1. Introduction
Nails, once considered a necessary evil, have become a fashion statement today. The need to maintain nail-hygiene is paramount to one’s overall health and well-being. Besides problems of etiquette, unkempt and unwashed nails can lead to a host of medical problems and adversely impact an individual’s health. Aesthetic need for healthy-looking nails has made the consumer aware and conscious of nail hygiene and the harmful effects of numerous nail disorders. Corpus unguis, commonly known as the nail plate, is considered to be a hydrophilic gel matrix [
3]. It can be macroscopically segmented into three layers: dorsal, intermediate, and ventral in the ratio 3: 5: [
2], respectively [
3]. The nail is comprised of 9-35% water, 0.1-1% lipid, and 10.60% disulfide linkage [
3]. It has a maximum swelling capacity of 25% and a water loss rate of 1.94 mg/cm2 /h3
The fungal infection, Onychomycosis, is a superficial filamentous infection which falls under the category of dermatophytosis. Dermatophytes account for causing around 90-95% infections, and 50-80% of incidences of onychomycosis are attributed to dermatophytes [
4,
5,
6]. The prevalence of onychomycosis is 50% in a population of patients suffering from nail disorders [
8]. The destructive invasive mode of action adopted by fungi, target the inhibition of the human biosynthetic pathway [
9]. Abnormal nail thickening and a substantial nail plate discoloration are manifestations of Onychomycosis [
10]. Age is an inevitable predisposing factor for Onychomycosis, however conditions such as diabetes, HIV infection, immunosuppression, presence of athlete’s foot, obesity, smoking, outdoor recreational activities, and tight clothing also prevail [
6,
9]. The lion's share of the Onychomycosis is caused by Trichophyton. Rubrum [
6,
9]. This specific strain requires a concentration of 0.19μg/mL of Itraconazole to inhibit 90% of the fungal organisms [
11].
Itraconazole is known to be an efficient broad-spectrum antifungal agent whose mechanism of action involves impeding CYP450 enzymes that play a critical role in the production of ergosterol, a principal component of a fungal cell wall [
9,
12].
Penetration enhancers, namely, Papain, Salicylic acid, and Urea are keratolytic agents that are utilized as chemical methods to enhance the transungual diffusion of an antifungal agent through the intimidating nail barrier [
4,
6,
14]. Nail lacquers are an upcoming drug delivery system intended for transungual mode of action. They not only have the potential to effectively treat nail disorders but also conceal the repulsive appearance of the affected nail plate.
Previous studies conducted suggested that Sally Hansen Ultimate Shield Base & Topcoat (reference) was a compatible vehicle to accommodate salicylic acid, urea, and papain in combination with Itraconazole [
5,
8,
15,
16]. The drying time was set at 5 minutes by the text, and the pH criteria were targeted to be within a range of 5-6 [
5,
8,
15,
16].
Problem: Systemic therapies for onychomycosis have shown to have significant harmful effects on the liver [
6]. Associated drawbacks are large dose requirements, high recurrence rates, drug interactions, osmotic diarrhea, and expense [
17].
Hypothesis: Inclusion of Papain, Salicylic acid, and Urea in an Itraconazole (1%w/w) nail lacquer will have the highest transungual penetration on application to the nail plate.
2. Materials and Methods
Itraconazole Hydrochloride Salt preparation [
18]: The salt of Itraconazole was chemically synthesized by an acid addition reaction to enhance the physicochemical properties of the antifungal agent18. The first step involved in the formulation of the hydrochloride salt of Itraconazole was the addition of 10.8mL of Dichloromethane to a measured amount of 1.20898g (1.71331mmol) Itraconazole (MW: 705.641g/mol) in an amber-colored rotary evaporator flask. The flask was stoppered and swirled to allow solubilization of Itraconazole in the solvent18. The second step included the addition of 0.33mL (13.70648mmol) concentrated hydrochloric acid (36%) (MW: 36.47g/mol) to the solution [
18]. The amber-colored rotatory flask containing the suspension was clamped onto a stand with its bottom touching the surface of the water bath. The third step involved heating the suspension under reflux at 50 degrees Celsius for 10minutes at a speed of 100rpm. On completion of the time duration, a vacuum was applied, and the rotating speed of the flask was reduced to 85rpm. Finally, the precipitate of the salt was formed, and the rotatory evaporator flask was dried in a vacuum oven at 60 degrees Celsius for 1 hour. The Itraconazole hydrochloride salt chemically synthesized was transferred into an amber-colored glass bottle, sealed and labeled well.
