Submitted:
12 August 2023
Posted:
15 August 2023
You are already at the latest version
Abstract
Keywords:
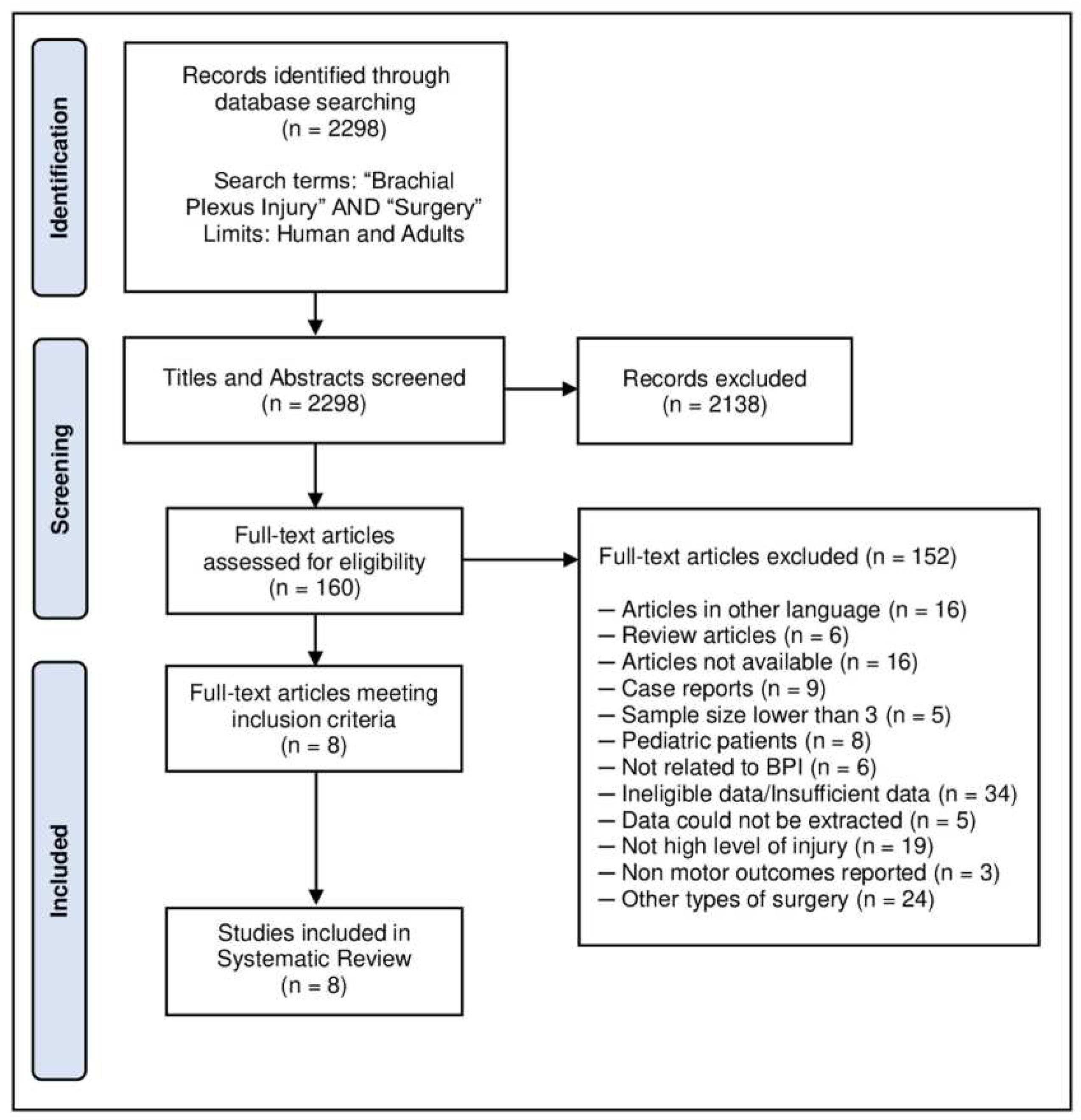
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Limthongthang R, Bachoura A, Songcharoen P, Osterman AL. Adult brachial plexus injury: evaluation and management. Orthop Clin North Am 2013;44:591-603. [CrossRef]
- Andrisevic E, Taniguchi M, Partington MD, Agel J, Van Heest AE. Neurolysis alone as the treatment for neuroma-in-continuity with more than 50% conduction in infants with upper trunk brachial plexus birth palsy. J Neurosurg Pediatr 2014;132:229-237. [CrossRef]
- Millesi H, Rath T, Reihsner R, Zoch G. Microsurgical neurolysis: its anatomical and physiological basis and its classification. Microsurgery. 1993;14:430-439. [CrossRef]
- Clarke HM, Al-Qattan MM, Curtis CG, Zuker RM. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg 1997;97:974-984. [CrossRef]
- Armas-Salazar A, García-Jerónimo AI, Villegas-López FA, Navarro-Olvera JL, Carrillo-Ruiz JD. Clinical outcomes report in different brachial plexus injury surgeries: a systematic review. Neurosurg Rev 2022;45:411-419. [CrossRef]
- Morgan R, Elliot I, Banala V, Dy C, Harris B, Ouellette EA. Pain Relief after Surgical Decompression of the Distal Brachial Plexus. J Brachial Plex Peripher Nerve Inj 2020;15:e22-e32. [CrossRef]
- Armas-Salazar A. Téllez-León N. García-Jerónimo AI. Villegas-López FA. Navarro-Olvera JL. Carrillo-Ruiz JD (2022) Neuropathic pain relief after surgical neurolysis in patients with traumatic brachial plexus injuries: A preliminary report. Pain Res Manag (Accepted).
