Submitted:
14 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
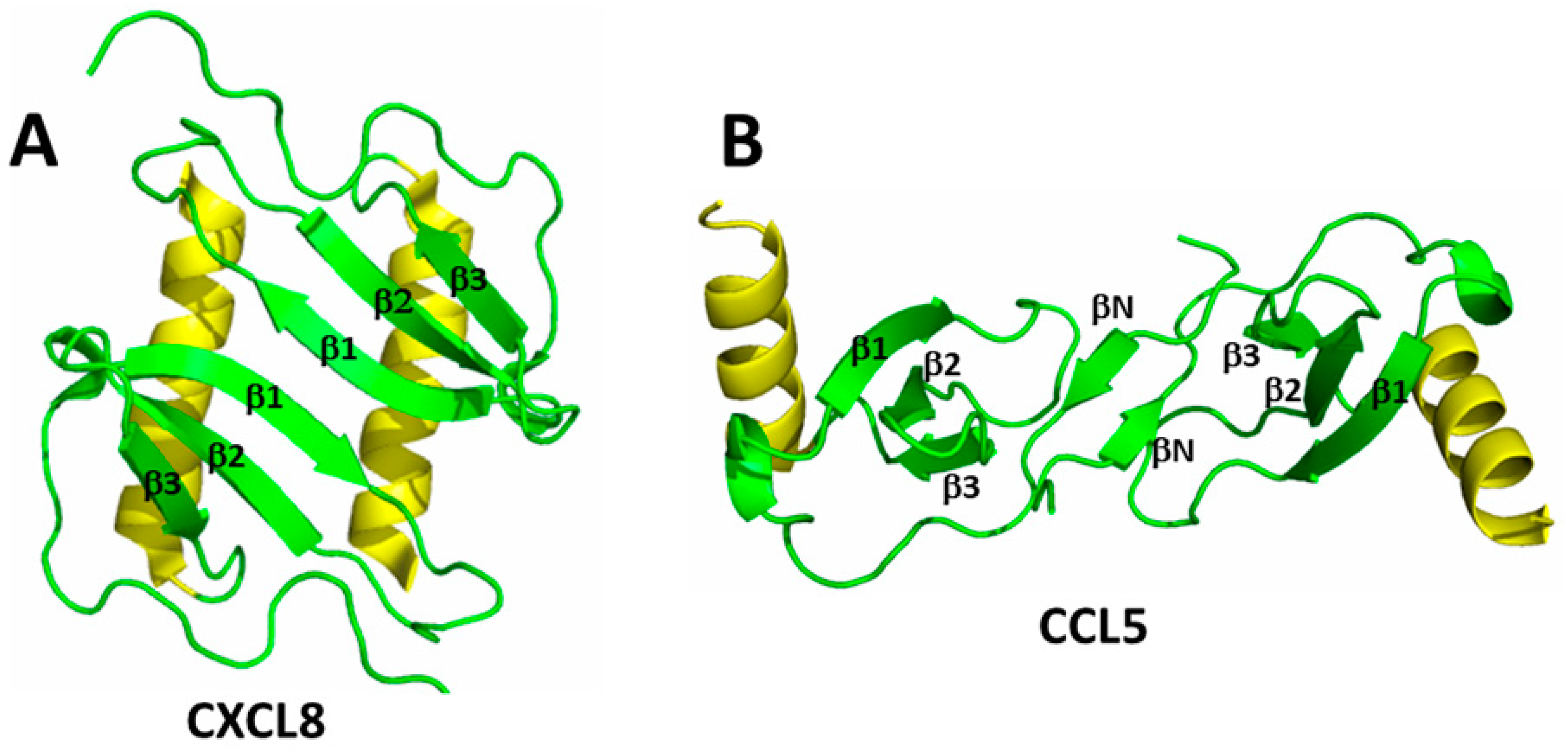
Chemokines
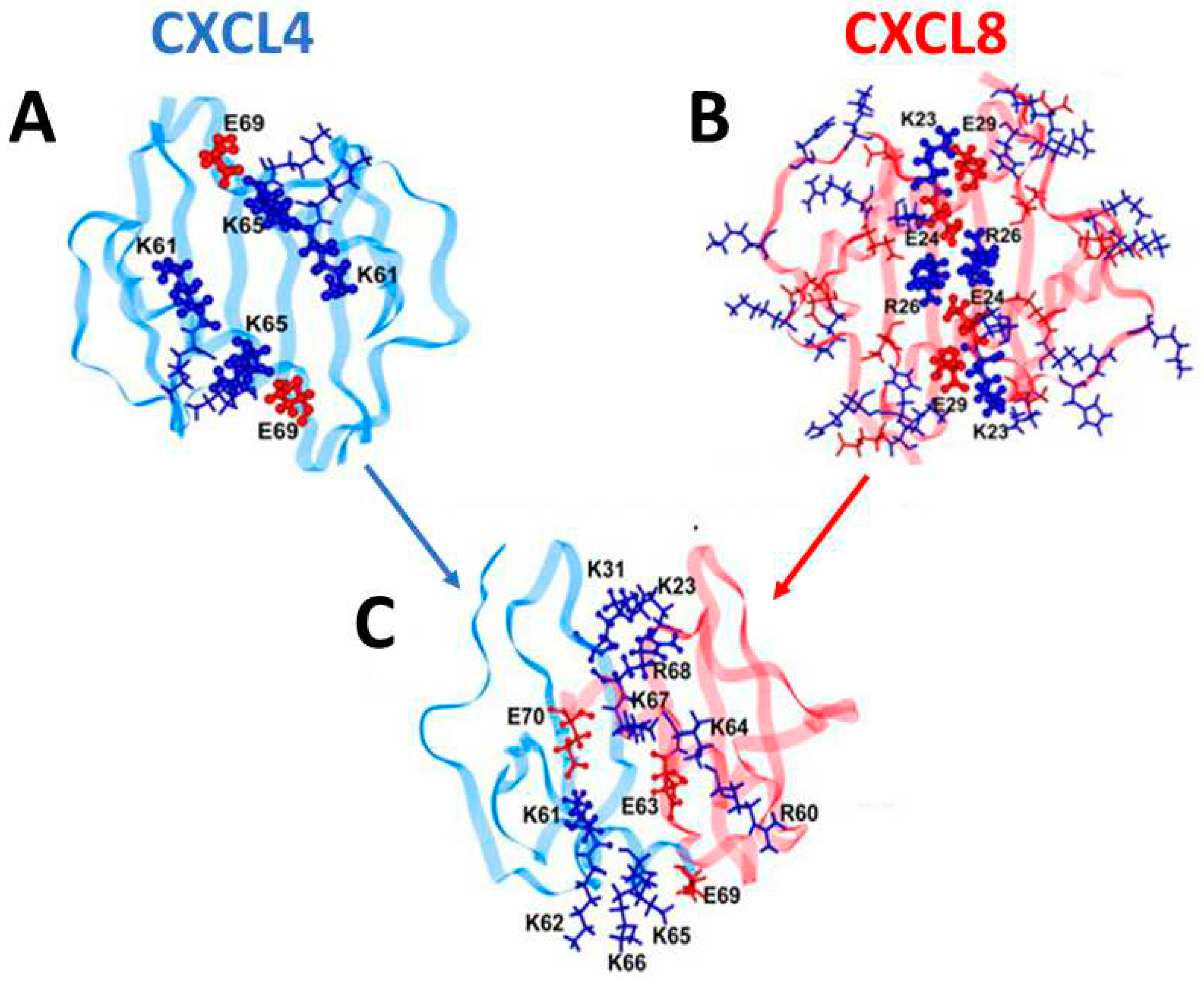
Chemokine Heterodimers.