Quantification of Dichloromethane [
20,
23]: A gas chromatographic system was used for the quantification of Dichloromethane. The column flow was set at 1.6 mL/min with a front inlet pressure of 16.72 and signal valve of 9.6. The parameters for the front inlet and outlet temperature were set at 140 and 250 degrees Celsius, respectively. The oven temperature was 40 degrees Celsius, and the overall run time was 40 minutes. Standards were prepared of concentrations, 6ppm, 60ppm, and 600ppm.
Quantification of Itraconazole Hydrochloride synthesized (λ_max=264) [
19]: A high-performance liquid chromatography system was used. An isocratic method was used for which column, column temperature, and flow rate were C18 (150 x 4.6mm, 3µ), 40 degrees Celsius, and 1mL/min, respectively. The mobile phase composition employed was 0.05% Trifluoroacetic acid in water for mobile phase A and 0.05% Trifluoroacetic acid in acetonitrile for mobile phase B (50% mobile phase A and 50% mobile phase B) Standards of concentrations 0.05mg/mL, 0.1mg/mL and 0.5mg/mL were prepared and samples of itraconazole and itraconazole hydrochloride were prepared by diluting 1mg in 10mL of diluent (0.05% TFA in 50:50 ACN: Water) individually. The standards and samples had an injection volume of 10µL.
Quantification of water content in drug synthesized: A mass of itraconazole hydrochloride (35.4720 mg) was measured for water content over a temperature ramp of 45 to 75 degrees Celsius.
Formulation protocol
Table 1.
Design Of Experiment to aid optimization using JMP®.
Table 1.
Design Of Experiment to aid optimization using JMP®.
| Run |
Urea |
Pain |
Salicylic acid |
Drug extracted |
| 1 |
2.8 |
5 |
0 |
- |
| 2 |
2.8 |
0 |
5 |
- |
| 3 |
0 |
5 |
5 |
- |
| 4 |
2.8 |
5 |
5 |
- |
| 5 |
2.8 |
0 |
5 |
- |
| 6 |
0 |
5 |
0 |
- |
| 7 |
2.8 |
5 |
0 |
- |
| 8 |
0 |
0 |
0 |
- |
| 9 |
0 |
0 |
0 |
- |
| 10 |
2.8 |
0 |
0 |
- |
| 11 |
0 |
0 |
5 |
- |
| 12 |
0 |
5 |
5 |
- |
Optimized nail lacquer formulation
Table 2.
Optimized nail lacquer formulation.
Table 2.
Optimized nail lacquer formulation.
| Ingredient |
Theoretical Quantity |
| Itraconazole hydrochloride |
1 %w/w |
| Urea |
2.8 %w/w |
| Papain |
5 %w/w |
| Salicylic acid |
5 %w/w |
| Isopropyl alcohol: Water (50:50) |
10 %v/w |
| Glycerin |
13.2 %v/w |
| Ethanol pure |
14 % v/w |
| Water |
14 % v/w |
| Sally Hansen Ultimate Shield Top Coat |
Qs 100 %w/w |
To formulate the optimized 1 % w/w Itraconazole lacquer, a measured amount of glycerin was added to water in a glass vial (Vial 1) and vortexed. A weighed quantity of Urea was added to the water-glycerin solution and mixed well. Papain was accurately weighed and added to the solution and vortexed gently to ensure the solution was homogenous. In another glass vial (Vial 2), a weighed quantity of Salicylic acid was solubilized in pure ethanol. To this solution, previously solubilized Itraconazole hydrochloride hydroalcoholic solution (50:50, Isopropanol: Water) was added and sonicated until homogenous. The theoretical amount of Sally Hansen Ultimate Shield Top Coat (vehicle) was divided equally and incorporated into each of the two glass vials. The final step of formulation involved incorporating Vial 2 into Vial 1, followed by a gentle vortex.
Diffusion study protocol [
19]: The procured nail clippings were altered to the dimensions of the custom-made diffusion cell, which was used as a support. The segment of the nail clipping to be coated with the nail lacquer was marked, and the nails were swiped clean, three times using distilled water. The nail clippings were then patted dry and weighed individually on the analytical weighing balance. The cleaned nails were aligned (dorsal side upwards) onto the custom-made diffusion cell, following which three coats of the formulated lacquers were applied.
The nails treated with lacquer were set aside for five days following which the nails were swiped clean, patted dry, and weighed. The drug extraction procedure involved trituration of the nail clippings using a mortar/pestle and transference into 1mL of diluent (0.05% TFA in 50:50 Acetonitrile: Water. The extracts were agitated for 24 hours in a light protected environment. The supernatant was sampled out and analyzed using the HPLC method elaborated in the quantification of transungual drug penetration section. The cleaning procedure was checked by conducting a drug extraction study at 0th hour on the procured nail clipping.
