1. Introduction
The genus
Spodoptera is composed of several species considered to be important agricultural pests, mainly in tropical and subtropical regions [
1]. In Brazil, four species of the
Spodoptera complex —
Spodoptera cosmioides (Walker, 1858),
Spodoptera eridania (Stoll, 1782),
Spodoptera albula (Walker, 1857) and
Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) — attack soybean and other economically important crops such as maize and cotton [
2]. Nonetheless,
Spodoptera species are considered secondary pests of soybean in Brazil and other South American countries [
3].
In 2013, the MON 87701 × MON 89788 soybean event (trade name Intacta RR2 PRO
®), expressing the insecticidal protein Cry1Ac from
Bacillus thuringiensis Berliner, became available to farmers in Brazil. This genetically modified (GM) soybean confers protection against
Anticarsia gemmatalis (Hübner, 1818) (Lepidoptera: Erebidae) and against
Chrysodeixis includens (Walker, [1858]),
Chloridea virescens (Fabricius, 1781) and
Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae) [
4,
5,
6,
7,
8], being cultivated on more than 36 million hectares per season in Brazil [
9]. During the 2021/2022 season, the event MON 87751 × MON 87708 × MON 87701 × MON 89788 (trade name Intacta 2 Xtend
®), expressing Cry1A.105, Cry2Ab2 and Cry1Ac proteins, was also released for commercial used in Brazil, extending the protection conferred by Bt soybean to the species
S. cosmioides [
6,
10].
Previous reports stated that Cry1A.105/Cry2Ab2/Cry1Ac and Cry1Ac soybean technologies had reduced lethality against
S. eridania and
S. frugiperda [
5,
8,
10]. This can be explained by their naturally low susceptibility to Bt proteins [
5,
11]. According to Horikoshi et al. [
8], more than 98% of the insects sampled from reproductive stages of Cry1Ac soybean in Brazil were
Spodoptera, including
S. cosmioides,
S. eridania and
S. frugiperda. Specifically for
S. frugiperda, the resistance to Bt proteins expressed in maize affects the performance of Bt soybean technologies [
12,
13,
14,
15,
16]. Regardless of their relatively low lethality against
Spodoptera species, these Bt soybean technologies suppressed infestations of primary lepidopteran species in soybean in Brazil, enabling a decrease in the use of chemical insecticides and enhancing the natural biological control of other species [
11,
17].
Understanding both lethal and non-lethal effects of Bt proteins and insecticides on target and non-target lepidopteran pests is important for the best use of these control tactics in integrated pest management (IPM) programs. However, most studies have typically neglected non-lethal effects (= sublethal effects) in favor of more obvious lethal effects, which are measured through mortality analysis. Sublethal effects can be defined as those caused by low residual doses, which can exert effects on biological, physiological and biochemical processes as well on insect development and reproduction [
18,
19,
20]. For example, sublethal effects of insecticides and Bt proteins extended larval development and reduced larval biomass, adult fecundity and population growth of several
Spodoptera species [
5,
10,
11,
15,
16,
21,
22,
23]. Therefore, elucidating the overall effects (i.e., both lethal and sublethal) of Bt proteins and insecticides on pest species is essential for the design of robust IPM programs. Thus, we evaluated the dose effects of flubendiamide and thiodicarb against
S. cosmioides, S. eridania,
S. albula and
S. frugiperda that survived on Bt and non-Bt soybean.
2. Materials and Methods
2.1. Collection and Rearing of Spodoptera Species
Brazilian populations of
S. cosmioides, S. eridania and S. albula were collected from non-Bt soybean fields from the 2019/2020 to 2021/2022 seasons, whereas
S. frugiperda were sampled from non-Bt maize during the 2021/2022 season. After collection,
Spodoptera larvae were transported to the laboratory and reared on the artificial diet proposed by Greene et al. [
24]. After two generations under laboratory conditions,
S. frugiperda presented
60% survival on Bt maize expressing Cry1 and Cry2 insecticidal proteins, indicating that the field-collected population had some degree of resistance to Bt proteins.
2.2. Soybean Plant Types
Seeds of GM soybean MON 87701 × MON 89788 × MON 87751 × MON 87708, which expresses Cry1A.105/Cry2Ab2/Cry1Ac insecticidal proteins (NEO590 I2X; GDM Genética do Brasil Ltda, Passo Fundo, RS, Brazil); GM soybean MON 87701 × MON 89788, expressing Cry1Ac protein (A5547 IPRO; Bayer Crop Science, São Paulo, SP, Brazil); and non-Bt soybean (NA 5909 RG; Nidera Sementes Ltda, São Paulo, SP, Brazil) were sown under field conditions at a density of 10 plants/m within each row and spacing of 0.45 m between rows. Before bioassays, plants were tested for the expression of Bt proteins using detection kits (QuickStix™; EnviroLogix).
