Submitted:
12 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
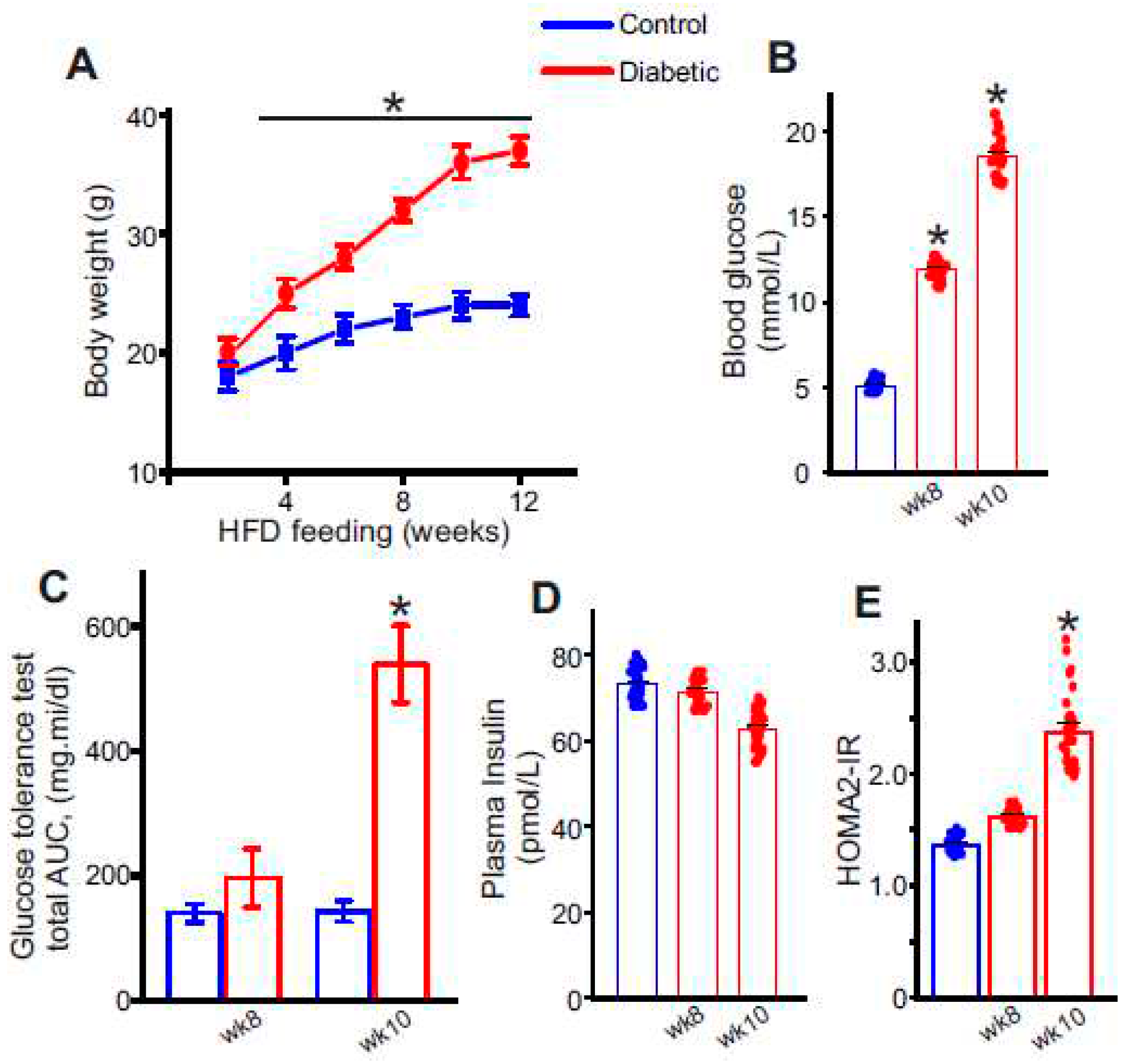
2.1. High-Fat Diet Combined with Low-Dose Streptozotocin Induces an Insulin-Resistant, T2D Phenotype in Mice
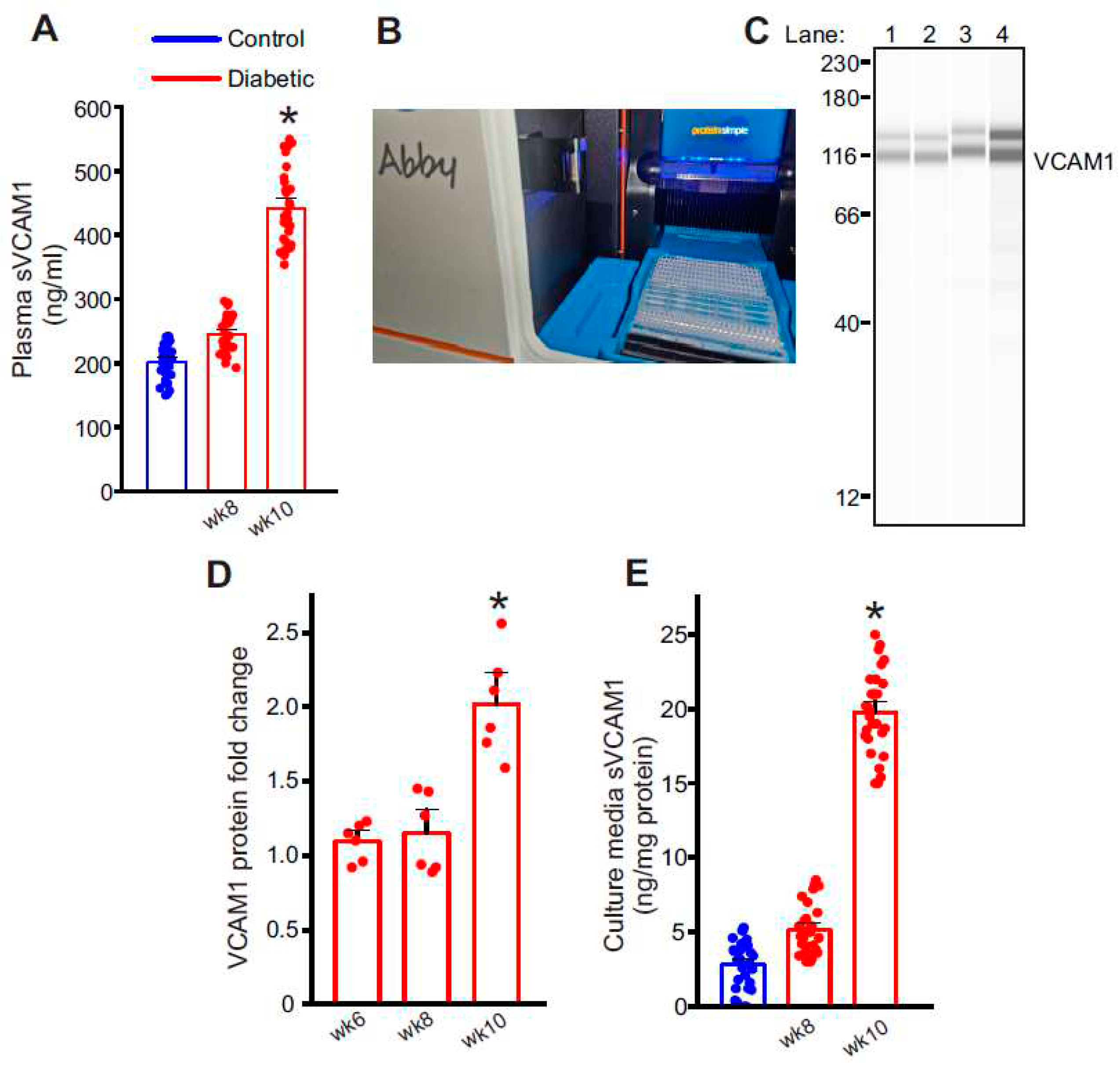
2.2. Plasma Soluble VCAM-1 (sVCAM1) Levels Increase with Insulin Resistance
2.3. Endothelial Cells as a Possible Source of Plasma sVCAM1 in Diabetic Animals