Quantification of transungual drug penetration (λ_max=264): The column, column temperature, and flow rate were C18 (150 x 4.6mm, 3µ), 40 degrees Celsius, and 1mL/min, respectively. The mobile phase composition employed was 0.05% TFA in water for mobile phase A and 0.05% TFA in ACN for mobile phase B (50% mobile phase A and 50% mobile phase B). Standards of concentrations 0.06µg/mL, 0.1µg/mL and 1µg/mL had an injection volume of 200µL, whereas 100µg/mL had 50µL and 500 µg/mL had 10µL injection volume. The nail extract samples had an injection volume of 200µL.
Quantification of drug content in lacquer formulation: The drug content assay was performed to determine the reasonable amount of drug present in the optimized lacquer formulation. The stipulated acceptable range of Itraconazole hydrochloride contained in the lacquer should not be less than 90% and not exceed 110% of the labeled quantity of the Itraconazole hydrochloride21. A standard calibration curve was constructed using Itraconazole hydrochloride standard solutions of concentrations, 0.01mg/mL(20µL), 0.5mg/mL(10µL), 1mg/mL(5µL) and 5mg/mL(5µL). The diluent used was 0.05% Trifluoroacetic acid in Acetonitrile: Water (50:50). The blank for standard solution was solely the diluent.
The sample solution was prepared by adding 0.35mL of sample in a centrifugal vial containing a 0.45µm membrane filter and spin centrifuging it at a speed of 10,000 rpm for 6 minutes. A second blank, excluding the drug and containing all the three penetration enhancers, was prepared and analyzed for comparison to the sample solutions. In the case of the accelerated stability study, a sample aliquot of 0.5mL was taken and centrifuged before analysis. The sample and standard solution were analyzed using an isocratic HPLC method with column, column flow rate, and temperature set at C18 (150 x 4.6mm, 3µ), 1mL/min, and 40 degrees Celsius respectively. The mobile phase composition employed was 0.05% TFA in water for mobile phase A and 0.05% TFA in ACN for mobile phase B (45% mobile phase A and 55% mobile phase B). The second blank and sample had an injection volume of 0.5µL
Spreadability evaluation22: A drop of the reference, Sally Hansen's ultimate shield topcoat, and sample optimize formulations were poured onto different glass slides. The vertical distance traveled by the drops of the optimized lacquer formulation was measured and compared to the reference lacquer.
Precipitation evaluation: The experiment was conducted to ascertain the absence of any little precipitation in the optimized formulations that might hinder the therapeutic effect. A drop of the optimized lacquers was applied onto different glass slides, and coverslips were placed on it. The glass slides were observed under the microscope at three different magnification powers, 5X, 10X, and 20X.
pH evaluation: The formulated nail lacquers under investigation were tested for their natural pH using a litmus paper previously wetted using distilled water.
Study for accelerated stability: The six optimized nail lacquers subjected to refrigeration temperature (4 degrees Celsius) for three weeks. Quality control tests, namely, drug content, spreadability, check for precipitation, and pH, were conducted to account for the formulations’ stability, shelf life, and consistency when subjected to extreme temperature.
3. Results
3.1. Quantification of residual solvent, Dichloromethane <0.1ppm reported as not detected.
3.2. Quantification of Itraconazole Hydrochloride salt synthesized
Table 3.
Percentage yield results for Itraconazole Hydrochloride synthesized.
Table 3.
Percentage yield results for Itraconazole Hydrochloride synthesized.
| Material |
Reported results |
| Itraconazole Standard (AUC average) |
1950.78 |
| Itraconazole hydrochloride Sample (AUC average) |
1552.84 |
| Itraconazole hydrochloride Sample (Yield) |
9.07 mg |
| Itraconazole hydrochloride Sample (%Yield) |
90.60% |
Salt factor (2 HCl) = 1.10, Weight of Itraconazole = 1.21 g, Weight of Itraconazole 2HCl = 1.51 g. Therefore, the theoretical amount of Itraconazole = 1.33 g. The obtained percent yield was calculated to be 113%.
Optimization of itraconazole hydrochloride nail lacquer (DOE 1-12)
Table 4.
Nail extraction concentrations of DOE runs 1-6.
Table 4.
Nail extraction concentrations of DOE runs 1-6.
| DOE run |
Reported results (mcg/mL) |
| 1 |
0.00 |
| 2 |
0.00 |
| 3 |
0.50 |
| 4 |
0.85 |
| 5 |
0.00 |
| 6 |
0.00 |
Table 5.
Nail extraction concentrations of DOE runs 7-12.
Table 5.
Nail extraction concentrations of DOE runs 7-12.
| DOE run |
Reported results (mcg/mL) |
| 7 |
0.60 |
| 8 |
0.00 |
| 9 |
0.00 |
| 10 |
0.00 |
| 11 |
0.00 |
| 12 |
0.00 |
3.3. Proof of concept study
Table 6.
