Submitted:
14 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental materials
2.2. Juice production
2.3. Consumer evaluation
2.4. Chemical analyses
2.5. Colour parameters
2.6. Total polyphenol
2.7. Antioxidant activity
2.8. Statistical analysis
3. Results and Discussion
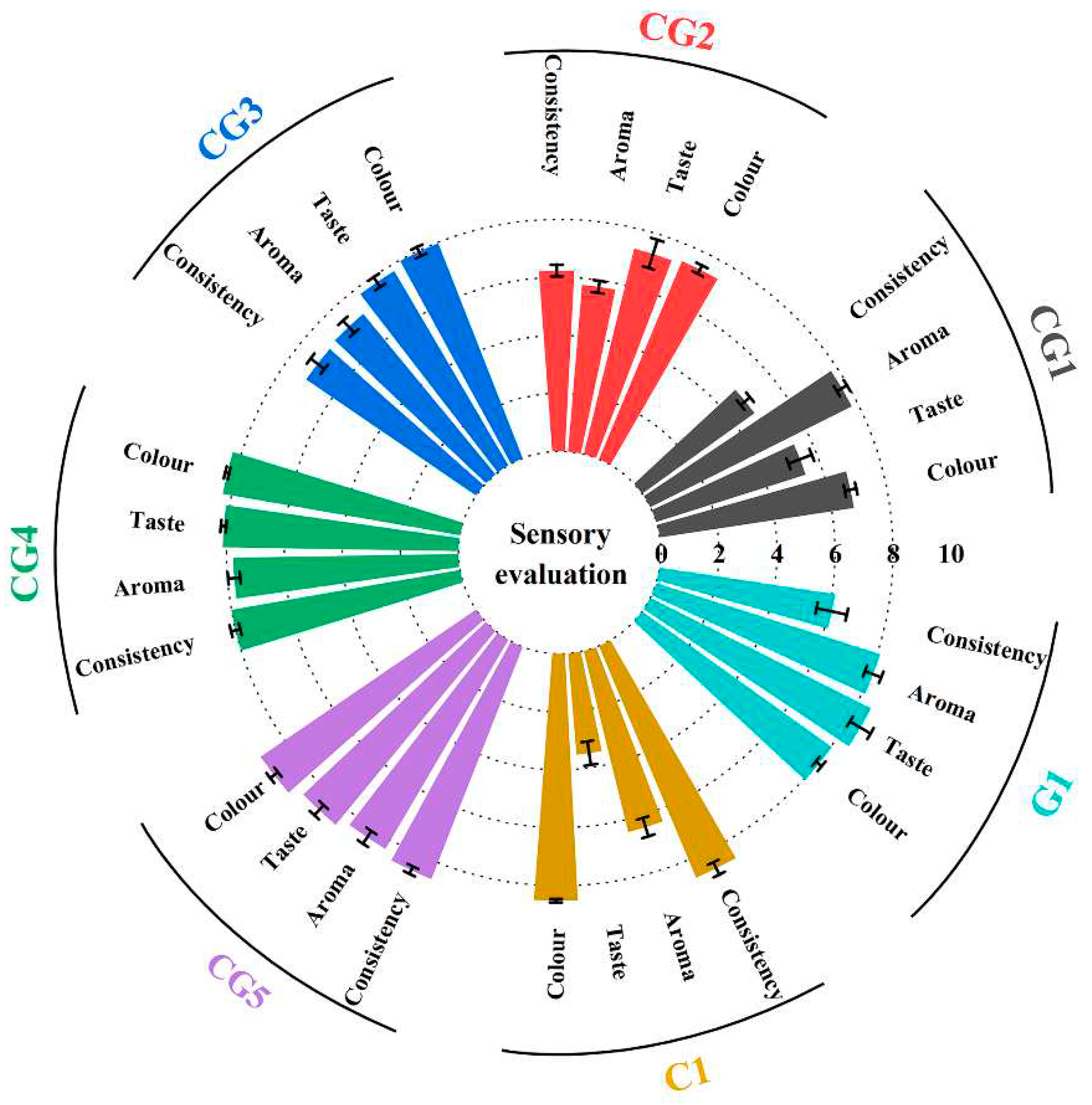
3.1. Consumer evaluation of the tested products
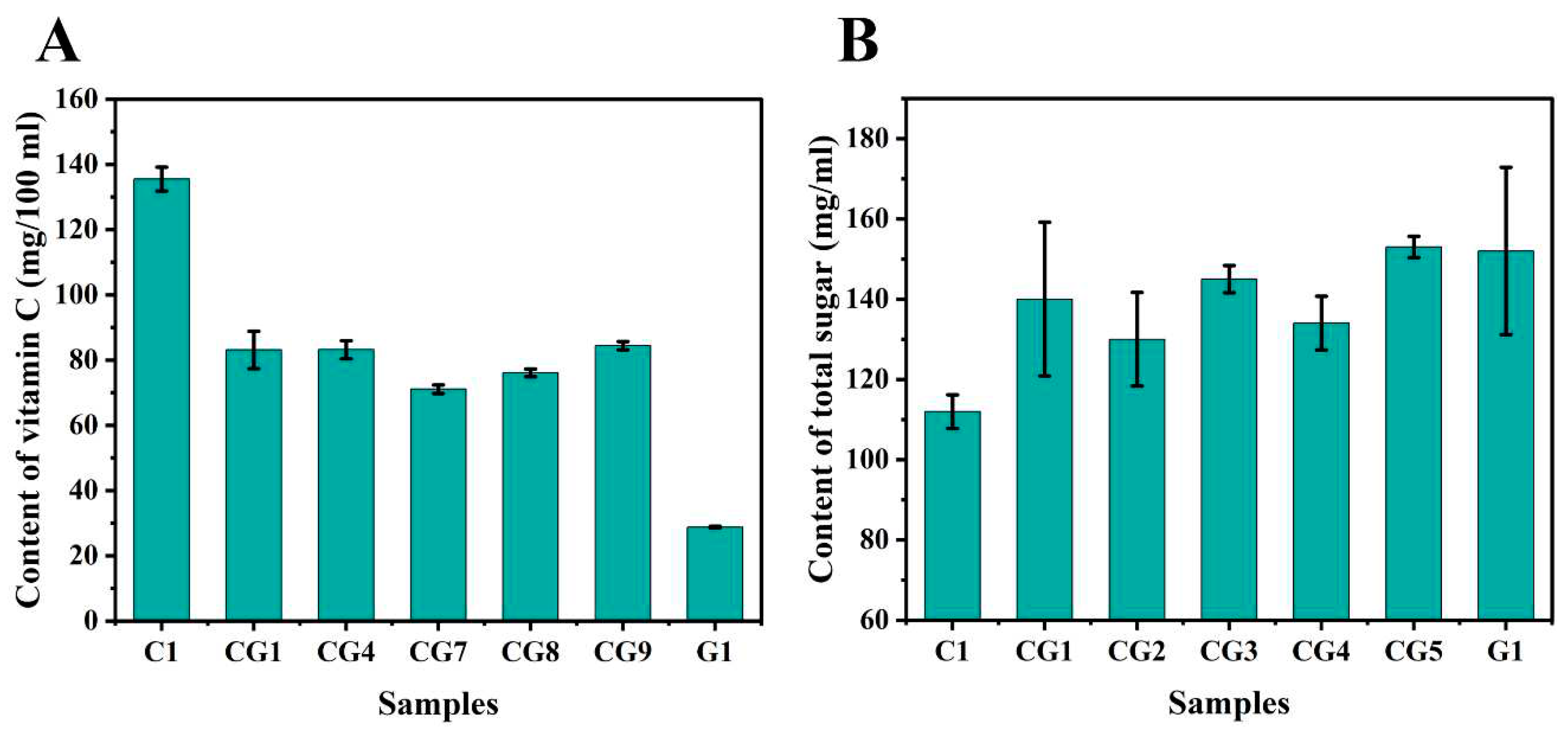
3.2. Chemical composition
3.3. Colour parameters of juices
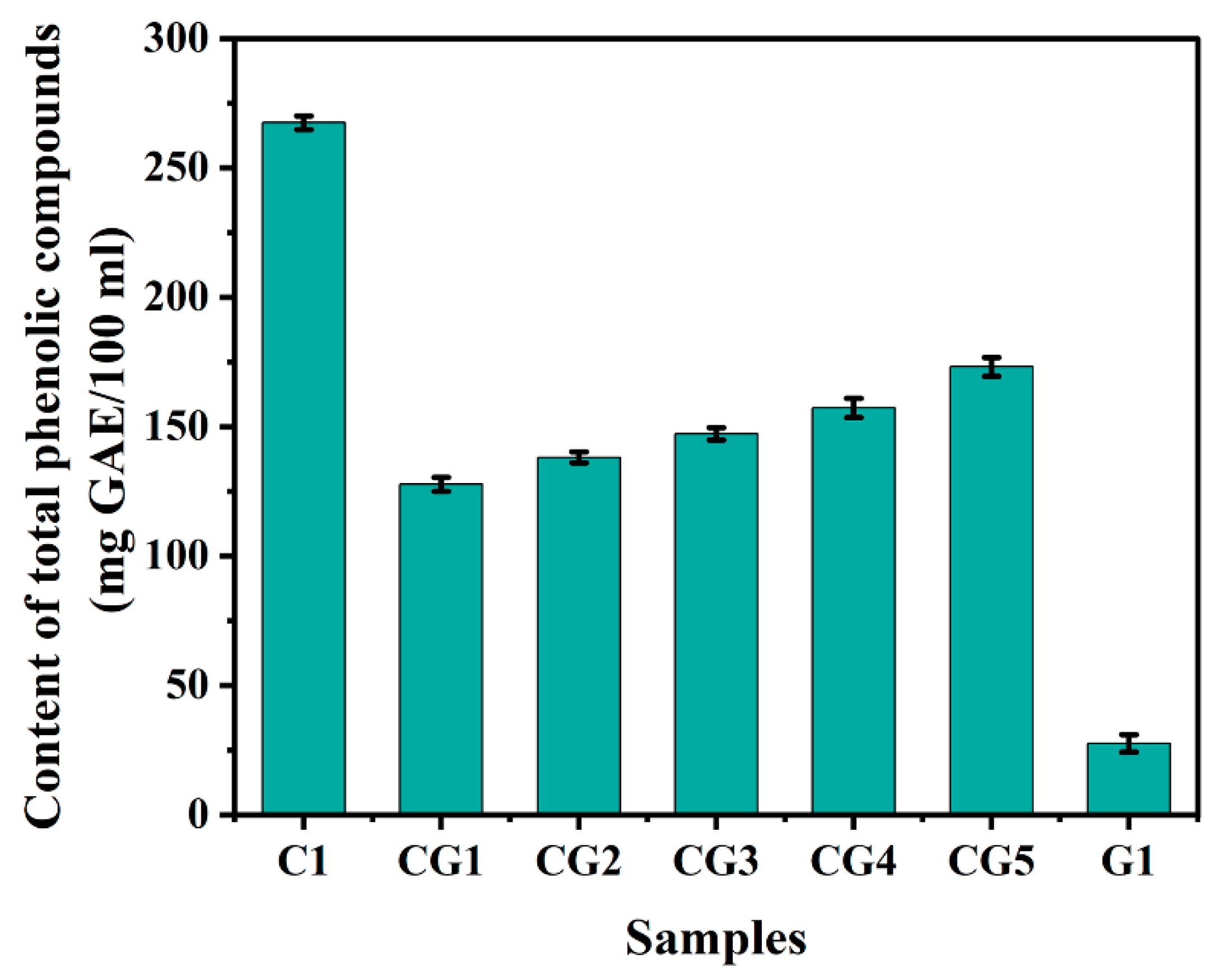
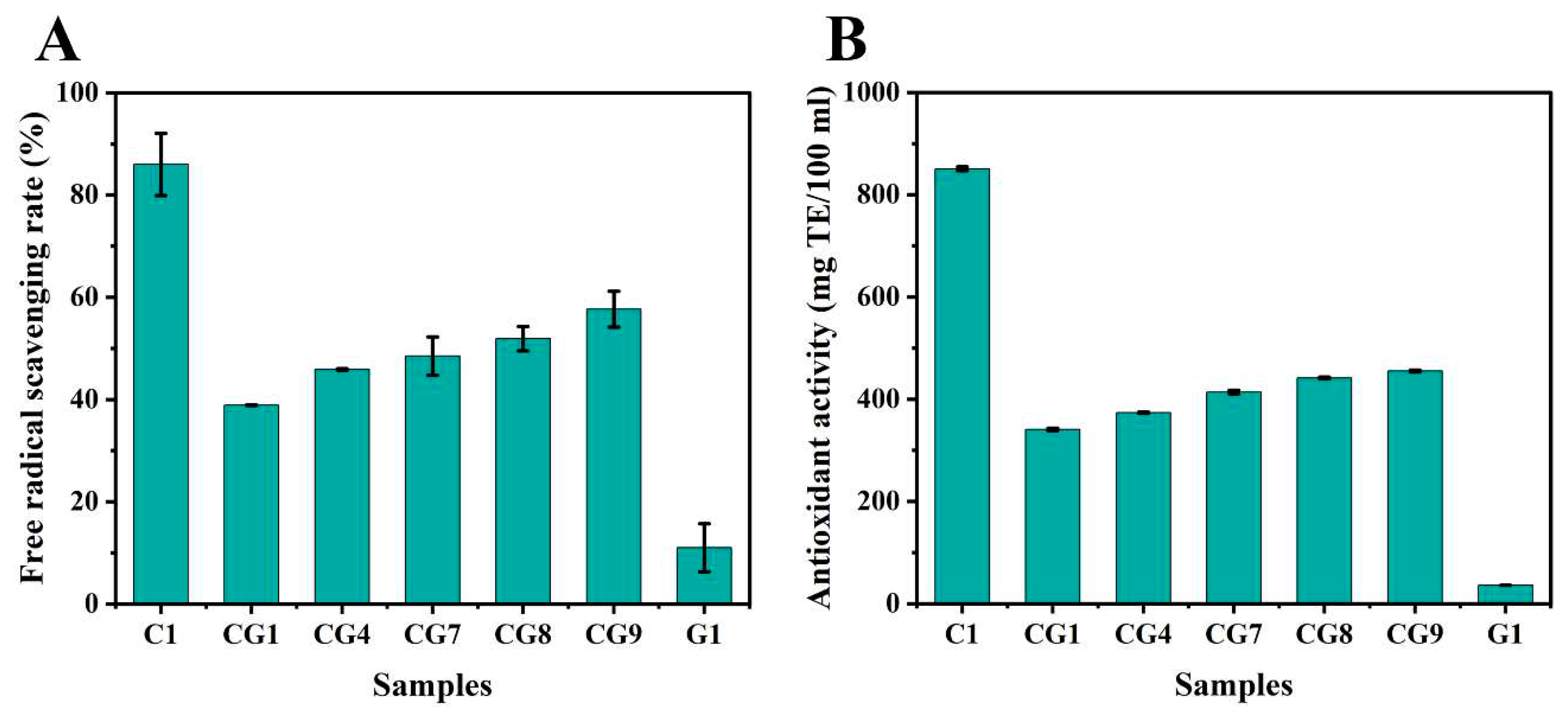
3.4. Total phenolic compounds and Antioxidant activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Zhao, W.; Li, W.; Geng, Q.; Zhao, R.; Yang, Y.G.; Lv, L.Y.; Chen, W.W. The Imbalance of Cytokines and Lower Levels of Tregs in Elderly Male Primary Osteoporosis. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Aliguer, N.; Falguera, M. An integrated approach to current trends in food consumption: Moving toward functional and organic products? Food Control 2012, 26, 274–281. [Google Scholar] [CrossRef]
- Layman, D.K. Eating patterns, diet quality and energy balance A perspective about applications and future directions for the food industry. Physiol. Behav. 2014, 134, 126–130. [Google Scholar] [CrossRef]
- Arihara, K. FUNCTIONAL FOODS. In Encyclopedia of Meat Sciences (Second Edition), Dikeman, M., Devine, C., Eds.; Academic Press: Oxford, 2014; pp. 32–36. [Google Scholar]
- Gupta, S.; Parvez, N.; Sharma, P. Nutraceuticals as Functional Foods. Journal of Nutritional Therapeutics 2015, 4, 64–72. [Google Scholar] [CrossRef]