Functional Considerations.
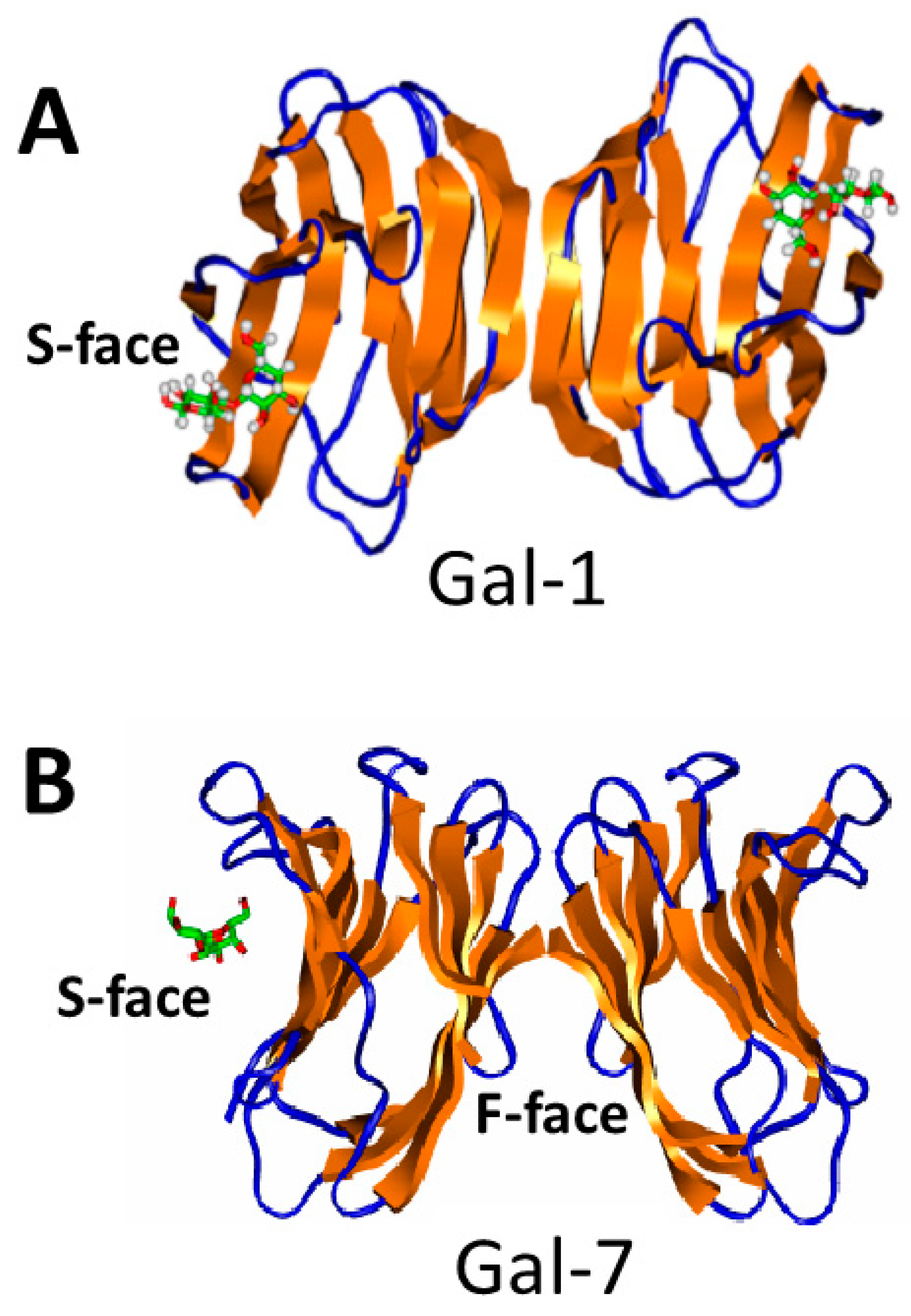
Galectins
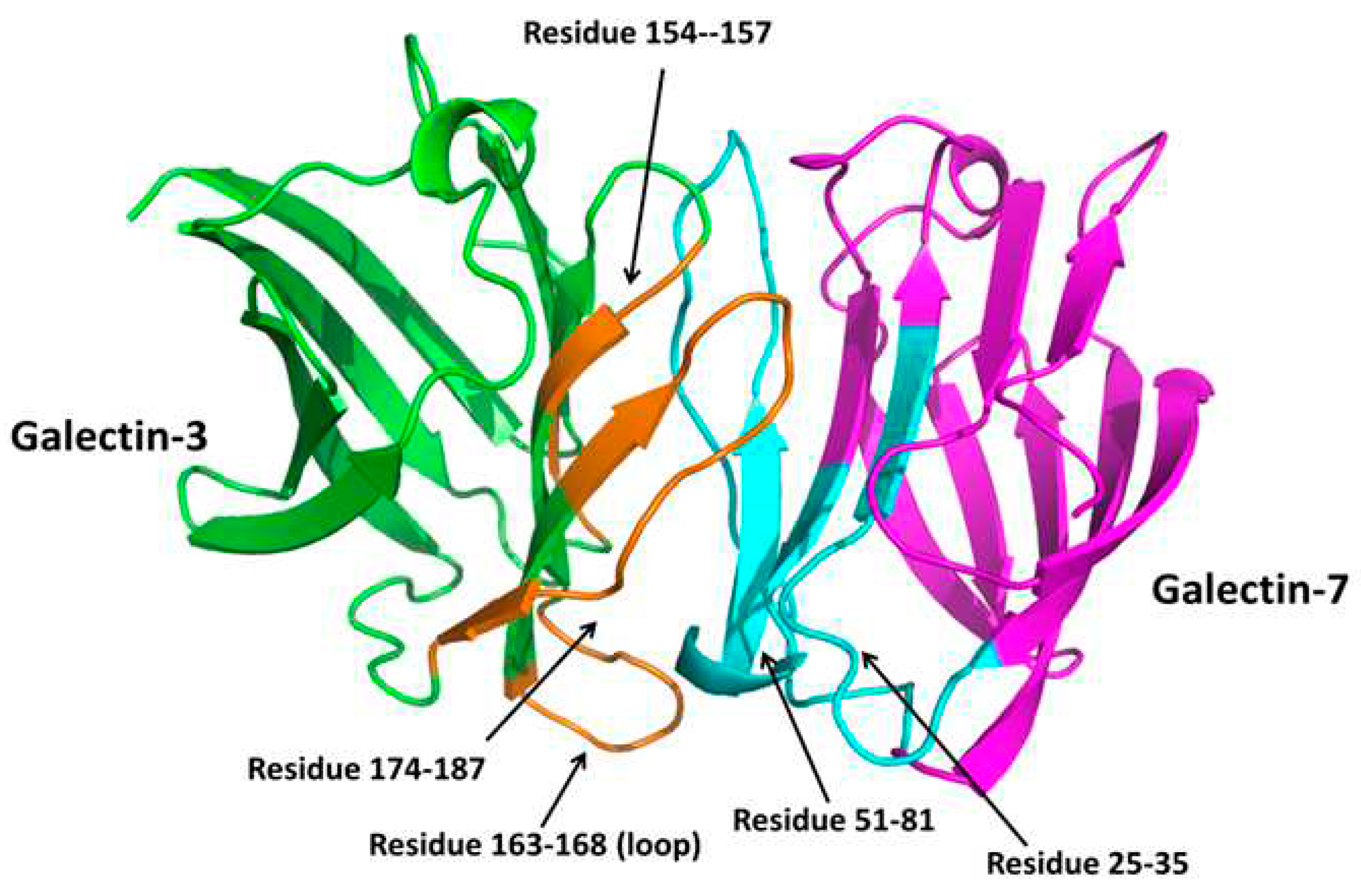
Galectin-Galectin Heterodimers.