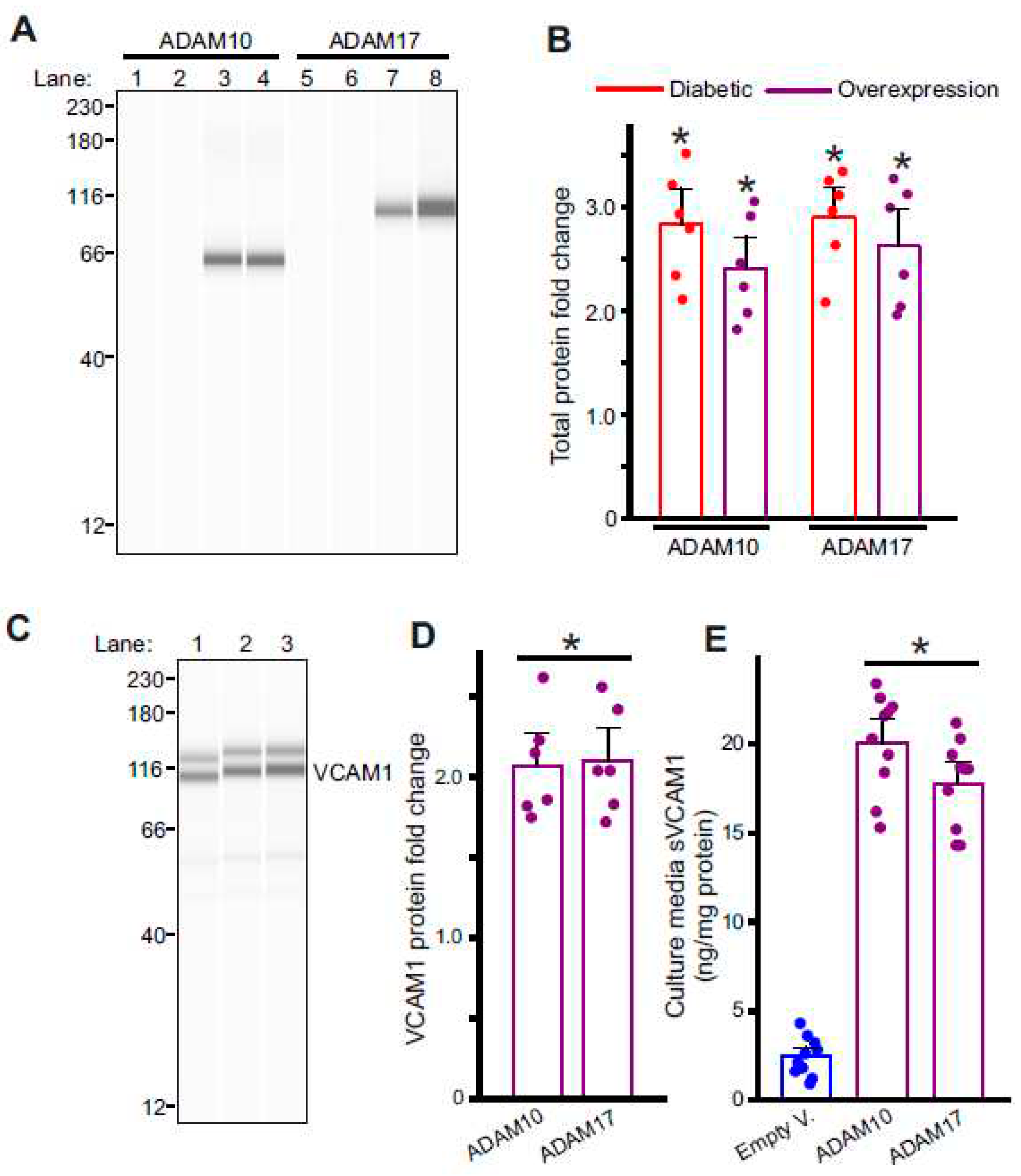
2.4. Diabetic Endothelial Cells Have Upregulated ADAM10 and ADAM17 Expression
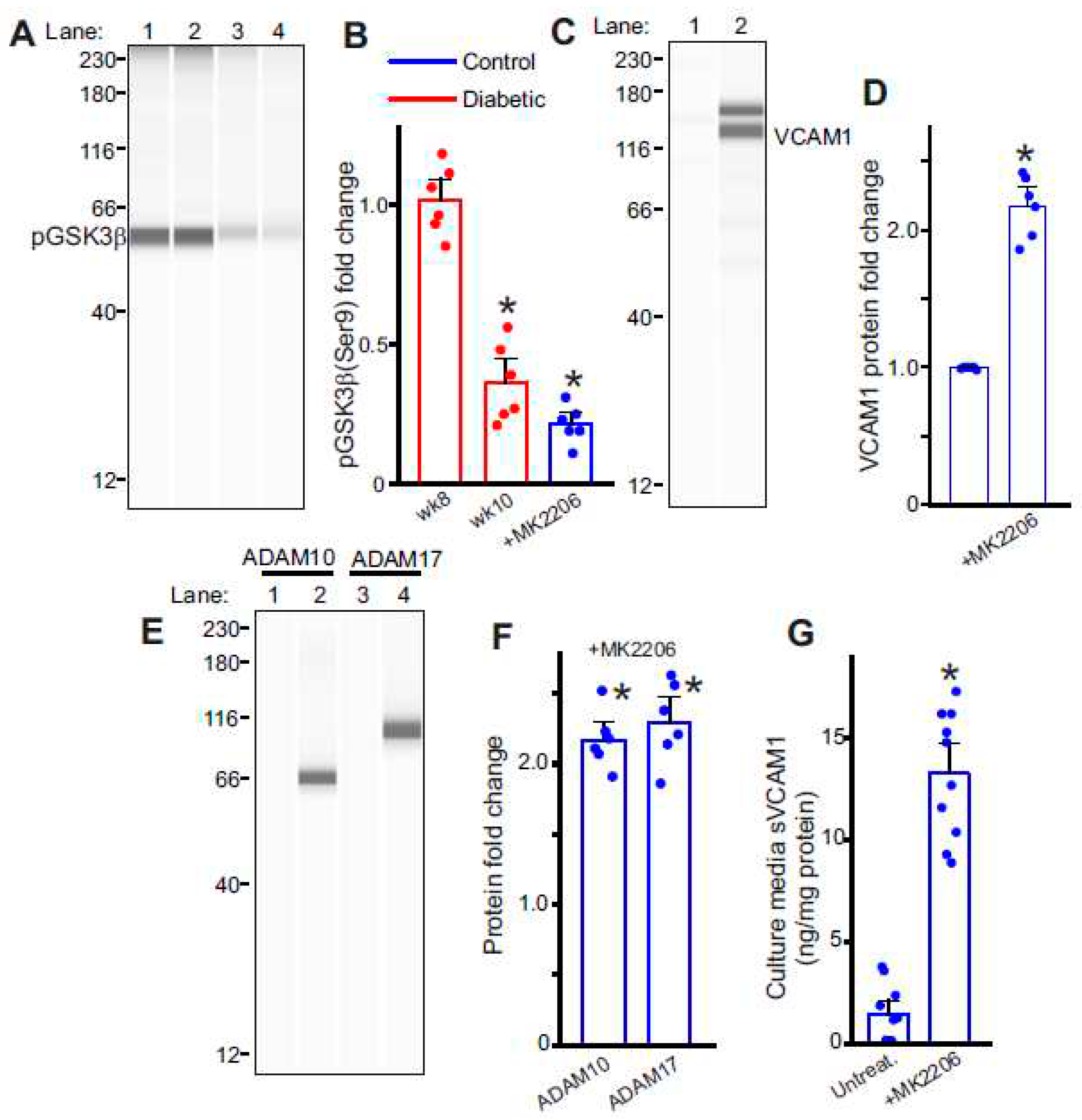
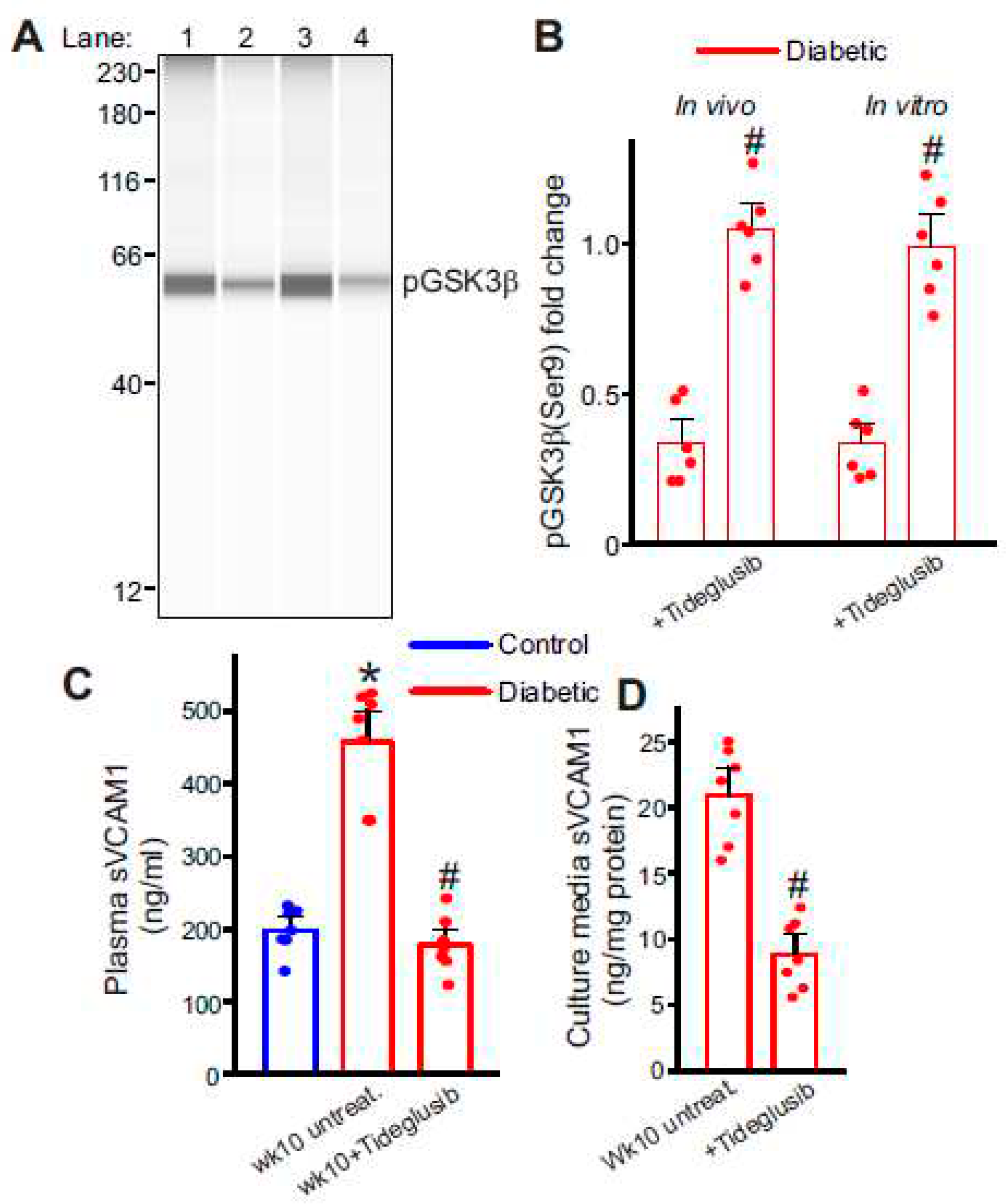
2.5. Diabetic Endothelial Cell GSK3β Regulates the Expression of ADAM10/17 and VCAM1
2.6. Selective Inhibition of GSK3β Downregulates Plasma sVCAM1 Levels.
3. Discussion
4. Materials and Methods
4.1. Animal Usage
4.2. Tissue Preparation