- Hardy, G. Nutraceuticals and functional foods: introduction and meaning. Nutrition (Burbank, Los Angeles County, Calif.) 2000, 16, 688–689. [Google Scholar] [CrossRef]
- Topping, D. Cereal complex carbohydrates and their contribution to human health. J. Cereal Sci. 2007, 46, 220–229. [Google Scholar] [CrossRef]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudone, L.; Gaivelyte, K.; Viskelis, P.; Janulis, V. Phenolic and antioxidant profiles of rowan (Sorbus L.) fruits. Nat. Prod. Res. 2014, 28, 1231–1240. [Google Scholar] [CrossRef]
- Liang, D.; Wang, J.X.; Li, D.J.; Shi, J.; Jing, J.; Shan, B.E.; He, Y.T. Lung Cancer in Never-Smokers: A Multicenter Case-Control Study in North China. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J.P. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef]
- Leporini, M.; Loizzo, M.R.; Sicari, V.; Pellicano, T.M.; Reitano, A.; Dugay, A.; Deguin, B.; Tundis, R. Citrus x Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing. ANTIOXIDANTS 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Wei, Y.; Feng, X.G.; Fan, J.F.; Chen, X.N. Composition, anti-LDL oxidation, and non-enzymatic glycosylation inhibitory activities of the flavonoids from Mesembryanthemum crystallinum. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmianski, J. The influence of addition of cranberrybush juice to pear juice on chemical composition and antioxidant properties. J. Food Sci. Technol.-Mysore 2018, 55, 3399–3407. [Google Scholar] [CrossRef] [PubMed]