2.3. Insecticides
The commercial products flubendiamide (Belt® 480 g active ingredient (a.i.)/L) – ryanodine receptor modulator (IRAC MoA group 28) – and thiodicarb (Larvin 800 WG g a.i./kg) – acetylcholinesterase inhibitor (IRAC MoA sub-group 1A) – commonly applied to control lepidopteran soybean pests were provided by Bayer S.A. (São Paulo, SP, Brazil) and used to perform bioassays.
2.4. Bioassays
To measure the mortality of
Spodoptera species on different soybean types, trifoliate leaves of Cry1A.105/Cry2Ab2/Cry1Ac soybean, Cry1Ac soybean and non-Bt soybean were excised from field grown plants at the V
5–8 growth stages [
25]. In the laboratory, trifoliate leaves were placed in 250-mL plastic pots over a gelled mixture of agar–water (2% agar), with a piece of filter paper separating the leaves from the agar–water layer. The leaves in each pot were infested with a total of 10 neonates (<24 h old) and pots were placed in a climatic chamber at 25 ± 2 °C, 60% ± 10% RH and 14:10 h light:dark photoperiod. Leaves were replaced every 2 d, until all neonates had either died or progressed to L1 or L2.
Surviving L1 and L2 larvae were then transferred to leaves of the same soybean type that were sprayed with the field label dose of flubendiamide (70 mL/ha) or thiodicarb (400 g/ha), sprayed with 50% of these doses, or unsprayed. To obtain these leaves, insecticides were applied to soybean plants cultivated under field conditions with a pressurized-CO2 backpack sprayer with a 2-m bar and 0.5 m nozzle spacing (XR 110.02 fan-type nozzle tips; TeeJet Technologies Co., Glendale Heights, IL, USA), simulating a spray volume of 150 L/ha. Approximately 50 min after spraying, trifoliate leaves from the upper-third part of plants were collected and, in the laboratory, were placed in 250-mL plastic pots as previously described. Unsprayed field-grown leaves of each soybean type were used as controls. Each pot was infested with 10 L1 or L2 larvae that had survived on unsprayed leaves of the same soybean type and then maintained in a climatic chamber under the same conditions described above. Larvae surviving 4 d of exposure to insecticide (or 4 d on unsprayed control leaves) were placed into 42-well plastic plates (1 larva/well) containing leaves collected from the same soybean type (Cry1A.105/Cry2Ab2/Cry1Ac, Cry1Ac or non-Bt) and treatment (insecticide and dose level) on which they previously survived. These leaves were collected from the plants initially sprayed with insecticide, to simulate the residual effect of the insecticides under field conditions. Leaves were replaced every 2 d until larval mortality or pupation.
The experimental design was randomized with 7 replicates of 10 L1 or L2 larvae per insect species, soybean type and insecticide type/dose. The following lethal and sublethal effects were assessed: mortality at 10 d on Cry1A.105/Cry2Ab2/Cry1Ac, Cry1Ac and non-Bt soybean, survival from L1 and L2 stages to adult post-exposure to sprayed and sprayed leaves, developmental time from L1 or L2 stage to adult, number of eggs laid per female (fecundity) and egg-hatching percent (fertility). To evaluate fecundity and fertility, 4 to 10 couples from each treatment were paired in PVC cages (23-cm height × 10-cm diameter; one couple/cage) coated with white paper and closed at the top with sheer fabric. Adults were fed with a 10% honey/aqueous solution provided on cotton. Eggs were collected and counted every 2 d until the death of the female moth. To measure egg-hatching percentage, 50 to 250 eggs from the second oviposition were obtained from each couple and placed in 100-mL plastic cups containing a piece of moistened filter paper.
2.5. Data Analysis
Mortality on Cry1A.105/Cry2Ab2/Cry1Ac, Cry1Ac and non-Bt soybean leaves sprayed with insecticides was corrected based on the mortality on unsprayed non-Bt soybean leaves according to Abbott’s formula [
26]. Data on mortality, survival to adult phase, developmental time, fecundity and fertility were then subjected to non-parametric analysis, and means differences were estimated by the Least Square Means Statement (LSMEANS option of PROC GLM) using a Tukey–Kramer test at
p < 0.05 [
27].