4.3. Endothelial Cell (EC) Isolation and Culture
4.4. Plasmid Vectors and EC Overexpression
4.6. AbbyTM Capillary Electrophoresis Immunoassay (Simple Western)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maric-Bilkan C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clinical science (London, England : 1979). 2017;131:833-846. [CrossRef]
- Kautzky-Willer A, Harreiter J. Sex and gender differences in therapy of type 2 diabetes. Diabetes research and clinical practice. 2017;131:230-241. [CrossRef]
- Ramos TN, Bullard DC, Barnum SR. ICAM-1: Isoforms and Phenotypes. The Journal of Immunology. 2014;192:4469. [CrossRef]
- Kallmann B.A. HV, Toyka K.V., Rieckmann P. Soluble VCAM-1 Release Indicates Inflammatory Blood-Brain Barrier Pathology and Further Modulates Adhesion. In: Hommes O.R., Comi G. (eds) Early Indicators Early Treatments Neuroprotection in Multiple Sclerosis. Topics in Neuroscience. 2004:115-117.
- Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). The Journal of biological chemistry. 2003;278:37459-37464. [CrossRef]
- Bruno CM, Valenti M, Bertino G, Ardiri A, Bruno F, Cunsolo M, Pulvirenti D, Neri S. Plasma ICAM-1 and VCAM-1 levels in type 2 diabetic patients with and without microalbuminuria. Minerva Med. 2008;99:1-5.