Functional Considerations.
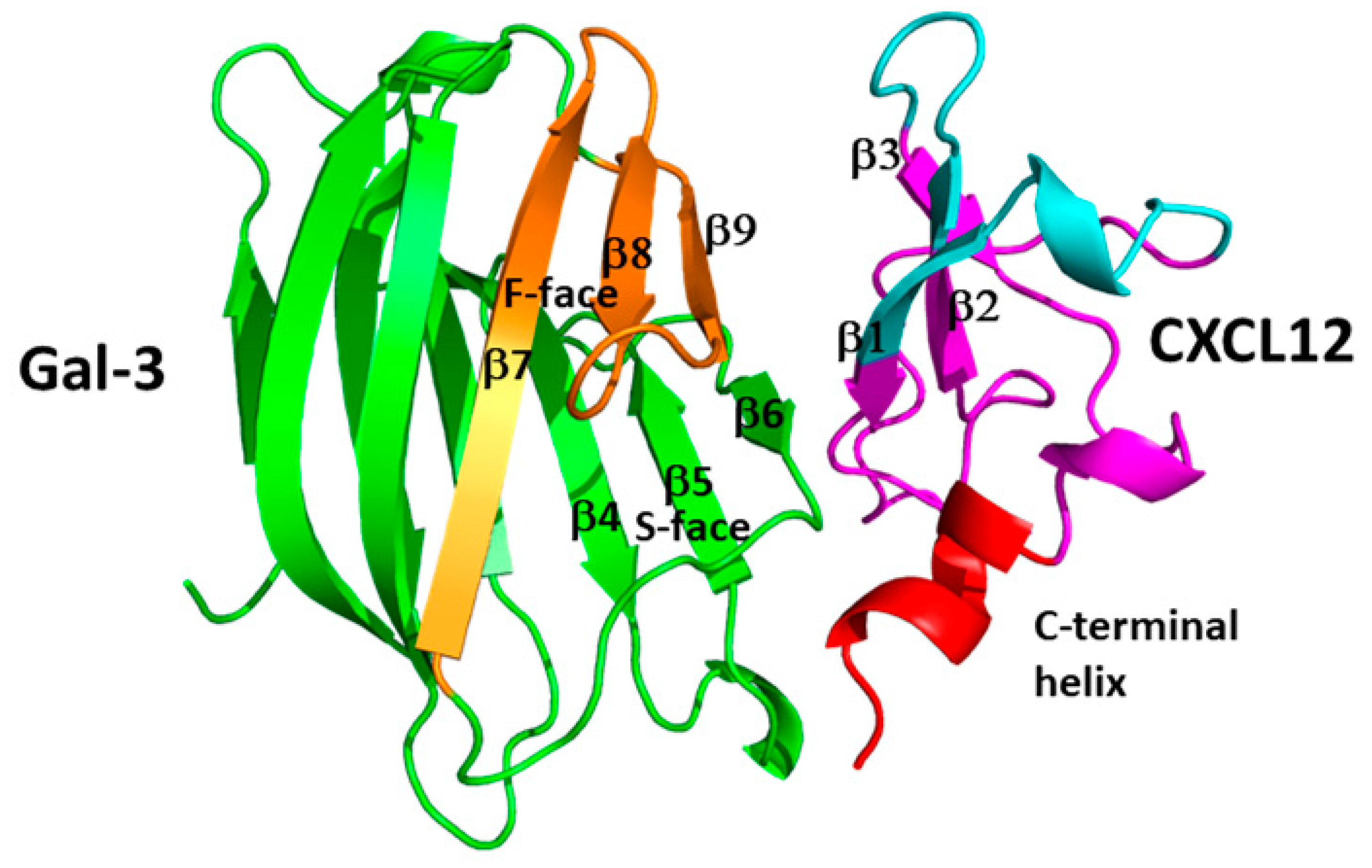
Chemokine–Galectin Heterodimers
Conclusions
References
- Guan, E., Wang, J., Norcross, M.A. Identification of Human Macrophage Inflammatory Proteins 1α and 1β as a Native Secreted Heterodimer. J. Biol. Chem. 2001, 276, 12404–12409. [CrossRef]
- Dudek, A.Z., Nesmelova, I., Mayo, K.H., Verfaillie, C.M., Pitchford, E., Slungaard, A. Platelet Factor 4 Promotes Adhesion of Hematopoietic Progenitor Cells and Binds IL-8: Novel Mechanisms for Modulation of Hematopoiesis. Blood 2003, 101, 4687-4694. [CrossRef]
- Nesmelova, I., Sham, Y., Dudek, A.Z., van Eijk, L.I., Wu, G., Slungaard, A., Mortari, F., Griffioen, A.W., Mayo, K.H. Platelet Factor 4 and Interleukin-8 CXC Chemokine Heterodimer Formation Modulates Function at the Quaternary Structural Level. J. Biol. Chem. 2005, 280, 4948-4958. [CrossRef]
- Nesmelova, I.V., Sham, Y., Gao, J., Mayo, K.H. CXC-chemokines associate with CC-chemokines to form mixed heterodimers: RANTES and PF4 monomers associate as CC-type heterodimers. J. Biol. Chem. 2008, 283, 24155-24166. [CrossRef]
- Koenen, R., von Hundelhausen, P., Nesmelova, I.V., Zernecke, A., Liehn, E.A., Sarabi, A., Kramp, B.K., Piccinini, A., Paludan, S.R., Kowalska, M.A., Kungl, A.J., Hackeng, T.M., Mayo, K.H., and Weber, C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nature Med. 2009, 15, 97-103.
- von Hundelshausen, P., Agten, S., Eckardt, V., Schmitt, M., Blanchet, X., Neideck, C., Ippel, H., Bidzhekov, K., Wichapong, K., Faussner, A., Drechsler, M., Grommes, J., Li, H., Dijkgraaf, I., Nicolaes, G., Döring, Y., Soehnlein, O., Heemskerk, J., Koenen, R., Mayo, K.H., Hackeng, T., Weber, C. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci. Transl. Med. 2017, 9, 384-392. [CrossRef]
- Miller, M.C., Ludwig, A.K., Wichapong, K., Kaltner, H., Kopitz, J., Gabius, H.-J., Mayo, K.H. Adhesion/growth-regulatory galectins tested in combination: evidence for synergistic functional cooperation and subunit swapping to form heterodimers. Biochem. J. 2018, 475, 1003-1018.
- Dings, R.P.M., Kumar, N., Mikkelson, S., Gabius, H.-J., Mayo, K.H. Stimulating cellular galectins networks by mixing galectins in vitro reveals synergistic activity. Biochem. Biophys. Reports 2021, 28, 101-116.