- Will, F.; Roth, M.; Olk, M.; Ludwig, M.; Dietrich, H. Processing and analytical characterisation of pulp-enriched cloudy apple juices. LWT-Food Sci. Technol. 2008, 41, 2057–2063. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, H.Y.; Mi, J.; Qin, X.Y.; Zhao, J.H.; Zhang, D.K.; Guo, C.; He, X.R.; An, W.; Cao, Y.L.; et al. Genome-Wide Identification and Analysis of the BBX Gene Family and Its Role in Carotenoid Biosynthesis in Wolfberry (Lycium barbarum L.). Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Wang, B.; He, J.L.; Zhang, S.J.; Li, L.L. Nondestructive prediction and visualization of total flavonoids content in Cerasus Humilis fruit during storage periods based on hyperspectral imaging technique. J. Food Process Eng. 2021, 44. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, J.; Shao, F.; Fu, S.; Sun, C.; Yuan, L.; Xie, C. Potential suitable distribution area and ecological characteristics of Cerasus humilis, an excellent tree species for windproof and sand fixation. Journal of Beijing Forestry University 2018, 40, 66–74. [Google Scholar]
- Mo, C.; Li, W.D.; He, Y.X.; Ye, L.Q.; Zhang, Z.S.; Jin, J.S. Variability in the sugar and organic acid composition of the fruit of 57 genotypes of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok]. J. Horticult. Sci. Biotechnol. 2015, 90, 419–426. [Google Scholar] [CrossRef]
- Li, W.D.; Li, O.; Mo, C.; Jiang, Y.S.; He, Y.; Zhang, A.R.; Chen, L.M.; Jin, J.S. Mineral element composition of 27 Chinese dwarf cherry (Cerasus humilis (Bge.) Sok.) genotypes collected in China. J. Horticult. Sci. Biotechnol. 2014, 89, 674–678. [Google Scholar] [CrossRef]