3. Results
3.1. Lethal Effects of Flubendiamide Against Spodoptera Species Surviving on Different Soybean Types
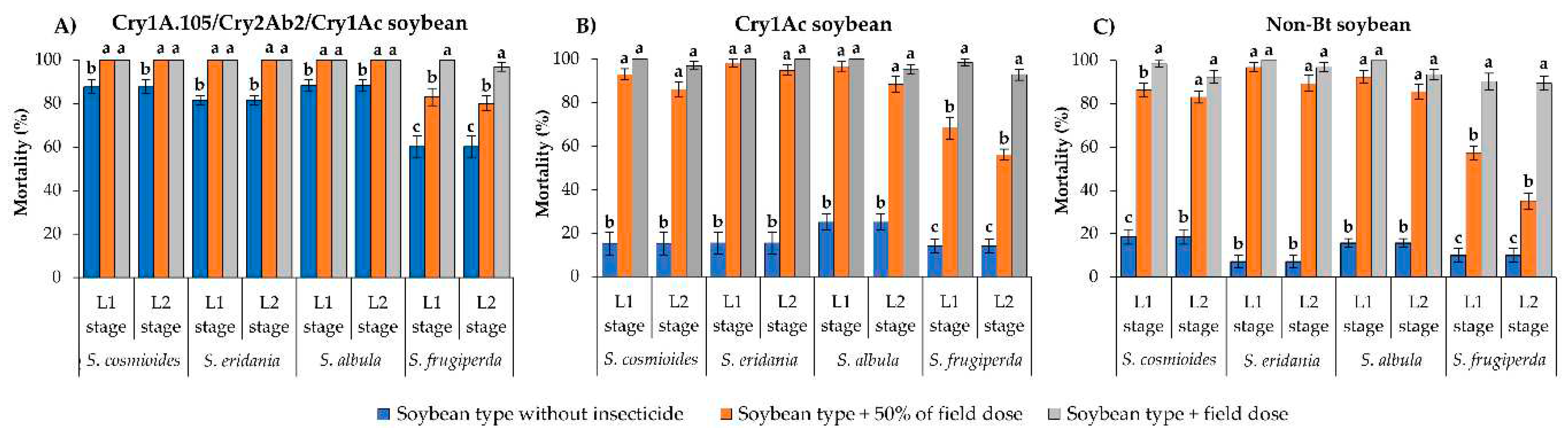
The mortality rate of S. cosmioides, S. eridania and S. albula ranged from 81.3 to 88.0% after 10 d on unsprayed Cry1A.105/Cry2Ab2/Cry1Ac soybean leaves (
Figure 1A). However, on this same soybean type, surviving L1 and L2 larvae presented significantly higher mortality (100%) on leaves sprayed with the field label dose of flubendiamide (70 mL/ha) or with 50% of this dose than on unsprayed leaves (F = 15.40; df = 2, 18; p < 0.0001 for S. cosmioides; F = 72.63; df = 2, 18; p < 0.0001 for S. eridania; F = 18.85; df = 2, 18; p < 0.0001 for S. albula) (
Figure 1A). In contrast, S. frugiperda showed
60% mortality after 10 d on unsprayed Cry1A.105/Cry2Ab2/Cry1Ac soybean leaves, but mortality rate increased to more than 80% when leaves were sprayed with either dose of flubendiamide (F = 30.31; df = 2, 18; p < 0.0001 for L1 stage; F = 24.87; df = 2, 18; p < 0.0001 for L2 stage) (
Figure 1A).
On unsprayed Cry1Ac soybean, all Spodoptera species evaluated presented <25% mortality after 10 d (
Figure 1B). The mortality rates of S. cosmioides, S. eridania and S. albula at L1 and L2 stages developing on Cry1Ac soybean leaves were significantly higher on leaves sprayed with the field label dose of flubendiamide or with 50% of this dose (>86% mortality) than on unsprayed leaves of the same soybean type (F = 199.14–141.50; df = 2, 18; p < 0.0001 for S. cosmioides; F = 239.73–191.42; df = 2, 18; p <0.0001 for S. eridania; F = 168.02–98.39; df = 2, 18; p < 0.0001 for S. albula) (
Figure 1B). For S. frugiperda, the mortality rate was <14% on Cry1Ac soybean leaves but increased to >93% when surviving L1 and L2 larvae were exposed to same soybean type sprayed with the field label dose of flubendiamide (F = 145.76; df = 2, 18; p < 0.0001 for L1 stage; F = 207.77; df = 2, 18; p < 0.0001 for L2 stage) (
Figure 1B).
Similar to previous results, on non-Bt soybean sprayed with either dose of flubendiamide, the mortality rates of L1 and L2 larvae of S. cosmioides, S. eridania and S. albula were >83%, being significantly higher than on non-Bt soybean leaves without insecticide (<19% mortality) (F = 235.54–175.02; df = 2, 18; p < 0.0001 for S. cosmioides; F = 347.49–268.08; df = 2, 18; p < 0.0001 for S. eridania; F = 370.28–259.29; df = 2, 18; p < 0.0001 for S. albula) (
Figure 1C). For S. frugiperda, the mortality rates of L1 and L2 larval stages on non-Bt soybean leaves sprayed with the field label dose of flubendiamide (90% mortality) were significantly higher than on leaves sprayed with 50% of this dose (<57% mortality) and on unsprayed leaves (10% mortality) (F = 143.20; df = 2, 18; p < 0.0001 for L1 stage; F = 146.83; df = 2, 18; p < 0.0001 for L2 stage) (
Figure 1C).
