severe acute respiratory illness among children< 5 years of age without influenza in Niger. BMC infectious diseases 2015, 15, 1–7. [Google Scholar]
- Gessner, B.D.; Shindo, N.; Briand, S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. The Lancet infectious diseases 2011, 11, 223–235. [Google Scholar] [CrossRef]
- Tchatchouang, S.; Nzouankeu, A.; Kenmoe, S.; Ngando, L.; Penlap, V.; Fonkoua, M.C.; Njouom, R. Bacterial aetiologies of lower respiratory tract infections among adults in Yaoundé, Cameroon. BioMed research international 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Lagare, A.; Ousmane, S.; Dano, I.D.; Issaka, B.; Issa, I.; Mainassara, H.B.; Mamadou, S. Molecular detection of respiratory pathogens among children aged younger than 5 years hospitalized with febrile acute respiratory infections: A prospective hospital-based observational study in Niamey, Niger. Health Science Reports 2019, 2, e137. [Google Scholar] [CrossRef] [PubMed]
- Birindwa, A.M.; Kasereka, J.K.; Gonzales-Siles, L.; Geravandi, S.; Mwilo, M.; Tudiakwile, L.K.; Skovbjerg, S. Bacteria and viruses in the upper respiratory tract of Congolese children with radiologically confirmed pneumonia. BMC Infectious Diseases 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Mhimbira, F.; Hiza, H.; Mbuba, E.; Hella, J.; Kamwela, L.; Sasamalo, M.; Fenner, L. Prevalence and clinical significance of respiratory viruses and bacteria detected in tuberculosis patients compared to household contact controls in Tanzania: a cohort study. Clinical microbiology and infection 2018, 25, 107–e1. [Google Scholar] [CrossRef]
- Razanajatovo, N.H.; Guillebaud, J.; Harimanana, A.; Rajatonirina, S.; Ratsima, E.H.; Andrianirina, Z.Z.; Heraud, J.M. Epidemiology of severe acute respiratory infections from hospital-based surveillance in Madagascar, November 2010 to July 2013. PLoS One 2018, 13, e0205124. [Google Scholar] [CrossRef]
- Lekana-Douki, S.E.; Mouinga-Ondémé, A.; Nkoghe, D.; Drosten, C.; Drexler, J.F.; Kazanji, M.; Leroy, E.M. Early introduction and delayed dissemination of pandemic influenza, Gabon. Emerging Infectious Diseases 2013, 19, 644. [Google Scholar] [CrossRef] [PubMed]
- Lekana-Douki, S.E.; Nkoghe, D.; Drosten, C.; Ngoungou, E.B.; Drexler, J.F.; Leroy, E.M. Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC infectious diseases 2014, 14, 1–11. [Google Scholar] [CrossRef]
- Breiman, R.F.; Cosmas, L.; Njenga, M.K.; Williamson, J.; Mott, J.A.; Katz, M.A.; Feikin, D.R. Severe acute respiratory infection in children in a densely populated urban slum in Kenya, 2007-2011. BMC infectious diseases 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Serengbe, G.B.; Gody, J.C.; Fioboy, R.; Nakoune, E. Viral etiology of acute respiratory infections in children in Bangui. Archives de Pediatrie 2014, 22, 324. [Google Scholar] [CrossRef]
- Kenmoe, S.; Tchendjou, P.; Vernet, M.A.; Moyo-Tetang, S.; Mossus, T.; Njankouo-Ripa, M.; Njouom, R. Viral etiology of severe acute respiratory infections in hospitalized children in Cameroon, 2011-2013. Influenza and other respiratory viruses 2016, 10, 386–393. [Google Scholar] [CrossRef]
- Uzoamaka, M.; Ngozi, O.; Johnbull, O.S.; Martin, O. Bacterial etiology of lower respiratory tract infections and their antimicrobial susceptibility. The American Journal of the Medical Sciences 2017, 354, 471–475. [Google Scholar] [CrossRef]