- Li, W.D.; Li, O.; Zhang, A.R.; Li, L.; Hao, J.H.; Jin, J.S.; Yin, S.J. Genotypic diversity of phenolic compounds and antioxidant capacity of Chinese dwarf cherry (Cerasus humilis (Bge.) Sok.) in China. Sci. Hortic. 2014, 175, 208–213. [Google Scholar] [CrossRef]
- Zhou, J.H. Research on the Clarification Technology of Prunus humilis Juice. Journal of Anhui Agricultural Sciences 2009. [Google Scholar]
- Guo, C.Z.; Wang, P.F.; Zhang, J.C.; Guo, X.W.; Mu, X.P.; Du, J.J. Organic acid metabolism in Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.] is controlled by a complex gene regulatory network. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Wojdylo, A.; Chmielewska, J.; Oszmianski, J. The influence of yeast type and storage temperature on content of phenolic compounds, antioxidant activity, colour and sensory attributes of chokeberry wine. Eur. Food Res. Technol. 2017, 243, 2199–2209. [Google Scholar] [CrossRef]
- Vigneshwaran, G.; More, P.R.; Arya, S.S. Non-thermal hydrodynamic cavitation processing of tomato juice for physicochemical, bioactive, and enzyme stability: Effect of process conditions, kinetics, and shelf-life extension. Curr. Res. Food Sci. 2022, 5, 313–324. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Wojdylo, A.; Teleszko, M.; Oszmianski, J. Physicochemical characterisation of quince fruits for industrial use: yield, turbidity, viscosity and colour properties of juices. Int. J. Food Sci. Technol. 2014, 49, 1818–1824. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmianski, J.; Wisniewski, R. Comparison of Phenolic Content and Antioxidant Capacity of Bear Garlic (Allium ursinum L.) in Different Maturity Stages. J. Food Process Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Suja, K.P.; Jayalekshmy, A.; Arumughan, C. Antioxidant activity of sesame cake extract. Food Chem. 2005, 91, 213–219. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan-Selenium Nanoparticle (Cs-Se NP) Foliar Spray Alleviates Salt Stress in Bitter Melon. Nanomaterials 2021, 11. [Google Scholar] [CrossRef]
- Fu, H.B.; Mu, X.P.; Wang, P.F.; Zhang, J.C.; Fu, B.C.; Du, J.J. Fruit quality and antioxidant potential of Prunus humilis Bunge accessions. PLoS One 2020, 15. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Ouyang, X.Y.; Laaksonen, O.; Liu, X.Y.; Shao, Y.; Zhao, H.F.; Zhang, B.L.; Zhu, B.Q. Effect of Lactobacillus acidophilus, Oenococcus oeni, and Lactobacillus brevis on Composition of Bog Bilberry Juice. Foods 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A. Bioactive compounds vs. organoleptic assessment of ‘smoothies’-type products prepared from selected fruit species. Int. J. Food Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Mu, X.P.; Wang, P.F.; Du, J.J.; Gao, Y.G.; Zhang, J.C. Comparison of fruit organic acids and metabolism-related gene expression between Cerasus humilis (Bge.) Sok and Cerasus glandulosa (Thunb.) Lois. PLoS One 2018, 13. [Google Scholar] [CrossRef]
- Schiassi, M.; Lago, A.M.T.; de Souza, V.R.; Meles, J.D.; de Resende, J.V.; Queiroz, F. Mixed fruit juices from Cerrado: Optimization based on sensory properties, bioactive compounds and antioxidant capacity. Br. Food J. 2018, 120, 2334–2348. [Google Scholar] [CrossRef]