- Eckardt, V., Miller, M.C., Blanchet, X., Duan, R., Leberzammer, J., Duchene, J., Soehnlein, O., Megens, R.T., Ludwig, A.K., Dregni, A., Faussner, A., Wichapong, K., Ippel, H., Dijkgraaf, I., Kaltner, H., Doring, Y., Bidzhekov, K., Hackeng, T.M., Weber, C., Gabius, H.-J., von Hundelshausen, P., Mayo, K.H. Chemokines and galectins form heterodimers to modulate inflammation. EMBO Rep. 2020, 21, e47852. [CrossRef]
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [CrossRef]
- Mackay, C.R. Chemokines: immunology’s high impact factors. Nat. Immunol. 2001, 2, 95–101. [CrossRef]
- Youn, B.S., Mantel, C., Broxmeyer, H.E. Chemokines, chemokine receptors and hematopoiesis. Immunol. Rev. 2000, 177, 150–174. [CrossRef]
- Koch, A.E., Polverini, P.J., Kunkel, S.L., Harlow, L.A., DiPietro, L.A., Elner, V.M., Elner, S.G., and Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [CrossRef]
- Belperio, J.A., Keane, M.P., Arenberg, D.A., Addison, C.L., Ehlert, J.E., Burdick, M.D., and Strieter, R.M. CXC Chemokines in Angiogenesis. J. Leukocyte Biol. 2000, 68, 1–8. [CrossRef]
- Zlotnik, A., Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 2000, 12, 121–127. [CrossRef]
- Clore, G.M., Gronenborn, A.M. Three-dimensional structures of α- and β-chemokines. FASEB J. 1995, 9, 57–62.
- Thomas, M.A., Buelow, B.J., Nevins, A.M., Jones, S.E., Peterson, F.C., Gundry, R.L., Grayson, M.H., Volkman, B.F. Structure-function analysis of CCL28 in the development of post-viral asthma. J. Biol. Chem. 2015, 290, 4528-4536. [CrossRef]
- Clark-Lewis, I., Kim, K.S., Rajarathnam, K., Gong, J.H., Dewald, B., Moser, B., Baggiolini, M., and Sykes, B.D. Structure-activity relationships of chemokines. J. Leukocyte Biol. 1995, 57, 703–711. [CrossRef]
- Wang, X., Watson, C., Sharp, J.S., Handel, T.M., Prestegard, J.H. Oligomeric structure of the chemokine CCL5/RANTES from NMR, MS, and SAXS data. Structure 2011, 19, 1138-1148.
- Jansma, A.L., Kirkpatrick, J.P., Hsu, A.R., Handel, T.M., Nietlispach, D. NMR analysis of the structure, dynamics, and unique oligomerization properties of the chemokine CCL27. J. Biol. Chem. 2010, 285, 14424-14437. [CrossRef]
- Clore, G.M., Appella, E., Yamada, M., Matsushima, K., Gronenborn, A.M. Three dimensional structure of interleukin 8 in solution. Biochemistry 1990, 29, 1689–1696. [CrossRef]
- Liang, W.G., Triandafillou, C.G., Huang, T.Y., Zulueta, M.M., Banerjee, S., Dinner, A.R., Hung, S.C., Tang, W.J. Crystal structure of CC chemokine 5 (CCL5). Proc. Natl. Acad. Sci. USA 2016, 113, 5000-5005.
- Skelton, N.J., Aspiras, F., Ogez, J., Schall, T.J. Proton NMR assignments and solution conformation of RANTES, a chemokine of the CC type. Biochemistry 1995, 34, 5329–5342. [CrossRef]
- Mayo, K.H., Roongta, V., Barker, S., Milius, R., Ilyina, E., Quinlan, C., La Rosa, G., Daly, T. NMR Solution Structure of the 32 kD Tetrameric Platelet Factor-4 ELR-Motif N-terminal Chimer: A Symmetric Tetramer. Biochemistry 1995, 34, 11399-11409.
- Yang, Y., Mayo, K.H., Daly, T., Barry, J.K., La Rosa, G.J. Subunit Association and Structural Analysis of Platelet Basic Protein and Related Proteins Investigated by 1H-NMR Spectroscopy and Circular Dichroism. J. Biol. Chem. 1994, 269, 20110-20118. [CrossRef]
- Swaminathan, G.J., Holloway, D.E., Colvin, R.A., Campanella, K., Papageorgiou, A.C., Luster, A.D., Acharya, K.R. Crystal Structures of Oligomeric Forms of the IP-10/CXCL10 Chemokine. Structure 2003, 11, 521–532.
- Zhang, X., Chen, L., Bancroft, D.P., Lai, C.K., Maione, T.E. Crystal structure of recombinant human platelet factor 4. Biochemistry 1994, 33, 8361–8366. [CrossRef]
- Paoletti, S., Petkovic, V., Sebastiani, S., Danelon, M.G., Uguccioni, M., Gerber, B.O. A rich chemokine environment strongly enhances leukocyte migration and activities. Blood 2005, 105, 3405–3412. [CrossRef]
- Campanella, G.S.V., Grimm, J., Manice, L.A., Colvin, R.A., Medoff, B.D., Woitkiewicz, G.R., Weissleder, R., Luster, A.D. Oligomerization of CXCL10 Is Necessary for Endothelial Cell Presentation and In Vivo Activity. J. Immunol. 2006, 177, 6991–6998. [CrossRef]
- Rek, A., Brandner, B., Geretti, E., Kungl, A.J. A biophysical insight into the RANTES–glycosaminoglycan interaction. Biochim. Biophys. Acta 2009, 1794, 577–582. [CrossRef]
- Ren, M., Guo, Q., Guo, L., Lenz, M., Qian, F., Koenen, R.R., Xu, H., Schilling, A.B., Weber, C., Ye, R.D., Dinner, A.R., Tang, W.-J. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP1 by insulin-degrading enzyme. EMBO J. 2010, 29, 3952–3966. [CrossRef]
- Mayo, K.H., Chen, M.-J. Human Platelet Factor 4 Monomer-Dimer-Tetramer Equilibria Investigated by NMR Spectroscopy. Biochemistry 1989, 28, 9469-9478. [CrossRef]
- Chen, M.J., Mayo, K.H. Human Platelet Factor 4 Subunit Association-Dissociation Thermodynamics and Kinetics. Biochemistry 1991, 30, 6402-6411. [CrossRef]
- Mayo, K.H. Low Affinity Platelet Factor 4 1H-NMR Derived Aggregate Equilibria Indicate Physiological Preference for Monomers over Dimers and Tetramers. Biochemistry 1991, 30, 925-934.
- Young, H., Roongta, V., Daly, T. J., Mayo, K.H. NMR Structure and Dynamics of Monomeric Neutrophil Activating Peptide-2. Biochem. J. 1999, 338, 591-598.
- Yang, Y., Mayo, K.H. Alcohol-Induced Protein Folding Transitions in Platelet Factor- 4: The O-State. Biochemistry 1993, 32, 8661-8671. [CrossRef]
- Yang, Y., Barker, S., Chen, M.-J., Mayo, K.H. Effect of Low Molecular Weight Aliphatic Alcohols and Related Compounds on Platelet Factor-4 Subunit Association. J. Biol. Chem. 1993, 268, 9223-9229. [CrossRef]
- Veldkamp, C.T., Ziarek, J.J., Su, J., Basnet, H., Lennertz, R., Weiner, J.J., Peterson, F.C., Baker, J.E., Volkman, B.F. Monomeric structure of the cardio-protective chemokine SDF-1/CXCL12. Protein Sci. 2009, 18, 1359-1369.
- Veldkamp, C.T., Peterson, F.C., Pelzek, A.J., Volkman, B.F. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL12) is altered by pH, phosphate, sulfate, and heparin. Protein Sci. 2005, 14, 1071-1081.