- Midha R, Grochmal J. Surgery for nerve injury: current and future perspectives. J Neurosurg 2019;130:675-685. [CrossRef]
- Li GY, Xue MQ, Wang JW, Zeng XY, Qin J, Sha K. Traumatic brachial plexus injury: a study of 510 surgical cases from multicenter services in Guangxi, China. Acta Neurochir (Wien) 2019;161:899-906. [CrossRef]
- Rempel DM, Diao E. Entrapment neuropathies: pathophysiology and pathogenesis. J Electromyogr Kinesiol 2004;14:71-75. [CrossRef]
- James MA. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am 2007;32:154-156. https://doi.org/10.1016/j.jhsa.2006.11.008. [CrossRef]
- McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement [published correction appears in JAMA. 2019 Nov 26;322(20):2026]. JAMA 2018;319:388-396. [CrossRef]
- Tsao B. The electrodiagnosis of cervical and lumbosacral radiculopathy. Neurol Clin 2007;25:473-494. [CrossRef]
- Altaf F, Mannan K, Bharania P, Sewell MD, Di Mascio L, Sinisi M. Severe brachial plexus injuries in rugby. Injury 2012;43:272-273. [CrossRef]
- Dubuisson AS, Kline DG. Brachial plexus injury: a survey of 100 consecutive cases from a single service. Neurosurgery 2002;51:673-683.
- Gousheh J. The treatment of war injuries of the brachial plexus. J Hand Surg Am 1995;20:S68-S76. https://doi.org/10.1016/s0363-5023(95)80173-1. [CrossRef]
- Gutkowska O, Martynkiewicz J, Mizia S, Bąk M, Gosk J. Results of Operative Treatment of Brachial Plexus Injury Resulting from Shoulder Dislocation: A Study with A Long-Term Follow-Up. World Neurosurg 2017;105:623-631. [CrossRef]
- Kim DH, Han K, Tiel RL, Murovic JA, Kline DG. Surgical outcomes of 654 ulnar nerve lesions. J Neurosurg 2003;98:993-1004. [CrossRef]
- Stewart MP, Birch R. Penetrating missile injuries of the brachial plexus. J Bone Joint Surg Br 2001;83:517-524. [CrossRef]
- Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci 2011;31:5325-5334. [CrossRef]
- Martin E, Senders JT, DiRisio AC, Smith TR, Broekman MLD. Timing of surgery in traumatic brachial plexus injury: a systematic review [published online ahead of print, 2018 ]. J Neurosurg 2018;1-13. [CrossRef]
- Čebron U, Mayer JA, Lu C, Daigeler A, Prahm C, Kolbenschlag J. Treatment Trends of Adult Brachial Plexus Injury: A Bibliometric Analysis. Plast Reconstr Surg Glob Open 2021;9:e3803. [CrossRef]
- Warade AC, Jha AK, Pattankar S, Desai K. Radiation-induced brachial plexus neuropathy: A review. Neurol India 2019;67(Supplement):S47-S52. [CrossRef]
- Baxter B, Gillingwater TH, Parson SH. Rapid loss of motor nerve terminals following hypoxia-reperfusion injury occurs via mechanisms distinct from classic Wallerian degeneration. J Anat 2008;212:827-835. [CrossRef]
- Giuffre JL, Kakar S Bishop AT, Spinner RJ, Shin AY. Current Concepts of the Treatment of Adult Brachial Plexus Injuries. American Society for Surgery of the Hand, 2010;35A:678–688. [CrossRef]
- Hems TEJ. Timing of surgical reconstruction for closed traumatic injury to the supraclavicular brachial plexus. Abstract. The Journal of Hand Surgery (European Volume), 2015;40E:568-572. [CrossRef]