3.2. Lethal Effects of Thiodicarb Against Spodoptera Species Surviving on Different Soybean Types
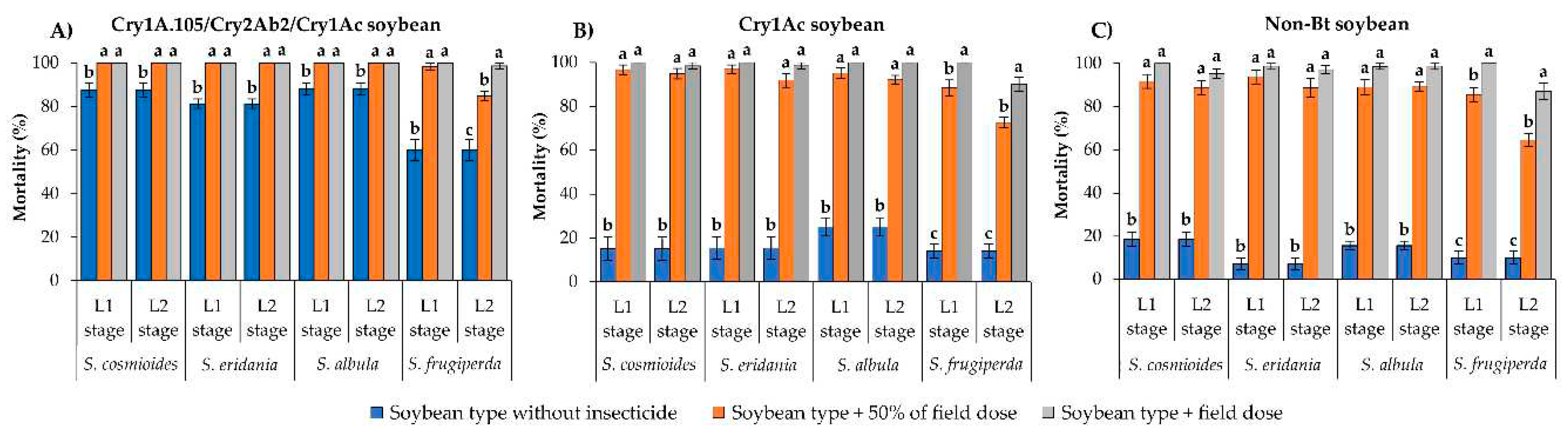
The mortality rates of S. cosmioides, S. eridania and S. albula on unsprayed Cry1A.105/Cry2Ab2/Cry1Ac soybean leaves were >81% after 10 d of exposure (
Figure 2A). However, surviving L1 and L2 larvae of these species presented significantly higher mortality (100%) when exposed to leaves of the same soybean type sprayed with the field label dose of thiodicarb (400 g/ha) or with 50% of this dose (F = 15.40; df = 2, 18; p < 0.0001 for S. cosmioides; F = 72.63; df = 2, 18; p < 0.0001 for S. eridania; F = 18.85; df = 2, 18; p < 0.0001 for S. albula) (
Figure 2A). Spodoptera frugiperda had
60% mortality on unsprayed Cry1A.105/Cry2Ab2/Cry1Ac soybean but surviving L1 larvae exposed to the same soybean type sprayed with either dose of thiodicarb showed >98% mortality (F = 57.08; df = 2, 18; p < 0.0001). In contrast, L2 larvae presented lower mortality on leaves sprayed with 50% of the thiodicarb field label dose (83.9% mortality) than on those sprayed with the field label dose (98.7% mortality) (F = 35.57; df = 2, 18; p < 0.0001) (
Figure 2A).
Mortality rates of S. cosmioides, S. eridania and S. albula were <25% on unsprayed Cry1Ac soybean leaves after 10 d of exposure (
Figure 2B). However, surviving L1 and L2 larvae of these species presented mortality ranging from 91.8% to 100% on Cry1Ac soybean leaves sprayed with either dose of thiodicarb, differing significantly from the mortality on unsprayed leaves of the same soybean type (F = 217.16–186.78; df = 2, 18; p < 0.0001 for S. cosmioides; F = 228.06–164.26; df = 2, 18; p <0.0001 for S. eridania; F = 161.03–151.13; df = 2, 18; p < 0.0001 for S. albula) (
Figure 2B). Similarly, the mortality of L1 and L2 stages of S. frugiperda on unsprayed Cry1Ac soybean leaves was <15%, but these larval stages presented >72.6% mortality on Cry1Ac leaves sprayed with either dose of thiodicarb (F = 143.20; df = 2, 18; p < 0.0001 for L1 stage; F = 146.83; df = 2, 18; p < 0.0001 for L2 stage) (
Figure 2B).
With the exception of the L2 stage of S. frugiperda developing on non-Bt soybean sprayed with 50% of the field label dose of thiodicarb (64.6% mortality), all other Spodoptera species presented >88.6% mortality when exposed to either dose of thiodicarb, differing significantly from those that developed on unsprayed non-Bt leaves (<19% mortality) (F = 272.14–190.82; df = 2, 18; p < 0.0001 for S. cosmioides; F = 380.34–236.36; df = 2, 18; p < 0.0001 for S. eridania; F = 344.46–361.43; df = 2, 18; p < 0.0001 for S. albula) (
Figure 2C).