- Troncoso MF, Ortiz-Quintero J, Garrido-Moreno V, Sanhueza-Olivares F, Guerrero-Moncayo A, Chiong M, Castro PF, García L, Gabrielli L, Corbalán R, et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2021;1867:166170. [CrossRef]
- Schmidt AM, Crandall J, Hori O, Cao R, Lakatta E. Elevated plasma levels of vascular cell adhesion molecule-1 (VCAM-1) in diabetic patients with microalbuminuria: a marker of vascular dysfunction and progressive vascular disease. Br J Haematol. 1996;92:747-750. [CrossRef]
- Xu Y, Hou H, Zhao L. The role of VCAM-1 in diabetic retinopathy: A systematic review and meta-analysis. Journal of diabetes and its complications. 2023;37:108380. [CrossRef]
- Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. European heart journal. 2013;34:2436-2443. [CrossRef]
- Gustavsson C, Agardh CD, Zetterqvist AV, Nilsson J, Agardh E, Gomez MF. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PloS one. 2010;5:e12699. [CrossRef]
- Chen J, Liu Q, He J, Li Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front Immunol. 2022;13:958790. [CrossRef]
- Hegazy GA, Awan Z, Hashem E, Al-Ama N, Abunaji AB. Levels of soluble cell adhesion molecules in type 2 diabetes mellitus patients with macrovascular complications. J Int Med Res. 2020;48:300060519893858. [CrossRef]
- Hocaoglu-Emre FS, Saribal D, Yenmis G, Guvenen G. Vascular Cell Adhesion Molecule 1, Intercellular Adhesion Molecule 1, and Cluster of Differentiation 146 Levels in Patients with Type 2 Diabetes with Complications. Endocrinol Metab (Seoul). 2017;32:99-105. [CrossRef]
- Hackman A, Abe Y, Insull W, Pownall H, Smith L, Dunn K, Gotto AM, Ballantyne CM. Levels of Soluble Cell Adhesion Molecules in Patients With Dyslipidemia. Circulation. 1996;93:1334-1338. doi: doi:10.1161/01.CIR.93.7.1334.
- Koenen RR, Pruessmeyer J, Soehnlein O, Fraemohs L, Zernecke A, Schwarz N, Reiss K, Sarabi A, Lindbom L, Hackeng TM, et al. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood. 2009;113:4799-4809. [CrossRef]
- Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D'Souza SE. Tumor Necrosis Factor-α-converting Enzyme (TACE/ADAM-17) Mediates the Ectodomain Cleavage of Intercellular Adhesion Molecule-1 (ICAM-1)*. Journal of Biological Chemistry. 2006;281:3157-3164. [CrossRef]
- Maas SL, Donners M, van der Vorst EPC. ADAM10 and ADAM17, Major Regulators of Chronic Kidney Disease Induced Atherosclerosis? Int J Mol Sci. 2023;24. [CrossRef]
- Leo MD, Peixoto-Nieves D, Yin W, Raghavan S, Muralidharan P, Mata-Daboin A, Jaggar JH. TMEM16A channel upregulation in arterial smooth muscle cells produces vasoconstriction during diabetes. American journal of physiology Heart and circulatory physiology. 2021;320:H1089-h1101. [CrossRef]
- Nagy C, Einwallner E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J Vis Exp. 2018. [CrossRef]
- Bui TM, Wiesolek HL, Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787-799. [CrossRef]
- Berardi C, Wassel CL, Decker PA, Larson NB, Kirsch PS, Andrade M, Tsai MY, Pankow JS, Sale MM, Sicotte H, et al. Elevated Levels of Adhesion Proteins Are Associated With Low Ankle-Brachial Index. Angiology. 2017;68:322-329. [CrossRef]
- Kotteas EA, Boulas P, Gkiozos I, Tsagkouli S, Tsoukalas G, Syrigos KN. The Intercellular Cell Adhesion Molecule-1 (ICAM-1) in Lung Cancer: Implications for Disease Progression and Prognosis. Anticancer Research. 2014;34:4665.
- Champagne B, Tremblay P, Cantin A, St. Pierre Y. Proteolytic Cleavage of ICAM-1 by Human Neutrophil Elastase. The Journal of Immunology. 1998;161:6398.