- Noland SS, Bishop AT, Spinner, RJ, Shin AY. Adult Traumatic Brachial Plexus Injuries. Journal of the American Academy of Orthopaedic Surgeons, 2019;27:705-716. [CrossRef]
- Doğan M, Koçak M, Onursal Kılınç Ö, Ayvat F, Sütçü G, Ayvat E. et al. Functional range of motion in the upper extremity and trunk joints: Nine functional everyday tasks with inertial sensors. Gait Posture 2019;70:141-147. [CrossRef]
- Lea RD, Gerhardt JJ. Range-of-motion measurements. J Bone Joint Surg Am 1995;77:784-798. [CrossRef]
- Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum 2009;61:623-632. [CrossRef]
- Tsai YJ, Su FC, Hsiao CK, Tu YK. Comparison of objective muscle strength in C5-C6 and C5-C7 brachial plexus injury patients after double nerve transfer. Microsurgery 2015;35:107-114. [CrossRef]
- Martins RS, Siqueira MG, Heise CO, Foroni L, Teixeira MJ. A prospective study comparing single and double fascicular transfer to restore elbow flexion after brachial plexus injury. Neurosurgery 2013;72:709-715. [CrossRef]
- Kitajima I, Doi K, Hattori Y, Takka S, Estrella E. Evaluation of quality of life brachial plexus injury patients after reconstructive surgery. Hand Surgery, 2006;11:103-107. [CrossRef]
- West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol 1997;29:201-210. [CrossRef]
- Brophy RH, Wolfe SW. Planning brachial plexus surgery: treatment options and priorities. Hand Clin 2005;21:47-54. [CrossRef]

| BMRC motor grading scale |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patient | Gender | Age at time of surgery | Etiology (mechanism) |
Location of injury | Side affected | Interval Injury-Surgery (mos) |
Follow-up (mos) |
Pre-op | Post-op |
| 1 | Female | 29 | Post-Traumatic (VT) | C5-C6 | Left | 12 | 24 | 2 | 4 |
| 2 | Female | 62 | Radiotherapy | C5-T1 | Right | 5 | 48 | 2 | 2 |
| 3 | Male | 43 | Post-Traumatic (VT) | C5-C6-C7 | Left | 19 | 108 | 2 | 4 |
| 4 | Female | 21 | Outlet thoracic Sx. | C7-C8-T1 | Left | 14 | 12 | 1 | 4 |
| 5 | Male | 20 | Post-Traumatic (VT) | C5-T1 | Right | 6 | 60 | 0 | 4 |
| 6 | Male | 41 | Tumor (E) | C5-T1 | Left | 4 | 60 | 1 | 4 |
| 7 | Male | 29 | Outlet thoracic Sx. | C7-C8-T1 | Right | 10 | 12 | 3 | 5 |
| 8 | Male | 46 | Outlet thoracic Sx. | C7-C8-T1 | Right | 16 | 12 | 3 | 3 |
| 9 | Female | 28 | Outlet thoracic Sx. | C7-C8-T1 | Right | 11 | 36 | 3 | 5 |
| 10 | Male | 35 | Post-Traumatic (VT) | C5-T1 | Right | 7 | 156 | 0 | 0 |
| 11 | Female | 22 | Post-Traumatic (VT) | C5-T1 | Right | 9 | 18 | 2 | 4 |
| 12 | Male | 22 | Post-Traumatic (IT) | C5-C6 | Right | 8 | 3 | 3 | 2 |
| 13 | Male | 21 | Post-Traumatic (VT) | C5-C6-C7 | Right | 20 | 12 | 2 | 2 |
| 14 | Female | 56 | Tumor (A) | C5-C6 | Left | 5 | 48 | 4 | 5 |
| 15 | Female | 52 | Post-Traumatic (VT) | C5-C6 | Left | 6 | 48 | 4 | 4 |
| 16 | Male | 32 | Post-Traumatic (VT) | C5-C6 | Left | 6 | 72 | 2 | 4 |
| 17 | Male | 26 | Post-Traumatic (VT) | C5-C6-C7 | Right | 14 | 24 | 2 | 4 |
| 18 | Male | 28 | Post-Traumatic (SI) | C5-C6 | Right | 8 | 2 | 3 | 2 |