- Niang, M.N.; Diop, N.S.; Fall, A.; Kiori, D.E.; Sarr, F.D.; Sy, S.; Dia, N. Respiratory viruses in patients with influenza-like illness in Senegal: focus on human respiratory adenoviruses. PLoS One 2017, 12, e0174287. [Google Scholar] [CrossRef] [PubMed]
- Famoroti, T.; Sibanda, W.; Ndung'u, T. Prevalence and seasonality of common viral respiratory pathogens, including Cytomegalovirus in children, between 0-5 years of age in KwaZulu-Natal, an HIV endemic province in South Africa. BMC pediatrics 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Kadjo, H.A.; Adjogoua, E.; Dia, N.; Adagba, M.; Abdoulaye, O.; Daniel, S.; Dosso, M. Detection of non-influenza viruses in acute respiratory infections in children under five-year-old in Cote d'Ivoire (January-December 2013). African Journal of Infectious Diseases 2018, 12, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.M.; Wandaogo SC, M.; Poda, A.; Tamini, L.; Kyere, A.E.; Sagna, T.; Snoeck, C.J. Epidemiology and molecular characterization of influenza viruses in Burkina Faso, sub-Saharan Africa. Influenza and other respiratory viruses 2018, 12, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Obodai, E.; Odoom, J.K.; Adiku, T.; Goka, B.; Wolff, T.; Biere, B.; Reiche, J. The significance of human respiratory syncytial virus (HRSV) in children from Ghana with acute lower respiratory tract infection: a molecular epidemiological analysis, 2006 and 2013-2014. PLoS One 2018, 13, e0203788. [Google Scholar] [CrossRef] [PubMed]
- Lekana-Douki, S.E.; Behillil, S.; Enouf, V.; Leroy, E.M.; Berthet, N. Detection of human bocavirus-1 in both nasal and stool specimens from children under 5 years old with influenza-like illnesses or diarrhea in Gabon. BMC research notes 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Kabego, L.; Balol'Ebwami, S.; Kasengi, J.B.; Miyanga, S.; Bahati, Y.L.; Kambale, R.; de Beer, C. Human respiratory syncytial virus: prevalence, viral co-infections and risk factors for lower respiratory tract infections in children under 5 years of age at a general hospital in the Democratic Republic of Congo. Journal of Medical Microbiology 2018, 67, 514–522. [Google Scholar] [CrossRef]
- Kenmoe, S.; Vernet, M.A.; Le Goff, J.; Penlap, V.B.; Vabret, A.; Njouom, R. Molecular characterization of human adenovirus associated with acute respiratory infections in Cameroon from 2011 to 2014. Virology journal 2018, 15, 1–7. [Google Scholar] [CrossRef]
- Adema, I.W.; Kamau, E.; Uchi Nyiro, J.; Otieno, G.P.; Lewa, C.; Munywoki, P.K.; Nokes, D.J. Surveillance of respiratory viruses among children attending a primary school in rural coastal Kenya. Wellcome Open Research 2020, 5, 63. [Google Scholar] [CrossRef]
- Kengne-Nde, C.; Kenmoe, S.; Modiyinji, A.F.; Njouom, R. Prevalence of respiratory viruses using polymerase chain reaction in children with wheezing, a systematic review and meta-analysis. Plos one 2020, 15, e0243735. [Google Scholar] [CrossRef]