- Oka, Y.; Butnaru, M.; von Buchholtz, L.; Ryba, N.J.P.; Zuker, C.S. High salt recruits aversive taste pathways. Nature 2013, 494, 472–475. [Google Scholar] [CrossRef]
- Gerard, K.A.; Roberts, J.S. Microwave heating of apple mash to improve Juice yield and quality. LWT-Food Sci. Technol. 2004, 37, 551–557. [Google Scholar] [CrossRef]
- Mena, P.; Garcia-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Marti, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Qazi, M.W.; de Sousa, I.G.; Nunes, M.C.; Raymundo, A. Improving the Nutritional, Structural, and Sensory Properties of Gluten-Free Bread with Different Species of Microalgae. Foods 2022, 11. [Google Scholar] [CrossRef]
| No | Symbols | Product |
|---|---|---|
| 1 | C1 | 100% CJ |
| 2 | CG1 | 34% CJ + 66% GJ |
| 3 | CG2 | 37% CJ + 63% GJ |
| 4 | CG3 | 40% CJ + 60% GJ |
| 5 | CG4 | 44% CJ + 56% GJ |
| 6 | CG5 | 50% CJ + 50% GJ |
| 7 | G1 | 100% GJ |
| Samples | L* | a* | b* | ∆E | h° | ∆C |
|---|---|---|---|---|---|---|
| C1 | 40.06 | 66.94 | 66.74 | - | - | - |
| CG1 (34) | 62.50 | 60.09 | 30.23 | 43.40 | 26.70 | 37.15 |
| CG2 (37) | 61.56 | 61.65 | 33.09 | 40.28 | 28.24 | 34.06 |
| CG3 (40) | 59.38 | 63.91 | 36.12 | 36.33 | 29.47 | 30.77 |
| CG4 (44) | 56.69 | 65.11 | 40.63 | 31.01 | 31.97 | 26.17 |
| CG5 (50) | 53.69 | 66.64 | 46.49 | 24.40 | 34.89 | 20.25 |
| G1 | 99.82 | 0.45 | 3.17 | 109.70 | 80.92 | 91.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