| *Mean ± SD | 34.06 ± 13.01 | 10 ± 4.89 | 41.94 ± 39.84 | 2.17 ± 1.15 | 3.44 ± 1.34 | ||||
| Median (IQR) | 29 (21.75) | 8.5 (8) | 30 (48) | 2 (1.25) | 4 (2) | ||||
| Etiology (group) | Global | Traumatic | Miscellaneous* |
|---|---|---|---|
| Sample size (n) | 18 | 11 | 7 |
| Demographics | |||
| Age (yrs) | 34.06 ± 13.01 | 30 ± 10.05 | 40.42 ± 15.28 |
| Gender (♂) | 61.11% | 72.7% | 42.85% |
| Location | |||
| C5-C6 | 6 (33.3%) | 5 (45.46%) | 1 (14.28%) |
| C5-C6-C7 | 3 (16.7%) | 3 (27.27%) | 0 |
| C8-T1 | 4 (22.2%) | 0 | 4 (57.15%) |
| C5-T1 (complete) | 5 (27.8% | 3 (27.27%) | 2 (28.57%) |
| Side affected | |||
| Left | 7 (38.9%) | 4 (36.36%) | 3 (42.85%) |
| Right | 11 (61.1%) | 7 (63.64%) | 4 (57.15%) |
| Interval Injury-Surgery (mos) | 10 4.89 | 10.45 ± 5.14 | 9.28 ± 4.75 |
| Follow-up (mos) | 41.94 39.84 | 47.90 ± 48.44 | 32.57 ± 20.45 |
| Clinical outcomes | |||
| BMRC | |||
| Pre-operative status | 2.17 ± 1.15 | 2 ± 1.18 | 2.42 ± 1.13 |
| Post-operative status | 3.44 ± 1.34 | 3.09 ± 1.37 | 4 ± 1.15 |
| Statistical analysis | |||
| p-valuea | 0.003 | 0.045 | 0.017 |
| Effect size (d)b | 0.913 | 0.703 | 0.977 |
| Delta (∆)c | ↑ 58.52% | ↑ 35.27% | ↑ 39.5% |
| Author’s & year (group/subgroups) |
No. of subjects | Study design | Location of Compromise/Injury | Interval injury-surgery |
BMRC motor grading scale | Proportion of motor recovery (≥M3) |
Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Post-operative | |||||||||
| <M3 | ≥M3 | <M3 | ≥M3 | |||||||
| Current study | 18 | CS | C5-C6: 33.3%, C5-C7: 16.6%, C7-T1: 22.3%, C5-T1: 27.8% |
10 (4.89) mos b | 11 | 7 | 5 | 13 | 72.2% | 41.9 (39.8) b |
| Morgan R. et al. (2020) [23] |
21 | RCR | Distal Brachial Plexus (cord branches at the level of bicipital groove). | 11 mos c | 3 | 18 | 1 | 20 | 95.2% | 10 (6-85) mos c |
| Guang-Yao Li. et al. (2019) [17] |
73 a | CR | C5: 19.18%, C5-C7: 17.81%, C5-T1: 35.62%, Cord branches: 19.18%, Supra & infraclavicular: 8.22% | NM | 73 | NM | 15 | 58 | 79.4% | 47.95 (25–68) mos c |
| Gutkowska O. et al. (2017) [13] |
33 a | RCS | Cord branches at high level (proximal third of upper extremity) |
9.03 mos b | 33 | NM | 21 | 11 | 33.3% | 5.1 yrs b |
| Altaf F. (2012) et al. [1] |
6 a | STI | C5-T1: 38.46%, C5-C6: 23.07%, C5-C7: 38.46% | 12 days to 4.5 mos | NM | NM | NM | 6 | 100% | between 4 and 6 mos |
| Kim D. et al. (2003) [15] |
20 a | RCR | High (proximal third of upper extremity) ulnar nerve injuries | NS | 20 | NM | 4 | 16 | 80% | NM |
| Dubuisson A. et al. (2002) [10] |
11 a | RCR | C5-C6: 36.36%, C5-T1: 18.18%, Cord branches: 45.45% | 7 mos c | 11 | NM | 2 | 9 | 81.8% | 3 yrs of more |
| Stewart M. et al. (2000) [26] |
14 a | CS | C5-C6: 14.28%, C7: 7.14%, C5-T1: 21.42%, Cord branch: 57.14% | 7.35 mos b | NM | NM | 8 | 6 | 42.8% | at least two yrs |
| Gousheh J. et al. (1995) [12] |
44 a | U | C5: 27.27%, C6: 34.09%, C7: 18.18%, C8: 11.36%, C5-T1: 9.09%. | 3 wks to 4 mos | 44 | NM | 1 | 43 | 97.7% | 5 yrs or more |
| Total/Mean | 240 | 75.82% | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