- Buchwald, A.G.; Tamboura, B.; Tennant, S.M.; Haidara, F.C.; Coulibaly, F.; Doumbia, M.; Tapia, M.D. Epidemiology, risk factors, and outcomes of respiratory syncytial virus infections in newborns in Bamako, Mali. Clinical Infectious Diseases 2020, 70, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Obe, O.A.; Mutiu, B.W.; Amoo, A. Respiratory Syncytial Virus Infection among Children in Lagos, Nigeria. J Clin Immunol Microbiol 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Kouakou, V.; Kadjo, H.; N'nan Alla Oulo, F.D.; N'guessan AN, D. Surveillance of Respiratory Syncytial Virus in Children Aged 0-5 years in Côte d'Ivoire. American Journal of BioScience 2021, 9, 185. [Google Scholar] [CrossRef]
- Kenmoe, S.; Sadeuh-Mba, S.A.; Vernet, M.A.; Penlap Beng, V.; Vabret, A.; Njouom, R. Molecular epidemiology of Enteroviruses and Rhinoviruses in patients with acute respiratory infections in Yaounde, Cameroon. Influenza and other respiratory viruses 2021, 15, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Baillie, V.L.; Moore, D.P.; Mathunjwa, A.; Baggett, H.C.; Brooks, A.; Feikin, D.R.; Madhi, S.A. Epidemiology of the rhinovirus (RV) in African and Southeast Asian children: a case-control pneumonia etiology study. Viruses 2021, 13, 1249. [Google Scholar] [CrossRef]
- Ntagereka, P.B.; Basengere, R.A.; Baharanyi, T.C.; Kashosi, T.M.; Buhendwa JP, C.; Bisimwa, P.B.; Mukwege, D. Molecular evidence of coinfection with acute respiratory viruses and high prevalence of SARS-CoV-2 among patients presenting flu-like illness in Bukavu city, Democratic Republic of Congo. Canadian Journal of Infectious Diseases and Medical Microbiology 2022, 2022. [Google Scholar] [CrossRef]
- Kafintu-Kwashie, A.A.; Nii-Trebi, N.I.; Obodai, E.; Neizer, M.; Adiku, T.K.; Odoom, J.K. Molecular epidemiological surveillance of viral agents of acute lower respiratory tract infections in children in Accra, Ghana. BMC pediatrics 2022, 22, 364. [Google Scholar] [CrossRef]
- Nair, H.; Simões, E.A.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang JS, F.; Campbell, H. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. The Lancet 2013, 381, 1380–1390. [Google Scholar] [CrossRef]
- Kenmoe, S.; Kengne-Nde, C.; Ebogo-Belobo, J.T.; Mbaga, D.S.; Fatawou Modiyinji, A.; Njouom, R. Systematic review and meta-analysis of the prevalence of common respiratory viruses in children< 2 years with bronchiolitis in the pre-COVID-19 pandemic era. PLoS One 2020, 15, e0242302. [Google Scholar]
- Van der Zalm, M.M.; Uiterwaal, C.S.; Wilbrink, B.; Koopman, M.; Verheij, T.J.; van der Ent, C.K. The influence of neonatal lung function on rhinovirus-associated wheeze. American journal of respiratory and critical care medicine 2011, 183, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus research 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PloS one 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. Journal of Infection 2020, 81, 266–275. [Google Scholar] [CrossRef]
| References | Collection period | Year of publication | Study country | Age range | Zone / Sample size | Type of sampling | Prevalence of pathogens | Type of study |
|---|---|---|---|---|---|---|---|---|
| [16] Lekana-Douki et al. | 2009 - 2011 | 2013 | Gabon | No limit | Urban/966 | Nasal | Flu A (61%); Flu B (39%) | Cross-sectional/ Prospective |
| [17] Lekana-Douki et al. | 2010 - 2011 | 2014 | Gabon | No limit | (Urban) /1041 | Nasopharyngeal | HAdV (17.5%), HPIV 1-4 (16.8%), EV (14.7%), HRSV (13.5%), and Flu A (11.9%). | Cross-sectional |