3.3. Sublethal Effects of Insecticides Against Spodoptera Species Surviving on Different Soybean Types
3.3.1. Effects on Development
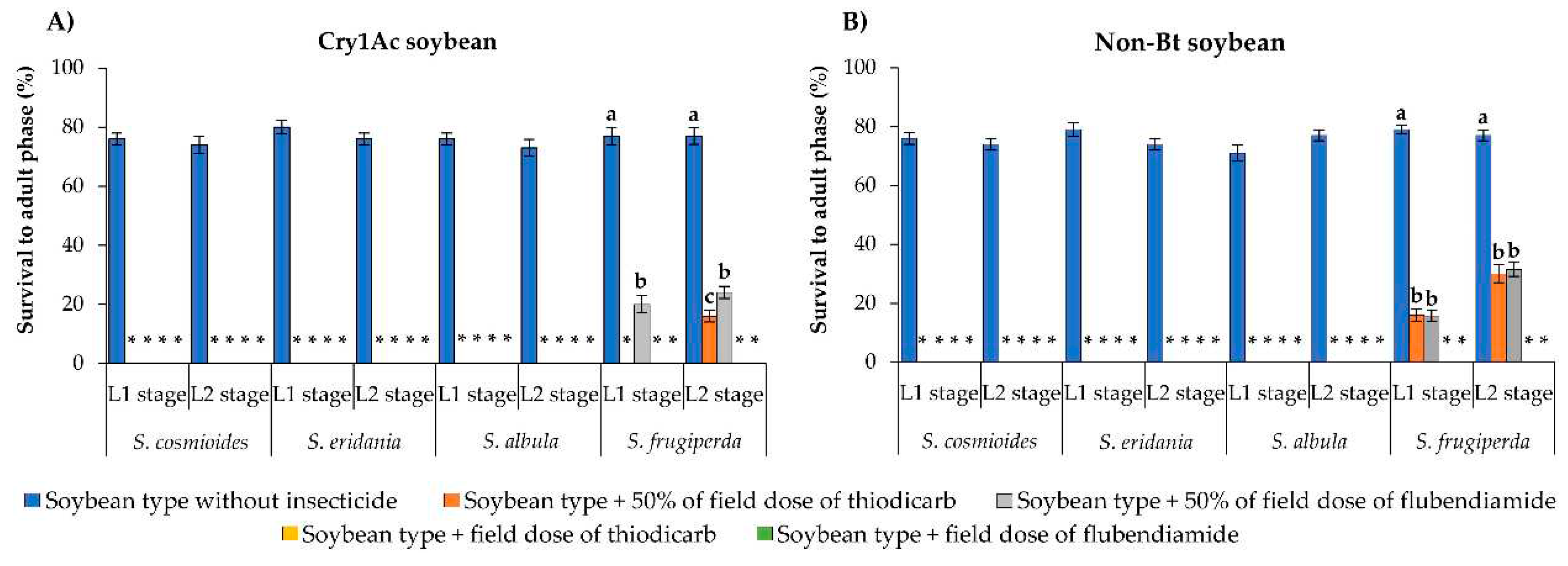
It is important to highlight that no L1 and L2 larvae of S. cosmioides, S. eridania or S. albula initially surviving on unsprayed Cry1A.105/Cry2Ab2/Cry1Ac soybean leaves developed to adulthood on either sprayed or unsprayed leaves, making it impossible to measure the sublethal effects of insecticides on these species. Only S. frugiperda larvae developing on Cry1A.105/Cry2Ab2/Cry1Ac soybean leaves without insecticide developed into adults (<63%).
On Cry1Ac and non-Bt soybeans, none of the Spodoptera larvae developed to adulthood on insecticide- sprayed leaves except for some S. frugiperda larvae exposed to leaves sprayed with 50% of the field label dose of flubendiamide or thiodicarb (
Figure 3A, B). On Cry1Ac soybean leaves, <24% of S. frugiperda larvae developed to adulthood after exposure to 50% of the field label dose of flubendiamide or thiodicarb, differing significantly from the frequency developing on leaves sprayed with the field label dose (no survival) or on unsprayed leaves (77% developed into adults) (F = 184.62–282.08; df = 1, 18; p < 0.0001) (
Figure 3A). Similar results were obtained on non-Bt soybean: <32% of larvae developed into adults on leaves sprayed with 50% of the field label dose of flubendiamide or thiodicarb, whereas on unsprayed leaves, survival to adulthood was
78% (F = 296.20–119.32; df = 2, 18; p < 0.0001). When surviving S. frugiperda larvae were exposed to non-Bt soybean leaves sprayed with field label doses of either insecticide, none developed to adulthood (
Figure 3B).