- Witte DR, Broekmans WM, Kardinaal AF, Klöpping-Ketelaars IA, van Poppel G, Bots ML, Kluft C, Princen JM. Soluble intercellular adhesion molecule 1 and flow-mediated dilatation are related to the estimated risk of coronary heart disease independently from each other. Atherosclerosis. 2003;170:147-153. [CrossRef]
- Semaan HB, Gurbel PA, Anderson JL, Muhlestein JB, Carlquist JF, Horne BD, Serebruany VL. Soluble VCAM-1 and E-Selectin, but Not ICAM-1 Discriminate Endothelial Injury in Patients with Documented Coronary Artery Disease. Cardiology. 2000;93:7-10. [CrossRef]
- Demerath E, Towne B, Blangero J, Siervogel RM. The relationship of soluble ICAM-1, VCAM-1, P-selectin and E-selectin to cardiovascular disease risk factors in healthy men and women. Annals of Human Biology. 2001;28:664-678. [CrossRef]
- Haarmann A, Nowak E, Deiß A, van der Pol S, Monoranu CM, Kooij G, Müller N, van der Valk P, Stoll G, de Vries HE, et al. Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin α-4-transduced outside-in signalling. Acta Neuropathol. 2015;129:639-652. [CrossRef]
- Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. The Lancet. 1998;351:88-92. [CrossRef]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr., Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219-4225. [CrossRef]
- Ling S, Nheu L, Komesaroff PA. Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini Rev Med Chem. 2012;12:175-183. [CrossRef]
- Allen S, Moran N. Cell Adhesion Molecules: Therapeutic Targets for Inhibition of Inflammatory States. Semin Thromb Hemost. 2015;41:563-571. [CrossRef]
- Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Frontiers in Immunology. 2019;10. [CrossRef]
- Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods in molecular biology (Clifton, NJ). 2012;821:421-433. [CrossRef]
- Gilbert ER, Fu Z, Liu D. Development of a nongenetic mouse model of type 2 diabetes. Experimental diabetes research. 2011;2011:416254. [CrossRef]
- Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:630-634.
- Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1:e78. [CrossRef]
- Leguina-Ruzzi A, Ortiz R, Velarde V. The streptozotocin-high fat diet induced diabetic mouse model exhibits severe skin damage and alterations in local lipid mediators. Biomed J. 2018;41:328-332. [CrossRef]
- Avtanski D, Pavlov VA, Tracey KJ, Poretsky L. Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Animal Model Exp Med. 2019;2:252-258. [CrossRef]
- Parilla JH, Willard JR, Barrow BM, Zraika S. A Mouse Model of Beta-Cell Dysfunction as Seen in Human Type 2 Diabetes. J Diabetes Res. 2018;2018:6106051. [CrossRef]
- Nath S, Ghosh SK, Choudhury Y. A murine model of type 2 diabetes mellitus developed using a combination of high fat diet and multiple low doses of streptozotocin treatment mimics the metabolic characteristics of type 2 diabetes mellitus in humans. J Pharmacol Toxicol Methods. 2017;84:20-30. [CrossRef]
- Heydemann A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. Journal of Diabetes Research. 2016;2016:2902351. [CrossRef]
- Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, Pappan K, Poitout V, Gannon M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. American Journal of Physiology-Endocrinology and Metabolism. 2015;308:E573-E582. [CrossRef]
- Christians JK, Lennie KI, Wild LK, Garcha R. Effects of high-fat diets on fetal growth in rodents: a systematic review. Reproductive Biology and Endocrinology. 2019;17:39. [CrossRef]
- Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr Diabetes. 2014;4:e135. [CrossRef]
- Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971-2006. Am J Clin Nutr. 2011;93:836-843. [CrossRef]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes care. 2004;27:1487-1495. [CrossRef]
- Matthews DR. Insulin resistance and beta-cell function--a clinical perspective. Diabetes, obesity & metabolism. 2001;3 Suppl 1:S28-33.
- Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol. 2015;6:620. [CrossRef]
- Shi M, Shi G-P. Different Roles of Mast Cells in Obesity and Diabetes: Lessons from Experimental Animals and Humans. Frontiers in Immunology. 2012;3:7.