Nail extraction concentrations of proof of concept run.
Table 6.
Nail extraction concentrations of proof of concept run.
| DOE run |
Drug extracted (mcg/mL) |
Drug/mg of nail |
| 1 |
1.89 |
0.13 |
| 2 |
1.83 |
0.10 |
| 3 |
1.81 |
0.14 |
| 4 |
1.80 |
0.10 |
| 5 |
1.82 |
0.10 |
| 6 |
1.81 |
0.11 |
3.4. Quality control evaluation for optimized nail lacquer
Table 7.
Drug content for the proof of concept for the optimized nail lacquer formulations.
Table 7.
Drug content for the proof of concept for the optimized nail lacquer formulations.
| Lacquer run |
Itraconazole content (%) |
Itraconazole/mg of nail |
| 1 |
101.63 |
50.13 |
| 2 |
101.44 |
51.67 |
| 3 |
94.66 |
50.07 |
| 4 |
101.09 |
50.1 |
| 5 |
98.72 |
50.14 |
| 6 |
98.58 |
50.04 |
Table 8.
Spreadability test results for Sally Hansen Ultimate Shield Base and Topcoat® (7 lengths and 4 widths).
Table 8.
Spreadability test results for Sally Hansen Ultimate Shield Base and Topcoat® (7 lengths and 4 widths).
| Replicate |
Distance (cm) |
| 1 |
4.00 cm |
| 2 |
4.10 cm |
| 3 |
4.03 cm |
Table 9.
Spreadability test results for optimized nail lacquer (7 lengths and 4 widths).
Table 9.
Spreadability test results for optimized nail lacquer (7 lengths and 4 widths).
| Lacquer run |
Distance (cm) |
Match (%) |
| 1 |
4.10 |
101.73 |
| 2 |
4.03 |
100.05 |
| 3 |
4.00 |
99.20 |
| 4 |
4.07 |
100.90 |
| 5 |
4.07 |
100.90 |
| 6 |
4.13 |
102.56 |
Check for precipitation.
The microscope slides observed under 5X, 10X, and 20X magnification power demonstrated the absence of precipitation and crystallization of the drug.
pH test
The pH of all the six proof of concept formulations were consistently within the stipulated range of 5-6.
Accelerated stability study data.
Drug content
Table 10.
Accelerated stability drug content.
Table 10.
Accelerated stability drug content.
| Lacquer run |
Itraconazole content (%) |
Itraconazole/mg of nail |
| 1 |
105.65 |
50.13 |
| 2 |
107.49 |
51.67 |
| 3 |
107.77 |
50.07 |
| 4 |
109.32 |
50.10 |
| 5 |
111.03 |
50.14 |
| 6 |
111.37 |
50.04 |
4. Discussion
The hydrochloride salt of itraconazole was synthesized to aid the incorporation of the various combinations of penetration enhancers, namely, Papain, Salicylic acid, and Urea is an antifungal nail lacquer containing 1 %w/w Itraconazole. The synthesis process of the salt involved the use of dichloromethane, a class II residual solvent with a concentration limit of 600ppm [
20]. Gas chromatography was conducted to verify that the synthesized Itraconazole hydrochloride salt had no traces of residual solvent present [
20]. The chromatograms shown in the appendix for gas chromatography indicate that the dichloromethane present in the synthesized salt was below the limit of quantitation.
A high-performance liquid chromatography (HPLC) method was employed to ascertain the percent recovery of the synthesized Itraconazole hydrochloride. Based on the linearity equation generated by the standard calibration curve for the percent recovery and taking into consideration the salt factor, the yield obtained was 113%. To account for the excess yield, a thermogravimetric analysis (TGA) was conducted to determine the residual water content. The outcome of the test indicated a water content of about 17.07%. The results obtained from the TGA were by the excess percent yield of the Itraconazole hydrochloride salt.
A fractional factorial design was used for formulation optimization in order to statistically evaluate the impact of a combination of different excipients on the experimental outcome of drag uptake within the constraints of a time bound experimental design. The fractional factorial design was used to reduce the number of experiments required to make optimization manageable and efficient. With a focus on key factors, this experimental design helped provide valuable insight into your main effects of multiple factors using a fraction of the full factorial design. In order to corroborate the optimization that was an outcome of the fractional factorial design, a proof-of-concept study was conducted, and the optimized formulation was tested for quality control parameters such as, drug content, drug uptake, pH, spreadability, and accelerated stability.