The developmental time to adulthood of L1 larvae of S. frugiperda surviving on leaves of Cry1Ac soybean sprayed with 50% of the field label dose of flubendiamide (29.3 ± 0.60 d) was significantly longer than that of larvae developing on the same soybean type without insecticide (25.6 ± 0.10 d) (F = 12.06; df = 1, 12; p = 0.0047). Similarly, longer developmental time to adulthood was observed for L1 larvae developing on non-Bt leaves sprayed with 50% of the field label dose of thiodicarb (30.6 ± 0.61 d) than for those on unsprayed leaves (27.1 ± 0.15 d) (F = 65.58; df = 1, 12; p = < 0.0001). In contrast, L1 larvae of S. frugiperda on non-Bt soybean had similar developmental times on leaves with 50% of the flubendiamide dose and on unsprayed leaves (27.1 ± 0.15 d and 28.2 ± 0.42 d, respectively) (F = 4.43; df = 1, 12; p = 0.0571). No L1 larvae survived to adulthood on Cry1Ac soybean leaves sprayed with 50% of the thiodicarb field label dose.
On the other hand, the immature stage of L2 larvae was 6 d longer on Cry1Ac soybean leaves sprayed with 50% of the thiodicarb field label dose than on unsprayed leaves (F = 175.84; df = 1, 12; p < 0.0001). In contrast, L2 larvae of S. frugiperda on Cry1Ac soybean leaves sprayed with 50% of the field label dose of flubendiamide (23.0 ± 0.31 d) or unsprayed leaves (22.3 ± 0.16 d) had similar developmental times to adulthood (F = 2.48; df = 1, 12; p = 0.1410). The development times were also similar between L2 larvae reared on non-Bt soybean sprayed with 50% of the field label dose of flubendiamide and those on unsprayed leaves (22.2 ± 0.29 d and 22.6 ± 0.18 d, respectively) (F = 1.07; df = 1, 12; p = 0.3213). However, L2 larvae reared on non-Bt soybean sprayed with 50% of the thiodicarb dose had 2 d longer developmental time until adulthood than those reared on unsprayed leaves (F = 22.56; df = 1, 12; p = 0.0005).
3.3.2. Effects on Reproduction
Sublethal effects on S. frugiperda reproduction rate were detected when larvae were exposed to 50% of the field label doses of flubendiamide or thiodicarb applied to Cry1Ac and non-Bt soybean (
Table 1). Adult females obtained from larvae that had developed on Cry1Ac soybean leaves sprayed with 50% of the field label dose of flubendiamide (from L1 and L2 larvae) or thiodicarb (only from L2 larvae) laid 25% fewer eggs than those from larvae fed on Cry1Ac soybean without insecticide (F = 5.91; df = 1, 12; p = 0.0316 for L1 stage; F = 6.27; df = 2, 18; p = 0.0086 for L2 stage). Similarly, females from L1 or L2 larvae reared on non-Bt soybean sprayed with a 50% dose of flubendiamide or thiodicarb also had reduced fecundity (24%–31% fewer eggs laid) compared with females from larvae raised on non-Bt soybean without insecticide (F = 9.33; df = 2, 15; p = 0.0023 for L1 stage; F = 19.71; df = 2, 20; p < 0.0001 for L2 stage) (
Table 1). However, regardless of the soybean type, the percentage of eggs hatching did not differ for females obtained from larvae developing on sprayed versus unsprayed soybean leaves (>82.6% eggs hatching) (p > 0.0503) (
Table 1).
4. Discussion
The GM soybean event MON 87701 × MON 89788 × MON 87751 × MON 87708, which expresses Cry1A.105/Cry2Ab2/Cry1Ac insecticidal proteins, showed high lethality against S. cosmioides, S. albula and S. eridania but had relative low lethality against S. frugiperda after 10 d of exposure. Except for S. frugiperda, no larvae of the tested Spodoptera species developed into adults on this soybean type. Results from previous studies also showed that no larvae of S. cosmioides, S. albula or S. eridania were able to develop to the adult stage on Cry1A.105/Cry2Ab2/Cry1Ac soybean [
10]. Nonetheless, none of the L1 and L2 larvae of Spodoptera species surviving on Cry1A.105/Cry2Ab2/Cry1Ac soybean for 10 d (
40% for S. frugiperda and <20% for other species tested) survived until the adult stage when exposed to leaves sprayed with flubendiamide or thiodicarb at either dose level. On Cry1A.105/Cry2Ab2/Cry1Ac soybean without insecticide, only S. frugiperda larvae could develop to adulthood.