- Crump, M.P., Rajarathnam, K., Kim, K.-S., Clark-Lewis, I., Sykes, B.D. Solution Structure of Eotaxin, a Chemokine That Selectively Recruits Eosinophils in Allergic Inflammation. J. Biol. Chem. 1998, 273, 22471-22479. [CrossRef]
- Ellyard, J.I., Simson, L., Bezos, A., Johnston, K., Freeman, C., Parish, C.R. Eotaxin Selectively Binds Heparin. J. Biol. Chem. 2007, 282, 15238–15247. [CrossRef]
- Proudfoot, A.E. The BBXB Motif of RANTES Is the Principal Site for Heparin Binding and Controls Receptor Selectivity. J. Biol. Chem. 2001, 276, 10620–10626. [CrossRef]
- Sheng, G.J., Oh, Y.I., Chang, S.-K., Hsieh-Wilson, L.C. Tunable Heparan Sulfate Mimetics for Modulating Chemokine Activity. J. Am. Chem. Soc. 2013, 135, 10898–10901. [CrossRef]
- Salanga, C.L., Handel, T.M. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp. Cell. Res. 2011, 317, 590-601. [CrossRef]
- Proudfoot, A.E. Chemokines and Glycosaminoglycans. Front. Immunol. 2015, 6, 246-258. [CrossRef]
- Shaw, J.P., Johnson, Z., Borlat, F., Zwahlen, C., Kungl, A., Roulin, K., Harrenga, A., Wells, T.N., Proudfoot, A.E. The X-Ray Structure of RANTES Heparin-Derived Disaccharides Allows the Rational Design of Chemokine Inhibitors. Structure 2004, 12, 2081–2093. [CrossRef]
- Seo, Y., Andaya, A., Bleiholder, C., Leary, J. A. Differentiation of CC vs CXC Chemokine Dimers with GAG Octasaccharide Binding Partners: An Ion Mobility Mass Spectrometry Approach. J. Am. Chem. Soc. 2013, 135, 4325–4332. [CrossRef]
- Mayo, K.H., Ilyina, E., Roongta, V., Dundas, M., Joseph, J., Lai, C.K., Maione, T., Daly, T. Heparin Binding to Platelet Factor-4. An NMR and Site-Directed Mutagenesis Study: Arginine Residues Crucial for Binding. Biochem. J. 1995, 312, 357-365.
- Dyer, D.P., Salanga, C.L., Volkman, B.F., Kawamura, T., Handel, T.M. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 2016, 26, 312-326. [CrossRef]
- Hoogewerf, A.J., Kuschert, G.S., Proudfoot, A.E., Borlat, F., Clark-Lewis, I., Power, C.A., Wells, T.N. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 1997, 36, 13570–13578. [CrossRef]
- Proudfoot, A.E., Handel, T.M., Johnson, Z., Lau, E.K., Li Wang, P., Clark-Lewis, I., Borlat, F., Wells, T.N., Kosco-Vilbois, M.H. (2003) Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [CrossRef]
- Crown, S.E., Yu, Y., Sweeney, M.D., Leary, J.A., Handel, T.M. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J. Biol. Chem. 2006, 281, 25438-25446. [CrossRef]
- Verkaar, F., van Offenbeek, J., van der Lee, M.M.C., van Lith, L.H.C.J., Watts, A.O., Rops, A.L.W.M.M., Aguilar, D.C., Ziarek, J.J., van der Vlag, J., Handel, T.M., Volkman, B.F., Proudfoot, A.E.I., Vischer, H.F., Zaman, G.J.R., Smit, M.J. Chemokine cooperativity is caused by competitive glycosaminoglycan binding. J. Immunol. 2014, 192, 3908-3914. [CrossRef]
- Mikhailov, D.V., Young, H., Linhardt, R.J., Mayo, K.H. Heparin Dodecasaccharide Binding to Platelet Factor-4 and Growth-related Protein-α: Induction of a Partially Folded State and Implications for Heparin-Induced Thromnocytopenia. J. Biol. Chem. 1999, 274, 25317-25329.
- Brandhofer, M., Hoffmann, A., Blanchet, X., Siminkovitch, E., Rohlfing, A.-K., El Bounkari, O., Nestele, J.A., Bild, A., Kontos, C., Hille, K., Rohde, V., Fröhlich, A., Golemi, J., Gokce, O., Krammer, C., Scheiermann, P., Tsilimparis, Sachs, N., N., Kempf, W., Mägdefessel, L., Otabil, M.K., Megens, R.T.A., Ippel, H., Koenen, R.R., Luo, J., Engelmann, B., Mayo, K.H., Gawaz, M., Kapurniotu, A., Weber, C., von Hundelshausen, P., Bernhagen, J. Heterocomplexes between the Atypical Chemokine MIF and the CXC-Motif Chemokine CXCL4L1 Regulate Inflammation and Thrombus Formation. Cell. Mol. Life Sci. 2022, 79, 512. [CrossRef]
- Jansma, A., Handel, T.M., Hamel, D.J. Chapter 2. Homo- and hetero-oligomerization of chemokines. Methods Enzymol. 2009, 461, 31-50.
- Koenen, R.R., Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat. Rev. Drug Discovery 2010, 9, 141–153. [CrossRef]
- Raman, D., Sobolik-Delmaire, T., Richmond, A. Chemokines in health and disease. Exper. Cell. Res. 2011, 317, 575–589. [CrossRef]
- Viola, A., Luster, A.D. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Ann. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [CrossRef]
- Allen, S.J., Crown, S.E., Handel, T.M. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007, 25, 787-820.
- Park, S.H., Das, B.B., Casagrande, F., Tian, Y., Nothnagel, H.J., Chu, M., Kiefer, H., Maier, K., DeAngelis, A.A., Marassi, F.M., Opella, S.J. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 2012, 491, 779-783. [CrossRef]
- Thelen, M., Stein, J.V. How chemokines invite leukocytes to dance. Nature Immun. 2008, 9, 953–959. [CrossRef]
- Wu, B., Chien, E.Y., Mol, C.D., Fenalti, G., Liu, W., Katritch, V., Abagyan, R., Brooun, A., Wells, P., Bi, F.C., Hamel, D.J., Kuhn, P., Handel, T.M., Cherezov, V., Stevens, R.C. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science 2010, 330, 1066–1071. [CrossRef]
- Hemmerich, S., Paavola, C., Bloom, A., Bhakta, S., Freedman, R., Grunberger, D., Krstenansky, J., Lee, S., McCarley, D., Mulkins, M., Wong, B., Pease, J., Mizoue, L., Mirzadegan, T., Polsky, I., Thompson, K., Handel, T.M., Jarnagin, K. Identification of residues in the monocyte chemotactic protein-1 that contact the MCP-1 receptor, CCR2. Biochemistry 1999, 38, 13013–13025. [CrossRef]
- Qin, L., Kufareva, I., Holden, L.G., Wang, C., Zheng, Y., Zhao, C., Fenalti, G., Wu, H., Han, G.W., Cherezov, V., Abagyan, R., Stevens, R.C., Handel, T.M. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science 2015, 347, 1117–11122. [CrossRef]
- Strieter, R.M., Polverini, P.J., Kunkel, S.L., Arenberg, D.A., Burdick, M.D., Kasper, J., Dzuiba, J., VanDamme, J., Walz, A., Marriott, D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [CrossRef]
- Blanpain, C., Doranz, B.J., Bondue, A., Govaerts, C., De Leener, A. The core domain of chemokines bind CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J. Biol. Chem. 2002, 278, 5179–5187. [CrossRef]
- Gouwy, M. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J. Leukocyte Biol. 2004, 76, 185–194. [CrossRef]
- Gouwy, M., Struyf, S., Noppen, S., Schutyser, E., Springael, J.-Y., Parmentier, M., Proost, P., VanDamme, J. Synergy between Co-produced CC and CXC Chemokines in Monocyte Chemotaxis through Receptor-Mediated Events. Mol. Pharmacol. 2008, 74, 485–495.