- Wang J, Shi G-P. Mast cell stabilization: novel medication for obesity and diabetes. Diabetes/metabolism research and reviews. 2011;27:919-924. [CrossRef]
- Roberts AC, Porter KE. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diabetes and Vascular Disease Research. 2013;10:472-482. [CrossRef]
- Sena CM, Pereira AM, Seiça R. Endothelial dysfunction — A major mediator of diabetic vascular disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832:2216-2231. [CrossRef]
- Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. European Journal of Cell Biology. 2012;91:472-485. [CrossRef]
- Morsing SKH, Rademakers T, Brouns SLN, Stalborch ADV, Donners M, van Buul JD. ADAM10-Mediated Cleavage of ICAM-1 Is Involved in Neutrophil Transendothelial Migration. Cells. 2021;10. [CrossRef]
- Palau V, Jarrín J, Villanueva S, Benito D, Márquez E, Rodríguez E, Soler MJ, Oliveras A, Gimeno J, Sans L, et al. Endothelial ADAM17 Expression in the Progression of Kidney Injury in an Obese Mouse Model of Pre-Diabetes. Int J Mol Sci. 2021;23. [CrossRef]
- Abu El-Asrar AM, Nawaz MI, Ahmad A, De Zutter A, Siddiquei MM, Blanter M, Allegaert E, Gikandi PW, De Hertogh G, Van Damme J, et al. Evaluation of Proteoforms of the Transmembrane Chemokines CXCL16 and CX3CL1, Their Receptors, and Their Processing Metalloproteinases ADAM10 and ADAM17 in Proliferative Diabetic Retinopathy. Front Immunol. 2020;11:601639. [CrossRef]
- Shalaby L, Thounaojam M, Tawfik A, Li J, Hussein K, Jahng WJ, Al-Shabrawey M, Kwok HF, Bartoli M, Gutsaeva D. Role of Endothelial ADAM17 in Early Vascular Changes Associated with Diabetic Retinopathy. J Clin Med. 2020;9. [CrossRef]
- Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor perspectives in biology. 2014;6. [CrossRef]
- De Meyts P. The Insulin Receptor and Its Signal Transduction Network. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, et al., eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
- Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721-4731. [CrossRef]
- Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. The Journal of biological chemistry. 1997;272:30075-30082.
- Eto M, Kouroedov A, Cosentino F, Lüscher TF. Glycogen synthase kinase-3 mediates endothelial cell activation by tumor necrosis factor-alpha. Circulation. 2005;112:1316-1322. [CrossRef]
- del Ser T, Steinwachs KC, Gertz HJ, Andres MV, Gomez-Carrillo B, Medina M, Vericat JA, Redondo P, Fleet D, Leon T. Treatment of Alzheimer's disease with the GSK-3 inhibitor tideglusib: a pilot study. Journal of Alzheimer's disease : JAD. 2013;33:205-215. [CrossRef]
- Lovestone S, Boada M, Dubois B, Hull M, Rinne JO, Huppertz HJ, Calero M, Andres MV, Gomez-Carrillo B, Leon T, et al. A phase II trial of tideglusib in Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2015;45:75-88. [CrossRef]
- Tolosa E, Litvan I, Hoglinger GU, Burn D, Lees A, Andres MV, Gomez-Carrillo B, Leon T, Del Ser T. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2014;29:470-478. [CrossRef]
- Ghiarone T, Castorena-Gonzalez JA, Foote CA, Ramirez-Perez FI, Ferreira-Santos L, Cabral-Amador FJ, de la Torre R, Ganga RR, Wheeler AA, Manrique-Acevedo C, et al. ADAM17 cleaves the insulin receptor ectodomain on endothelial cells and causes vascular insulin resistance. American journal of physiology Heart and circulatory physiology. 2022;323:H688-h701. [CrossRef]
- MacKay CE, Floen M, Leo MD, Hasan R, Garrud TAC, Fernández-Peña C, Singh P, Malik KU, Jaggar JH. A plasma membrane-localized polycystin-1/polycystin-2 complex in endothelial cells elicits vasodilation. eLife. 2022;11:e74765. [CrossRef]
- Ritchie JL, Walters JL, Galliou JMC, Christian RJ, Qi S, Savenkova MI, Ibarra CK, Grogan SR, Fuchs RA. Basolateral amygdala corticotropin-releasing factor receptor type 1 regulates context-cocaine memory strength during reconsolidation in a sex-dependent manner. Neuropharmacology. 2021;200:108819. [CrossRef]
- Beekman C, Janson AA, Baghat A, van Deutekom JC, Datson NA. Use of capillary Western immunoassay (Wes) for quantification of dystrophin levels in skeletal muscle of healthy controls and individuals with Becker and Duchenne muscular dystrophy. PloS one. 2018;13:e0195850. [CrossRef]
- Krishnan V, Ali S, Gonzales AL, Thakore P, Griffin CS, Yamasaki E, Alvarado MG, Johnson MT, Trebak M, Earley S. STIM1-dependent peripheral coupling governs the contractility of vascular smooth muscle cells. eLife. 2022;11:e70278. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