The survival of S. frugiperda until the adult stage on Cry1A.105/Cry2Ab2/Cry1Ac soybean can be explained by the cross-crop resistance and high frequency of Cry protein resistance alleles in Brazilian populations of this species [
13,
15,
28]. These characteristics negatively affect the performance of further Bt plants, including cotton and other types of Bt soybean, against this species [
10,
14,
16]. These results indicated that, in growing areas cultivated with Cry1A.105/Cry2Ab2/Cry1Ac soybean, complementary chemical control is needed in situations of high infestation of S. frugiperda, but only when the number of surviving larvae exceeds the economic threshold level (20 large larvae (≥1.5 cm) per sample cloth in 1 m of soybean row, 30% defoliation at the vegetative stage or 15% defoliation at the reproductive stage) [
29]. In contrast to the results with Cry1A.105/Cry2Ab2/Cry1Ac soybean, MON 87701 × MON 89788 (Cry1Ac soybean) presented reduced lethality against all Spodoptera species, with more than 73% developing into adults. Previous studies also reported low lethality of this Bt soybean against Spodoptera species [
5,
8,
10]. Similar survival until the adult stage was also observed when Spodoptera species developed on non-Bt soybean leaves (>71% developed into adults). However, S. cosmioides, S. eridania and S. albula larvae surviving on Cry1Ac or non-Bt soybean did not produce adults when exposed to soybean leaves sprayed with either dose of flubendiamide or thiodicarb. Spodoptera frugiperda larvae surviving on these soybean types could produce adults when exposed to a 50% dose of either insecticide, but not when exposed to the full field label doses. These results indicate that, if outbreaks of these species are detected in Cry1Ac soybean fields, insecticide application is indicated when action thresholds are reached, since these species have high defoliation capacity (mainly S. cosmioides) and also attack flowers and pods during the reproductive stages, reducing soybean yield [
29,
30].
In addition to lethal effects, the exposure of insects to sublethal concentrations of insecticides can affect insect behavior, physiology and ecology [
18]. In our study, sublethal effects of Bt proteins and insecticides on S. frugiperda developing on soybean leaves (Bt and non-Bt) sprayed with 50% of field label dose of flubendiamide or thiodicarb were evident: these insects had an extended larval stage, and females had lower fecundity than insects that had developed on the same soybean leaf type without insecticide. Previous studies also reported substantial sublethal effects of chlorantraniliprole (same mode of action of flubendiamide), reducing the number of eggs laid by S. frugiperda [
31]. Chlorantraniliprole also prolonged the larval, pupal and generation times of S. exigua and it increased the life cycle time and reduced the fecundity of S. cosmioides [
32,
33], whereas flubendiamide reduced the fecundity and fertility of S. litura [
34,
35]. Studies also reported relevant sublethal effects of Bt proteins expressed on soybean, reducing the larval biomass, adult fecundity and population growth potential of several Spodoptera species, including S. frugiperda [
5,
10,
11,
16]. In summary, Spodoptera species surviving on Bt and non-Bt soybean exposed to non-lethal quantities of flubendiamide or thiodicarb had prolonged immature stages and decreased female reproductive performance. Combining control tactics is an important component of IPM, and the use of Bt plants can help manage lepidopteran pests that are not targeted by the Bt technologies but exhibit sublethal effects from these technologies that could enhance the level of control of chemical insecticides.
5. Conclusions
In this study, we documented lethal and sublethal effects of flubendiamide and thiodicarb on Spodoptera species surviving on different Bt and non-Bt soybean types. Lethal effects included the following: (i) after 10 d of exposure, Bt soybean expressing Cry1A.105/Cry2Ab2/Cry1Ac insecticidal proteins presented high lethality against larvae of S. cosmioides, S. albula and S. eridania, but relatively low lethality against S. frugiperda; (ii) Bt soybean expressing only Cry1Ac insecticidal protein had low lethality against larvae of all Spodoptera species tested; (iii) none of the Spodoptera larvae exposed to leaves sprayed with the field label dose of flubendiamide or thiodicarb survived to adulthood, regardless of soybean plant type; and (iv) among insecticide-exposed larvae, only S. frugiperda that survived on Cry1Ac and non-Bt soybean sprayed with 50% of the field label dose of flubendiamide or thiodicarb survived to adulthood. Sublethal effects on S. frugiperda of flubendiamide or thiodicarb applied to Cry1Ac and non-Bt soybean included prolonged immature stage and lower female fecundity, suggesting population suppression of this pest. In summary, the lethal and sublethal effects here reported can help to optimize the management of Spodoptera species on Bt and non-Bt soybean fields in Brazil and other South American countries.
Author Contributions
Conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing D.N.G. and O.B.; methodology, validation, investigation, writing—original draft preparation, visualization, V.E.P., P.G.A., M.A.G.W. and L.F.W.; conceptualization, writing—original draft preparation, writing—review and editing, R.J.H., S.M. and G.P.H.; resources, supervision, project administration and funding acquisition, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the National Council for the Improvement of Higher Education (CAPES)—Finance Code 001. This research was also funded by the National Council for Technological and Scientific Development (CNPq) (grant numbers 430483/2018-0 and 305464/2020-5).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the National Council for the Improvement of Higher Education (CAPES), which provided a scholarship to the first author. We are also grateful to the National Council for Technological and Scientific Development (CNPq) for the research fellowship to O.B. (Process 305464/2020-5).