- von Hundelshausen, P., Koenen, R.R., Sack, M., Mause, S.F., Adriaens, W., Proudfoot, A.E., Hackeng, T.M., Weber, C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930. [CrossRef]
- Agten, S.M., Koenen, R., Ippel, H., Eckert, V., von Hundelshausen, P., Mayo, K.H., Weber, C., Hackeng, T.M. Probing Functional Heteromeric Chemokine Protein-Protein Interactions through Conformation-assisted Oxime-Linkage. Angewante Chemie 2016, 55, 14963-14966.
- Nguyen, K.T.P., Volkman, B., Dréau, D., Nesmelova, I.V. A new obligate CXCL4-CXCL12 heterodimer for studying chemokine heterodimer activities and mechanisms. Sci. Rep. 2022, 12, 17204. [CrossRef]
- Stevens, R.L., Colombo, M., Gonzales, J.J., Hollander, W., Schmid, K. The glycosaminoglycans of the human artery and their changes in atherosclerosis. J. Clin. Invest. 1976, 58, 470-477. [CrossRef]
- Taylor, K.R., Gallo, R.L. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006, 20, 9– 22. [CrossRef]
- Handel, T.M., Johnson, Z., Crown, S.E., Lau, E.K., Proodfoot, A.E. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Ann. Rev. Biochem. 2005, 74, 385–410. [CrossRef]
- Gouwy, M., Struyf, S., Berghmans, N., Vanormelingen, C., Schols, D., Van Damme, J. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur. J. Immunol. 2011, 41, 963-973.
- Gouwy, M., Schiraldi, M., Struyf, S., Van Damme, J., Uguccioni, M. Possible mechanisms involved in chemokine synergy fine tuning the inflammatory response. Immunol. Lett. 2012, 145, 10-14. [CrossRef]
- Gouwy, M., Struyf, S., Leutenez, L., Pörtner, N., Sozzani, S., Van Damme, J. Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiol. 2014, 219, 218-229. [CrossRef]
- Mortier, A., Van Damme, J., Proost, P. Overview of the mechanisms regulating chemokine activity and availability. Immunol. Lett. 2012, 145, 2-9. [CrossRef]
- Barondes, S.H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. Galectins: a family of animal β-galactoside-binding lectins. Cell 1994, 76, 597-598. [CrossRef]
- Barondes, S.H. Galectins: a personal overview. Trends Glycosci. Glycotechnol. 1998, 9, 1–7. [CrossRef]
- Cooper, D.N., Barondes S.H. God must love galectins; he made so many of them. Glycobiology 1990, 9, 979-984. [CrossRef]
- Cooper, D.N. Galectinomics: finding themes in complexity. Biochim. Biophys. Acta. 2002, 1572, 209-231. [CrossRef]
- Cooper, D., Iqbal, A.J., Gittens, B.R., Cervone, C., Perretti, M. The effect of galectins on leukocyte trafficking in inflammation: sweet or sour? Ann. N.Y. Acad. Sci. 2012, 1253, 181–192.
- Camby, I., Le Mercier M., Lefranc F., Kiss R. Galectin-1: a small protein with major functions. Glycobiology 2006, 16, 137-157. [CrossRef]
- Teichberg, V.I., Silman I., Beitsch D.D., Resheff G. A β-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383-1387. [CrossRef]
- Hirabayashi, J., Kasai K. The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 1993, 3, 297-304.
- Kasai, K., Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J. Biochem. (Tokyo) 1996, 119, 1-8. [CrossRef]
- Ippel, H., Miller, M.C., Vértesy, S., Zheng, Y., Cañada, F.J., Suylen, D., Umemoto, K., Romano, C., Hackeng, T., Tai, G., Leffler, H., Kopitz, J., André, S., Kübler, D., Jiménez-Barbero, J., Oscarson, S., Gabius, H.-J., Mayo, K. H. Intra- and intermolecular interactions of human galectin-3: assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888-903. [CrossRef]
- Zhao, Z., Xu, X., Cheng, H., Miller, M.C., He, Z., Gu, H., Zhang, Z., Raz, A., Mayo, K.H., Tai, G., Zhou, Y. Galectin-3 N-terminal tail prolines modulate cell activity and glycan-mediated oligomerization/phase separation. Proc. Natl. Acad. Sci. USA 2021, 118 e2021074118. [CrossRef]
- Si, Y., Yao, Y., Ayala, G., Li, X., Han, Q., Zhang, W., Tai, G., Mayo, K.H., Zhou, Y., Su, J. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta 2021, 1865, e129755. [CrossRef]
- Si, Y., Li, Y., Yang, T., Li, X., Ayala, G.J., Mayo, K.H., Tai, G., Su, J., Zhou, Y. Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J. 2021, 288, 1041-1055. [CrossRef]
- Li, X., Yao, Y., Liu, T., Gu, K., Han, Q., Zhang, W., Ayala, G.J., Liu, Y., Na, H., Yu, J., Zhang, F., Mayo, K.H., Su, J. Actin binding to galectin-13/placental protein-13 occurs independently of the galectin canonical ligand binding site. Glycobiology 2021, 31, 1219-1229.
- Kadoya, T., Horie H. Structural and functional studies of galectin-1: a novel axonal regeneration-promoting activity for oxidized galectin-1. Curr. Drug Targets. 2005, 6, 375-383. [CrossRef]
- Hirabayashi, J., Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa β-galactoside-binding lectin. J. Biol. Chem. 1991, 266, 23648-23653. [CrossRef]
- Tracey, B.M., Feizi T., Abbott W.M., Carruthers R.A., Green B.N., Lawson A.M. Subunit molecular mass assignment of 14,654 Da to the soluble β-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J. Biol. Chem. 1992, 267, 10342-10347.
- Nesmelova, I.V., Ermakova, E., Daragan, V.A., Pang, M., Menéndez, M., Lagartera, L., Solís, D., Baum, L.G., Mayo, K.H. Lactose binding to galectin-1 modulates structural dynamics, increases conformational entropy, and occurs with apparent negative cooperativity. J. Mol. Biol. 2010, 397, 1209-1230. [CrossRef]
- Ermakova, E., Miller M.C., Nesmelova, I.V., Lopez-Merino, L., Berbís MA, Nesmelov, Y., Lagartera, L., Daragan, V.A., André S., Cañada F.J., Jiménez-Barbero J.,Solis, D., Gabius H.J., Mayo, K.H. Lactose Binding to Human Galectin-7 (p53-induced gene 1) Induces Long-range Effects through the Protein Resulting in Increased Dimer Stability and Evidence for Positive Cooperativity. Glycobiology 2013, 23, 508-523.
- Miller, M.C., Nesmelova, I.V., Daragan, V.A., Ippel, H., Michalak, M., Dregni, A., Kaltner, H., Kopitz, J., Gabius, H.-J., Mayo, K.H. Identification of Pro4 prolyl peptide bond isomerization in human galectin-7 and its impact on structural dynamics and glycan affinity. Biochem. J. 2020, 477, 3147–3165.