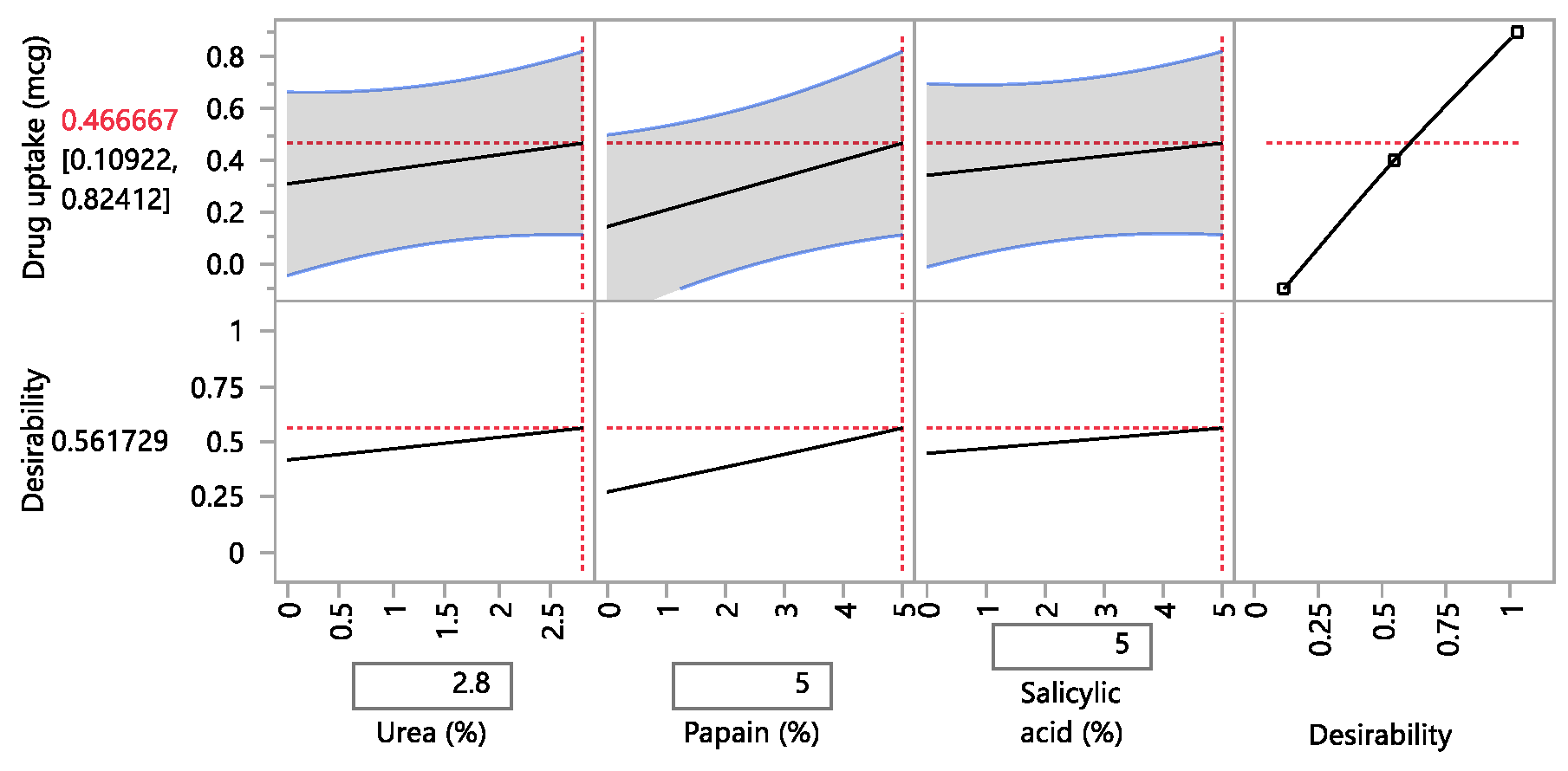
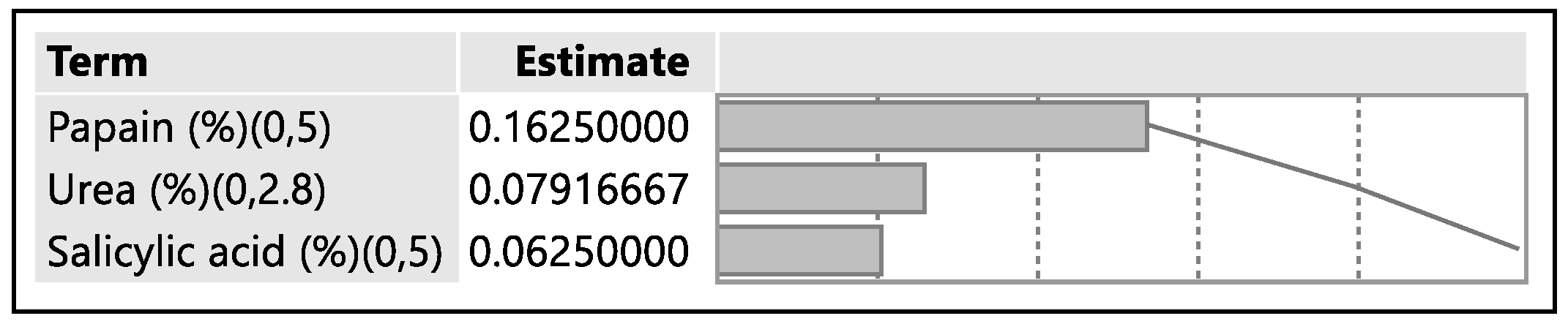
A design of experiment table was built using the JMP software to facilitate the optimization of the antifungal nail lacquer under test. The continuous input variables were the concentrations of the different penetration enhancers, Papain, Salicylic acid and Urea. The levels of Urea used were 0%, 2.8%, for Papain were 0% and 5% and Salicylic acid were 0% and 5%. The response variable was the drug diffused from the nail clipping after five days.