| [2] Ouédraogo et al. | 2010 - 2011 | 2014 | Burkina Faso | < 3 years | (Urban) /209 | Nasopharyngeal | HRV (59.1%); EV (25.5%); HRSV (16.1%); HMPV (9.4%) | Prospective |
| [9] Lagare et al. | 2010 -2012 | 2015 | Niger | < 5 years | (Urban) /160 | Nasopharyngeal | HRSV (35%); HRV (29%); HPIV (24%); S. pneumoniae (56%); H. inflenzae (12%) | Retrospective |
| [18] Breiman et al. | 2007 - 2011 | 2015 | Kenya | < 5 years | 2592 | Naso/ Oro -pharyngeal | HRV/EV ( 42% ); HRSV ( 25% ); HAdV ( 20% ); HMPV ( 13,7% ), Flu A ( 10,8% ) | Cross-sectional |
| [19] Serengbe et al. | 2013 | 2015 | CAR | < 5 years | 361 | Nasopharyngeal | HRV(47,5%);FluA/B (26,6%);HPIV-3(9,3%);HRSV(5,8% ); EV(4,3%); HAdV (2,9%); HBoV (1,4%); HCoV (1,4%) | Cross-sectional |
| [20] Kenmoe et al. | 2011 - 2013 | 2016 | Cameroon | ≤15 | (Urban) /347 | Nasopharyngeal | HRSV ( 13.2% ), HAdV ( 27.3% ), HboV ( 10.6% ), Flu A/B ( 9.8% ); HPIV ( 6.6% ); HCoV ( 5.7% ); HMPV ( 2.3% ); HRV/EV (11.5%) | Prospective |
| [21] Uzoamaka et al. | 2014 - 2016 | 2017 | Nigeria | No limit | (Peri-urban) / 954 |
Expectoration | Klebsiella pneumonia ( 49,9% ); Klebsiella spp/ Pseudomonas aeruginosa, ( 1.4% ) | Cross-sectional |
| [22] Niang et al. | 2012 - 2015 | 2017 | Senegal | No limit | (Urban) /6381 | Naso/ Oro -pharyngeal | HAdV ( 30,8% ); FluA/B ( 53,1% ); HRV ( 30% ); Ev ( 18,5% ); HRSV ( 13,5% ) | Cross-sectional, Prospective |
| [23] Famoroti et al. | 2011 - 2015 | 2018 | South Africa | 0 - 5 years | (Urban) /2172 | Expectoration /Nasopharyngeal |
HRSV (32.1%), HAdV (21.8%), HRV (15.4%), FluA swl (5.1%) | Retrospective |
| [24] Kadjo et al. | 2013 | 2018 | Ivory Coast | < 5 years | (Urban) /1340 | Nasopharyngeal | HRV (31.92%), HRSV (24.4%), HPIV (20.5%), HCoV 229E ( 12.05% ) | Cross-sectional |
| [25] Sanou et al. | 2014 - 2015 | 2018 | Burkina Faso | < 5 years | (Urban) / 924 | Nasopharyngeal | Flu A/B (15.1%), A(H3N2) (69.1%) A(H1N1) pdm09 (30.9%) | Cross-sectional |
| [26] Obodai et al. | 2006, 2013-2014 | 2018 | Ghana | < 5 years | (Urban) /552 | Nasopharyngeal | HRSV ( 23% ) | Cross-sectional |
| [27] Lekana-Douki et al. | 2018 | 2018 | Gabon | < 5 years | (Urban) / 810 | Nasopharyngeal | HBoV ( 4,4% ) | Retrospective |
| [28] Kabego et al. | 2016 | 2018 | DRC | < 5 years | (Urban) / 146 | Nasopharyngeal | HRSV ( 21.2 % ); HRV ( 16.4 % ); HPIV-3 ( 13.7% ) and HAdV ( 4.79 % ). | Cross-secctional, analytical/ Prospective |
| [14] Mhimbira et al. | 2013 - 2015 | 2018 | Tanzania | No limit | (Urban) /972 | Nasopharyngeal | HRV ( 9.3% ); Influenza A ( 3.1% ); HRSV A/B ( 1.9% ); H. influenzae ( 26.1% ); S. pneumoniae ( 21.5% ) | Prospective cohort |
| [29] Kenmoe et al. | 2011 - 2014 | 2018 | Cameroon | < 15 years | (Urban) / 811 | Nasopharyngeal | HAdV( 27.12% ) | Cross-sectional |
| [15] Razanajatovo et al. | 2010 - 2013 | 2018 | Madagascar | No limit | (Urban) / 876 | Nasopharyngeal, Expectorations Blood |
HRSV ( 37,7% ) ; FluA ( 18,4% ) ; HRV ( 13,5% ) ; HAdV ( 8,3% ) ; S. Pneumoniae ( 50,3% ) ; H. Influenzae b ( 21,4% ) ; Klebsiella ( 4,6% ) | Prospective |
| [12] Lagare, et al. | 2015 | 2019 | Niger | < 5 years | (Urban) / 638 | Expectoration /Nasopharyngeal |
HRSV ( 23,3 % ), HPIV ( 12,2% ), HRV ( 9,4 % ), HAdV ( 9,4 % ), Flu A ( 8,1 %) / S. pneumoniae ( 39%) , Staph. aureus ( 12,2% ), H. influenzae B ( 2,5% ) | Prospective |