- Bourne, Y., Bolgiano B., Liao D. I., Strecker G., Cantau P., Herzberg O., Feizi T. and Cambillau C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nat. Struct. Biol. 1994, 1, 863-870. [CrossRef]
- Nagae, M., Nishi N., Murata T., Usui T., Nakamura T., Wakatsuki S., Kato R. Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition. J. Biol. Chem. 2006, 281, 35884-35893. [CrossRef]
- Miller, M.C., Ribeiro, J.P., Roldós, V., Martín-Santamaría, S., Cañada, F.J., Nesmelova, I.A., André, S., Pang, M., Klyosov, A.A., Baum, L.G., Jiménez-Barbero, J., Gabius, H.-J., Mayo, K.H. Structural aspects of binding of α-linked digalactosides to human galectin-1. Glycobiology 2011, 21,1627-1641. [CrossRef]
- Zhang, Z., Miller, M.C., Xu, X., Zheng, Y., Song, C., Zhang, F., Zhou, Y., Tai, G., Mayo, K.H. NMR-based insight into galectin-3 binding to endothelial cell adhesion molecule CD146: evidence for non-cannonical interactions with the lectin’s CRD b-sandwich F-face. Glycobiology 2019, 29, 608-618.
- Miller, M., Nesmelova, I.V., Klyosov, A., Platt, D., Mayo, K.H. The carbohydrate binding domain on galectin-1 is more extensive for a complex glycan than for simple saccharides: implications for galectin-glycan interactions at the cell surface. Biochem. J. 2009, 421, 211-221. [CrossRef]
- Miller, M., Klyosov, A., Mayo, K.H. The α-galactomannan Davanat binds galectin-1 at asite different from the conventional galectin carbohydrate binding site. Glycobiology 2009, 19, 1034-1045.
- Miller, M.C., Klyosov, A., Mayo, K.H. Structural Features for α-galactomannan binding to galectin-1. Glycobiology 2012, 22, 543-551. [CrossRef]
- Miller, M.C., Ippel, H., Suylen, D., Klyosov, A.A., Traber, P.G., Hackeng, T., Mayo, K.H. Binding of Polysaccharides to Human Galectin-3 at a Non-Canonical Site in its Carbohydrate Recognition Domain. Glycobiology 2016, 26, 88-99.
- Miller, M.C., Zheng, Y., Yan, J., Zhou, Y., Tai, G., Mayo, K.H. Novel polysaccharide binding to the N-terminal tail of galection-3 is likely modulated by proline isomerization. Glycobiology 2017, 27, 1038-1051.
- Zhang, T., Miller, M.C., Lan, Y., Zheng, Y., Liu, F., Zhao, D., Su, J., Mayo, K.H., Tai, G., Zhou, Y. Macromolecular assemblies of complex polysaccharides with Galectin-3 and their synergestic effects on function. Biochem. J. 2017, 474, 3849-3868.
- Zheng, Y., Su, J., Miller, M.C., Zhang, T., Mayzel, M., Tai, G., Mayo, K.H., Zhou, Y. Topsy-turvy binding of negatively-charged homogalacturonan oligosaccharides to galectin-3. Glycobiology 2021, 31, 341-350. [CrossRef]
- Miller, M.C., Cai, C., Wichapong, K., Bhaduri, S., Pohl, N.L.B., Linhardt, R.J., Gabius, H.-J., Mayo, K.H. Structural insight into the binding of human galectins to corneal keratan sulfate, its desulfated form and related saccharides. Sci. Reports 2020, 10, 15708-15713. [CrossRef]
- Hirabayashi, J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W.E., Yagi F., Kasai K. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta. 2002, 1572, 232-254. [CrossRef]
- Yang, R.Y., Hsu D.K., Liu, F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737-6742. [CrossRef]
- Massa, S.M., Cooper D.N., Leffler H., Barondes S.H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 1993, 32, 260-267. [CrossRef]
- Kuklinski, S., Probstmeier R. Homophilic binding properties of galectin-3: involvement of the carbohydrate recognition domain. J. Neurochem. 1998, 70, 814-823. [CrossRef]
- Ahmad, N., Gabius H.-J., Andre S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F. and Brewer C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841-10847. [CrossRef]
- Rini, J.M. Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 551-577.
- Gitt, M.A., Wiser, M.F., Leffler, H., Herrmann, J., Xia, Y.R., Massa, S.M., Cooper D.N., Lusis A.J., Barondes, S.H. Sequence and mapping of galectin-5, a β-galactoside-binding lectin, found in rat erythrocytes. J. Biol. Chem. 1995, 270, 5032-5038. [CrossRef]
- Madsen, P., Rasmussen H.H., Flint T., Gromov P., Kruse T.A., Honore B., Vorum H., Celis J.E. Cloning, expression, and chromosome mapping of human galectin-7. J. Biol. Chem. 1995, 270, 5823-5829. [CrossRef]
- Leonidas, D.D., Vatzaki E.H., Vorum H., Celis J.E., Madsen P., Acharya K.R. Structural basis for the recognition of carbohydrates by human galectin-7. Biochemistry 1998, 37, 13930-13940. [CrossRef]
- Leonidas, D.D., Elbert, B.L., Zhou, Z., Leffler, H., Ackerman, S.J., Acharya K.R. Crystal structure of human Charcot-Leyden crystal protein, an eosinophil lysophospholipase, identifies it as a new member of the carbohydrate-binding family of galectins. Structure 1995, 3, 1379-1393. [CrossRef]
- Miura, T., Takahashi M., Horie H., Kurushima H., Tsuchimoto D., Sakumi K., Nakabeppu Y. Galectin-1β, a natural monomeric form of galectin-1 lacking its six amino-terminal residues promotes axonal regeneration but not cell death. Cell Death Differ. 2004, 11, 1076-1083. [CrossRef]
- Brewer, F.C. Binding and cross-linking properties of galectins. Biochim Biophys Acta. 2002, 1572, 255-262.
- Thiemann, S., Baum, L.G. The road less traveled: regulation of leukocyte migration across vascular and lymphatic endothelium by galectins. J. Clin. Immunol. 2011, 31, 2–9. [CrossRef]
- Thiemann, S., Baum, L.G. Galectins and immune responses-just how do they do those things they do? Annu. Rev. Immunol. 2016, 34, 243–264.
- Liu, F.T., Yang, R.Y., Hsu, D.K. Galectins in acute and chronic inflammation. Ann. N.Y. Acad. Sci. 2012, 1253, 80–91. [CrossRef]
- Kaltner, H., Toegel, S., Caballero, G.G., Manning, J.C., Ledeen, R.W., Gabius, H.-J. Galectins: their network and roles in immunity/tumor growth control. Histochem. Cell. Biol. 2017, 147, 239–256. [CrossRef]
- Demetriou, M., Nabi, I.R., Dennis, J.W. Galectins as adaptors: linking glycosylation and metabolism with extracellular cues. Trends Glycosci. Glycotechnol. 2018, 30, 167–177. [CrossRef]
- Kasai, K. Galectins: quadruple-faced proteins. Trends Glycosci. Glycotechnol. 2018, 30, 221– 223.
- de Jong, C., Gabius, H.-J., Baron, W. The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis. Cell. Mol. Life Sci. 2020, 77, 1289–1317. [CrossRef]
- Caballero, G., Kaltner, H., Kutzner, T.J., Ludwig, A.K., Manning, J.C., Schmidt, S., Sinowatz, F., Gabius, H.-J. How galectins have become multifunctional proteins. Histol. Histopathol 2020, 35, 509–539.
- Hong, M.-H., Weng, I.C., Liu, F.-T. Galectins as intracellular regulators of cellular responses through the detection of damaged endocytic vesicles. Trends Glycosci. Glycotechnol. 2018, 30, 179–184. [CrossRef]
- Sato, S. Cytosolic galectins and their release and roles as carbohydrate-binding proteins in host–pathogen interaction. Trends Glycosci. Glycotechnol. 2018, 30, 199–209. [CrossRef]
- Leffler, H. Galectins structure and function--a synopsis. Results Probl. Cell. Differ. 2001, 33, 57-83.