Two approaches were employed to quantify the amount of the drug Itraconazole hydrochloride diffuse through the nail plate. The first approach involved conducting a transungual penetration study using a custom-made diffusion cell. The custom-made diffusion cell unit comprised of 8 diffusion cells within it. The procured nail clippings were altered to the dimensions of the diffusion cell and the formulated lacquers, in adherence to the DOE table were applied onto the dorsal surface of each nail clipping. The receptor chamber was filled with phosphate buffer (pH 7.4), and preservatives, namely, methylparaben and propylparaben were added to impede any microbial growth. A temperature of 34 degrees Celsius was maintained, and an aliquot of 250µL was sampled out at 0th hour, to check for the absence of drug diffused and at 72nd hour for drug diffused. The approach of using a custom-made diffusion cell was ruled out on account of the drug, Itraconazole hydrochloride having a solubility of 0.0002 mg/mL in pH 7.4.
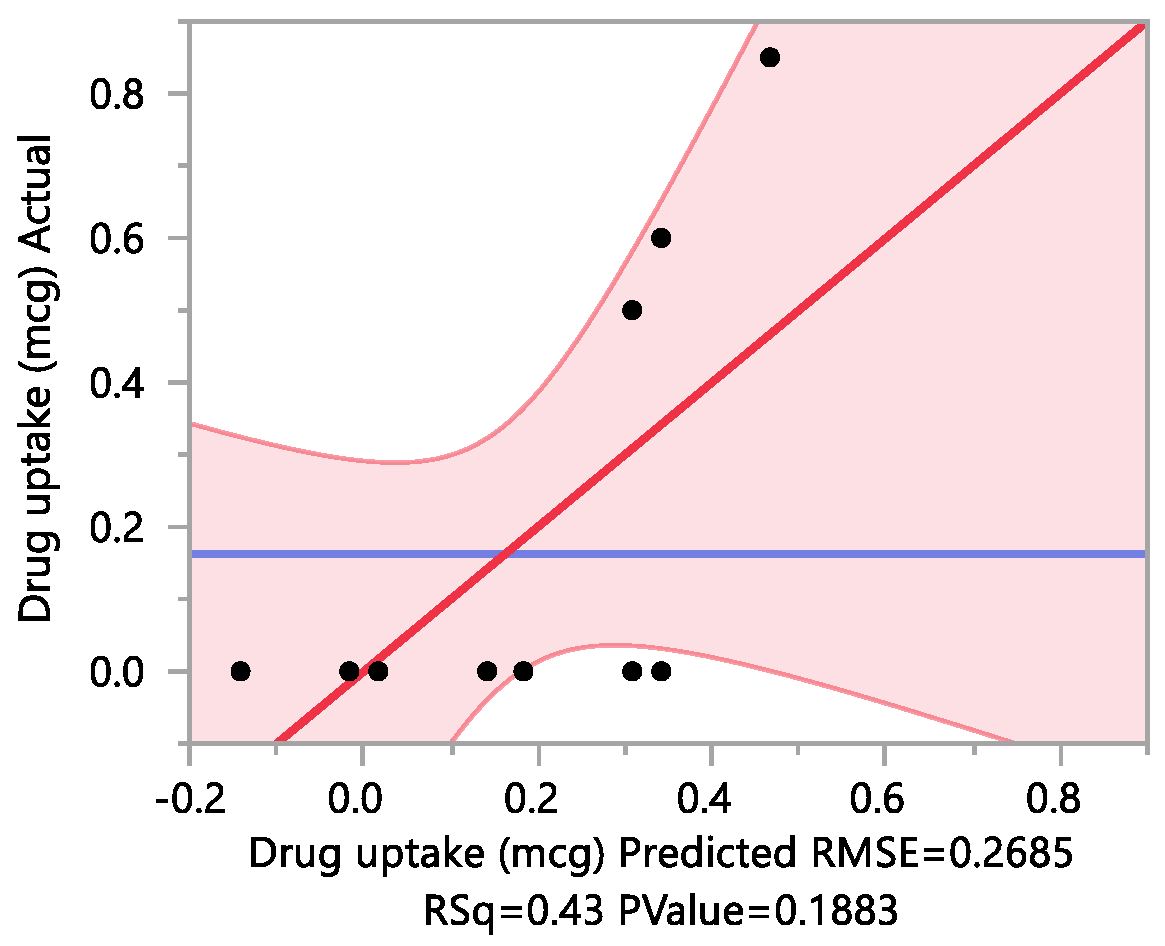
A second approach utilized the drug uptake (elaborated in the method section) on the same set of 12 nails. The results from this method indicated that the DOE runs for 3, 4, 7 had a drug uptake of 0.50 µg/mL, 0.85 µg/mL, and 0.60 µg/mL respectively.