| [11] Tchatchouang et al. | 2019 | 2019 | Cameroon | No limit | (Urban) /141 | Branco-alveolar lavage (BAV) | S. pneumoniae/ H. infuenzae (14.2%); K. pneumoniae (9.2%); Staph. aureus, (7.1%) | Foresight |
| [30] Adema et al. | 2017 - 2018 | 2020 | Kenya | < 20 years | (Urban) /781 | Nasopharyngeal | HRV (16.7%); HPIV (2.7%) ; HCoV (229E,NL63, OC43 ) (2.0%) ; HAdV (0.9%) ; HRSV (0.6%) | Longitudinal/ Cohort |
| [31] Kengne-Nde et al. | 2019 | 2020 | Cameroon | No limit | 1426 | Naso/ Oro -pharyngeal | HRV ( 35,6% ); HRSV ( 31,0% ); HBoV ( 8,1% ); HAdV ( 7,7% ); Flu A/B ( 6,5% ); HMPV ( 5,8% ); EV ( 4,3% ); HPIV 1-4 ( 3,8% ); HCoV ( 2,2% ) | Cohort, Case-control, Cross-sectional |
| [49] Kenmoe et al. | 2019 - 2020 | 2020 | Cameroon | < 2 years | 51 | Naso/ Oro -pharyngeal | HRSV ( 59% ); HRV ( 19,3% ); HBoV ( 8,2% ); HAdV ( 6,1% ); HMPV ( 5,4% ); HPIV ( 5,4% ); Flu A/B ( 3,2% ); HCoV ( 2,9% ); Ev ( 2,9% ) | Cross-sectional |
| [32] Buchwald et al. | 2011 - 2013 | 2020 | Mali | < 2 years | (Urban) /1333 | Naso/ Oro -pharyngeal | HRSV ( 37% ) | Cohort |
| [33] Obe et al. | 2021 | 2021 | Nigeria | < 5 years | (Urban) /200 | Nasopharyngeal | HRSV ( 22.5% ) | Cross-sectional |
| [8] Deberu et al. | 2018 - 2019 | 2021 | Ghana | No limit | (Urban) /264 | Expectoration | Klebsiella spp. (28%); M. tuberculosis (6.5%); Pseudomonas spp.( 15.2% ) | Retrospective |
| [34] Kouakou et al. | 2021 | 2021 | Ivory Coast | ≤ 5 years | (Urban/rural) / 5648 | Nasopharyngeal | HRSV ( 10% ) | Cross-sectional/ descriptive |
| [35] Kenmoe et al. | 2011 - 2014 | 2021 | Cameroon | No limit | (Urban) / 974 | Nasopharyngeal | HRV/EV ( 16.4% ) | Cross-sectional |
| [13] Birindwa et al. | 2015 - 2017 | 2021 | DRC | ≤ 5 years | (Urban) /2322 | Nasopharyngeal | H. influenzae ( 54% ); S. pneumoniae ( 96% ); HRV ( 73% ); EV ( 17% ); HRSV ( 7% ); | Cross-sectional |
| [36] Baillie et al. | 2011 - 2014 | 2021 | South Africa | ≤ 5 years | (Urban) /4232 | Naso/ Oro -pharyngeal | HRV ( 21% ) ; | Cross-sectional |
| [37] Ntagereka, et al. | 2021 | 2022 | DRC | No limit | (Urban) /1352 | Oro-pharyngeal | SARS-CoV-2 ( 13.9% ), Flu A ( 5.6% ), Flu B (0.9%) | Cross-sectional |
| [38] Kafintu-Kwashie et al. | 2015 - 2016 | 2022 | Ghana | < 5 years | (Urban) /188 | Nasopharyngeal | HRSV (11.4); HMPV ( 1.7 %); | Cross-sectional |
| Prevalence (%) | Number of studies | Number of countries | |
|---|---|---|---|
| VIRUS | |||
| Human Respiratory Syncytial Virus | 0,6 - 59 | 23 | 15 |
| Human rhinovirus | 9,3 - 73 | 17 | 11 |
| Influenza virus A/B | 0,9 - 69 | 18 | 11 |
| Human adenovirus | 0,9 - 30,8 | 14 | 9 |
| Para Human Influenza virus | 3,8 - 24 | 10 | 7 |
| Human Enterovirus | 2,9 - 25,5 | 9 | 7 |
| HCoV NL63 | 1,4 - 5,7 | 7 | 3 |
| HCoV OC43 | 1,4 - 5,7 | 7 | 3 |
| HCoV 229E | 2,0 - 12,05 | 7 | 4 |
| HCoV HKU-1 | 1,4 - 5,7 | 6 | 2 |
| Human metapneumovirus | 1,7 - 13,7 | 6 | 4 |
| Human bocavirus | 1,4 - 10,6 | 5 | 3 |
| BACTERIA | |||
| Streptococcuspneumoniae | 14,2 - 96 | 6 | 5 |
| Haemophilus influenzaetype b | 2,5 - 54 | 6 | 5 |
| Klebsiella pneumoniae | 1,4 - 49,9 | 5 | 4 |
| Staphylococcus aureus | 7,1 - 12,2 | 2 | 2 |
| Pnseudomonas aeruginosa | 1,4 - 15,2 | 2 | 2 |
| Mycobacterium tuberculosis | 0 - 6,5 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