- Liu, F.T., Rabinovich G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29-41. [CrossRef]
- Neri, D., Bicknell R. Tumour vascular targeting. Nat. Rev. Cancer 2005, 5, 436-446. [CrossRef]
- Perillo, N.L., Pace K.E., Seilhamer J.J., Baum L. G. Apoptosis of T cells mediated by galectin- 1. Nature 1995, 378, 736-739.
- Cherayil, B.J., Weiner S.J., Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 1989, 170, 1959-1972. [CrossRef]
- Gil, C.D., La, M., Perretti, M., Oliani, S.M. Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell. Biol. Int. 2006, 30, 338-344. [CrossRef]
- Karlsson, A., Follin P., Leffler H., Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 1998, 91, 3430-3438.
- Zhu, L.L., Zhao, X.Q., Jiang, C., You, Y., Chen, X.P., Jiang, Y.Y., Jia, X.M., Lin, X. C-type lectin receptors dectin-3 and dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013, 39, 324-334. [CrossRef]
- Ostrop, J., Lang, R. Contact, collaboration, and conflict: signal integration of Syk-coupled C- type lectin receptors. J. Immunol. 2017, 198, 1403-1414.
- Vértesy, S., Michalak, M., Miller, M.C., Schnölzer, M., André, S., Kopitz, J., Mayo, K.H., Gabius, H.-J. Structural significance of galectin design: impairment of homodimer stability by linker insertion and partial reversion by ligand presence. Prot. Eng. Des. Sel. 2015, 28, 199-210. [CrossRef]
- Zhang, S., Moussodia, R.O., Murzeau, C., Sun, H.J., Klein, M.L., Vértesy, S., André, S., Roy, R., Gabius, H.-J., Percec, V. Dissecting molecular aspects of cell interactions using glycodendrimersomes with programmable glycan presentation and engineered human lectins. Angew. Chem. Int. Ed. 2015, 54, 4036-4040. [CrossRef]
- Papaspyridonos, M., McNeill, E., de Bono, J.P., Smith, A., Burnand, K.G., Channon, K.M., Greaves, D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 433–440. [CrossRef]
- Masamune, A., Satoh, M., Hirabayashi, J., Kasai, K., Satoh, K., Shimosegawa, T. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 729-736. [CrossRef]
- Qian, D., Lu, Z., Xu, Q., Wu, P., Tian, L., Zhao, L., Cai, B., Yin, J., Wu, Y., Staveley-O’Carroll, K.F., Jiang, K., Miao, Y., Li, G. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett. 2017, 397, 43–51. [CrossRef]
- Filer, A., Bik, M., Parsonage, G.N., Fitton, J., Trebilcock, E., Howlett, K., Cook, M., Raza, K., Simmons, D.L., Thomas, A.M., Salmon, M., Scheel-Toellner, D., Lord, J.M., Rabinovich, G.A., Buckley, C.D. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheumatol. 2009, 60, 1604–1614. [CrossRef]
- Toegel, S., Weinmann, D., Andre, S., Walzer, S.M., Bilban, M., Schmidt, S., Chiari, C., Windhager, R., Krall, C., Bennani-Baiti, I.M., Gabius, H.-J. Galectin-1 couples glycobiology to inflammation in osteoarthritis through the activation of an NF-κB-regulated gene network. J. Immunol. 2016, 196, 1910–1921.
- Weinmann, D., Kenn, M., Schmidt, S., Schmidt, K., Walzer, S.M., Kubista, B., Windhager, R., Schreiner, W., Toegel, S., Gabius, H.-J. Galectin-8 induces functional disease markers in human osteoarthritis and cooperates with galectins-1 and -3. Cell. Mol. Life Sci. 2018, 75, 4187–4205. [CrossRef]
- Chen, C., Duckworth, C.A., Fu, B., Pritchard, D.M., Rhodes, J.M., Yu, L.G. Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br. J. Cancer 2014, 110, 741–752. [CrossRef]
- Cattaneo, V., Tribulatti, M.V., Carabelli, J., Carestia, A., Schattner, M., Campetella, O. Galectin-8 elicits pro-inflammatory activities in the endothelium. Glycobiology 2014, 24, 966–973. [CrossRef]
- Carabelli, J., Quattrocchi, V., D’Antuono, A., Zamorano, P., Tribulatti, M.V., Campetella, O. Galectin-8 activates dendritic cells and stimulates antigen-specific immune response elicitation. J. Leukoc. Biol. 2017, 102, 1237–1247. [CrossRef]
- Nesmelova, I.V., Pang, M., Baum, L.G., Mayo, K.H. 1H, 13C, and 15N backbone and side-chain chemical shift assignments for the 29 kDa human galectin-1 protein dimer. Biomol. NMR Assign. 2008, 2, 203-205. [CrossRef]
- Ippel, H., Miller, M.C., Berbis, M.A., Suylen, D., André, S., Hackeng, T.M., Cañada, F.J., Weber, C., Gabius, H.-J., Jiménez-Barbero, J., Mayo, K.H. 1H, 13C, and 15N backbone and side-chain chemical shift assignments for the 36 proline-containing, full length 29 kDa human chimera-type galectin-3. Biomol. NMR Assign. 2015, 9, 59-63. [CrossRef]
- Crump, M.P., Gong, J.H., Loetscher, P., Rajarathnam, K., Amara, A., Arenzana-Seisdedos, F., Virelizier. J.L., Baggiolini, M., Sykes, B.D., Clark-Lewis, I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997, 16, 6996–7007. [CrossRef]
- Gozansky, E.K., Louis, J.M., Caffrey, M., Clore, G.M. Mapping the binding of the N-terminal extracellular tail of the CXCR4 receptor to stromal cell-derived factor-1α. J. Mol. Biol. 2005, 345, 651–658. [CrossRef]
- Hsu, D.K., Zuberi, R.I., Liu, F.T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 1992, 267, 14167–14174. [CrossRef]
- Herrmann, J., Turck, C.W., Atchison, R.E., Huflejt, M.E., Poulter, L., Gitt, M.A., Burlingame, A.L., Barondes, S.H., Leffler, H. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-tyrosine-rich sequence with bacterial and tissue collagenase. J. Biol. Chem. 1993, 268, 26704–26711. [CrossRef]
- Ochieng, J., Fridman, R., Nangia-Makker, P., Kleiner, D.E., Liotta, L.A., Stetler-Stevenson, W.G., Raz, A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 1994, 33, 14109–14114. [CrossRef]
- Rajarathnam, K., Desai, U.R. Structural insights into how proteoglycans determine chemokine–CXCR1/CXCR2 interactions: progress and challenges. Front. Immunol. 2020, 11, 660-671.
- Talaga, M.L., Fan, N., Fueri, A.L., Brown, R.K., Bandyopadhyay, P., Dam, T.K. Multitasking human lectin galectin-3 interacts with sulfated glycosaminoglycans and chondroitin sulfate proteoglycans. Biochemistry 2016, 55, 4541–4551. [CrossRef]
- Peranzoni, E., Lemoine, J., Vimeux, L., Feuillet, V., Barrin, S., Kantari-Mimoun, C., Bercovici, N., Guerin, M., Biton, J., Ouakrim, H., Regnier, F., Lupo, A., Alifano, M., Damotte, D., Donnadieu, E. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, 4041–4050. [CrossRef]
- Gordon-Alonso, M., Hirsch, T., Wildmann, C., van der Bruggen, P. Galectin-3 captures interferon-γ in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793-798.
- von Hundelshausen, P., Wichapong, K., Gabius, H.-J., Mayo, K.H. The marriage of chemokines and galectins as functional heterodimers. Cell. Mol. Life Sci. 2021, 78, 8073–8095. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