5. Conclusions
The hypothesis was accepted for the current investigation as the results indicated that the optimized nail lacquer, comprising of 1% w/w itraconazole hydrochloride, 5% w/w Papain, 5% w/w Salicylic acid, and 2.8% w/w Urea had the highest transungual penetration into the nail on application to the dorsal surface of the nail plate. The antifungal nail lacquer adhered to the tests for drug content, spreadability, check for precipitation, pH, accelerated stability, and conformed as a whole to be reproducible and consistent.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
Wholehearted gratitude to Campbell University, NC and it’s research committee namely, Dr. Mali R Gupta, Dr. Antoinne Al-Achi, and Mr. Paul Johnson. Special thanks to the engineering department of Campbell University, NC for their support in developing a custom nail-diffusion cell apparatus. My sincere appreciation for my classmates for volunteering their nail clippings upon request. My parents and loved ones for their constant support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
JMP analysis for the design of experiment
| Urea (%w/w) |
Papain (%w/w) |
Salicylic acid (%w/w) |
Drug uptake (mcg) |
| 2.8 |
5 |
0 |
0.00 |
| 2.8 |
0 |
5 |
0.00 |
| 0 |
5 |
5 |
0.50 |
| 2.8 |
5 |
5 |
0.85 |
| 2.8 |
0 |
5 |
0.00 |
| 0 |
5 |
0 |
0.00 |
| 2.8 |
5 |
0 |
0.60 |
| 0 |
0 |
0 |
0.00 |
| 0 |
0 |
0 |
0.00 |
| 2.8 |
0 |
0 |
0.00 |
| 0 |
0 |
5 |
0.00 |
| 0 |
5 |
5 |
0.00 |
Response Drug uptake (mcg)
Actual by Predicted Plot
Effect Summary
| Source |
LogWorth |
|
PValue |
| Papain (%)(0,5) |
1.159 |
 |
0.06930 |
| Urea (%)(0,2.8) |
0.472 |
 |
0.33694 |
| Salicylic acid (%)(0,5) |
0.353 |
 |
0.44332 |
Lack Of Fit
| Source |
DF |
Sum of Squares |
Mean Square |
F Ratio |
| Lack Of Fit |
4 |
0.27166667 |
0.067917 |
0.8907 |
| Pure Error |
4 |
0.30500000 |
0.076250 |
Prob > F |
| Total Error |
8 |
0.57666667 |
|
0.5433 |
| |
|
|
|
Max RSq |
| |
|
|
|
0.6997 |
Residual by Predicted Plot
Studentized Residuals
Externally Studentized Residuals with 95% Simultaneous Limits (Bonferroni)
Summary of Fit
| RSquare |
0.432205 |
| RSquare Adj |
0.219282 |
| Root Mean Square Error |
0.268483 |
| Mean of Response |
0.1625 |
| Observations (or Sum Wgts) |
12 |
Parameter Estimates
| Term |
Estimate |
Std Error |
t Ratio |
Prob>|t| |
| Intercept |
0.1625 |
0.077504 |
2.10 |
0.0693 |
| Urea (%)(0,2.8) |
0.0791667 |
0.077504 |
1.02 |
0.3369 |
| Papain (%)(0,5) |
0.1625 |
0.077504 |
2.10 |
0.0693 |
| Salicylic acid (%)(0,5) |
0.0625 |
0.077504 |
0.81 |
0.4433 |
Prediction Profiler
Effect Screening
The parameter estimates have equal variances.
The parameter estimates are not correlated.
Parameter Estimate Population
| Term |
Estimate |
t Ratio |
Prob>|t| |
| Intercept |
0.1625000 |
2.0967 |
0.0693 |
| Urea (%)(0,2.8) |
0.0791667 |
1.0214 |
0.3369 |
| Papain (%)(0,5) |
0.1625000 |
2.0967 |
0.0693 |
| Salicylic acid (%)(0,5) |
0.0625000 |
0.8064 |
0.4433 |
Pareto Plot of Estimates
References
- Odds, F. C. (1993). Itraconazole—a new oral antifungal agent with a vast spectrum of activity in superficial and systemic mycoses. Journal of dermatological science, 5(2), 65-72. [CrossRef]
- Jessup, C. J., Warner, J., Isham, N., Hasan, I., & Ghannoum, M. A. (2000). Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. Journal of clinical microbiology, 38(1), 341-344. [CrossRef]
- Saner, M. V., Kulkarni, A. D., & Pardeshi, C. V. (2014). Insights into drug delivery across the nail plate barrier. Journal of Drug Targeting,22(9), 769-789. [CrossRef]
- Jacobsen, A. A., & Tosti, A. (2017). Predisposing Factors for Onychomycosis. Onychomycosis,11-19. [CrossRef]
- Osborne, C. S., Leitner, I., Favre, B., & Ryder, N. S. (2004). Antifungal drug response in anin vitromodel of dermatophyte nail infection. Medical Mycology,42(2), 159-163. [CrossRef]
- Rich, P. (2011). Hydrosoluble medicated nail lacquers: In vitro drug permeation and corresponding antimycotic activity. Yearbook of Dermatology and Dermatologic Surgery,2011, 259-260. [CrossRef]
- Salicylic acid. (n.d.). Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/salicylic_acid.
- Quintanar-Guerrero, D., Ganem-Quintanar, A., Tapia-Olguin, P., Kalia, Y. N., & Buri, P. (1998). The Effect of Keratolytic Agents on the Permeability of Three Imidazole Antimycotic Drugs Through the Human Nail. Drug Development and Industrial Pharmacy,24(7), 685-690. [CrossRef]
- Gupta, A. K., & Studholme, C. (2016). Novel investigational therapies for onychomycosis: An update. Expert Opinion on Investigational Drugs,25(3), 297-305. [CrossRef]
- Gupta, A. K., Sauder, D. N., & Shear, N. H. (1994). Antifungal agents: An overview. Part II. Journal of the American Academy of Dermatology,30(6), 911-933. [CrossRef]
- Aktas, A. E., Yigit, N., Aktas, A., & Gozubuyuk, S. G. (2014). Investigation of in vitro activity of five antifungal drugs against dermatophytes species isolated from clinical samples using the E-test method. The Eurasian journal of medicine, 46(1), 26. [CrossRef]
- https://www.drugbank.ca/drugs/DB01167.
- https://drfungus.org/knowledge-base/itraconazole/.
- Kobayashi, Y., Miyamoto, M., Sugibayashi, K., & Morimoto, Y. (1999). Drug Permeation through the Three Layers of the Human Nail Plate. Journal of Pharmacy and Pharmacology,51(3), 271-278. [CrossRef]
- Hamdan, J., & Hahn, R. (2006). Antifungal Drugs for Systemic Mycosis: An Overview of Mechanism of Action and Resistance. Anti-Infective Agents in Medicinal Chemistry,5(4), 403-412. [CrossRef]
- Itraconazole. (n.d.). Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/itraconazole.
- Kataria, P., Sharma, G., Thakur, K., Bansal, V., Dogra, S., & Katare, O. P. (2016). Emergence of nail lacquers as potential transungual delivery system in the management of onychomycosis. Expert opinion on drug delivery, 13(7), 937-952. [CrossRef]
- Bagavatula, H., Lankalapalli, S., Tenneti, V. V. K., Beeraka, N. M. R., & Bulusu, B. T. (2014). Comparative studies on solubility and dissolution enhancement of different itraconazole salts and their complexes. Advances in Pharmacology and Pharmacy, 2(6), 85-95. [CrossRef]
- Dr. Mali R Gupta, Sindhu Nair. Formulation of Itraconazole Emulgel as a Transungual Drug Delivery System (620 research).
- 20. USP-467-ICH-Q3C.
- https://www.drugfuture.com/Pharmacopoeia/USP35/data/v35300/usp35nf30s0_m43724.html.
- 2: Sawant Desai, IJPRBS, 2014; Volume 3(2), 2014; 22. Amrita Sawant Desai, IJPRBS, 2014; Volume 3(2): 200-214.
- 23. USP 467 Residual Solvent Method.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).