Conflicts of Interest
R.J.H., S.M. and G.P.H. are employed by Bayer Crop Science, but these authors declare no additional conflicts of interest. D.N.G., V.E.P., P.G.A., M.A.G.W., L.F.W. and O.B. declare no potential conflict of interest.
References
- Pogue, M.G. A world revision of the genus Spodoptera Guenée: (Lepidoptera: Noctuidae). In Memoirs of the American Entomological Society; American Entomological Society: Philadelphia, PA, USA, 2002; Volume 43, pp. 1–202. [Google Scholar]
- Parra, J.R.P.; Coelho Jr, A.; Cuervo-Rugno, J.B.; Garcia, A.G. , Moral, R.A.; Specht, A., Ed.; Neto, D.D. Important pest species of the Spodoptera complex: Biology, thermal requirements and ecological zoning. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Blanco, C.A.; Chiaravalle, W.; Dalla-Rizza, M.; Farias, J.R.; García-Degano, M.F.; Gastaminza, G.; Mota-Sánchez, D.; Murúa; M.G.; Omoto, C.; Pieralisi, B.K.; Rodríguez, J.; Rodríguez-Maciel, J.C.; Terán-Santofimio, H.; Terán-Vargas, A.P.; Valencia, S.J.; and Willink, E. Current situation of pests targeted by Bt crops in Latin America. Curr. Opin. Insect Sci. 2016, 15, 131–138. [CrossRef]
- Bernardi, O.; Malvestiti, G.S.; Dourado, P.M.; Oliveira, W.S.; Martinelli, S.; Berger, G.U.; Head, G.P.; Omoto, C. Assessment of the high-dose concept and level of control provided by MON 87701 × MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. 2012, 68, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, O.; Sorgatto, R.J.; Barbosa, A.D.; Domingues, F.A.; Dourado, P.M.; Carvalho, R.A.; Martinelli, S.; Head, G.P.; Omoto, C. Low susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera frugiperda (Lepidoptera: Noctuidae) to genet-ically-modified soybean expressing Cry1Ac protein. Crop Prot. 2014, 58, 33–40. [Google Scholar] [CrossRef]
- Dourado, P.M.; Bacalhau, F.B.; Amado, D.; Carvalho, R.A.; Martinelli, S.; Head, G.P.; Omoto, C. High susceptibility to Cry1Ac and low resistance allele frequency reduce the risk of resistance of Helicoverpa armigera to Bt soybean in Brazil. PLoS ONE 2016, 11, 0161388. [Google Scholar] [CrossRef]
- Bacalhau, F.B.; Dourado, P.M.; Horikoshi, R.J.; Carvalho, R.A.; Semeão, A.; Martinelli, S.; Berger, G.U.; Head, G.P.; Salvadori, J.R.; Bernardi, O. Performance of genetically modified soybean expressing the Cry1A.105, Cry2Ab2, and Cry1Ac proteins against key lepidopteran pests in Brazil. J. Econ. Entomol. 2020, 113, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, R.J.; Dourado, P.M.; Berger, G.U.; Fernandes, D.S.; Omoto, C.; Willse, A.; Martinelli, S.; Head, G.P.; Corrêa, A.S. Large-scale assessment of lepidopteran soybean pests and efficacy of Cry1Ac soybean in Brazil. Sci. Rep. 2021, 11, 15956. [Google Scholar] [CrossRef]
- Kynetec. FarmTrack Soybean 22/23. 2023. Available online: https://www.kynetec.com/ (accessed on 5 July 2023).
- Barcellos, G.A.; Hanich, M.R.; Pretto, V.E.; Weschenfelder, M.A.G.; Horikoshi, R.J.; Dourado, P.M.; Ovejero, R.F.L.; Berger, G.U.; Martinelli, S.; Head, G.P.; et al. Characterizing the lethal and sub-lethal effects of genetically₋modified soybean expressing Cry1A.105, Cry2Ab2, and Cry1Ac insecticidal proteins against Spodoptera species (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. 2022, 79, 548–559. [Google Scholar] [CrossRef]
- Horikoshi, R.J.; Dourado, P.M.; Bernardi, O.; Willse, A.; Godoy, W.A.C.; Omoto, C.; Bueno, A.F.; Martinelli, S.; Berger, G.U.; Head, G.P.; et al. Regional pest suppression associated with adoption of Cry1Ac soybean benefits pest management in tropical agriculture. Pest Manag. Sci. 2022, 78, 4166–4172. [Google Scholar] [CrossRef]
- Machado, E.P.; Rodrigues Junior, G.L.S.; Somavilla, J.C.; Führ, F.M.; Zago, S.L.; Marques, L.H.; Santos, A.C.; Nowatzki, T.; Dahmer, M.L.; Omoto, C.; et al. Survival and development of Spodoptera eridania, Spodoptera cosmioides and Spodoptera albula (Lepidoptera: Noctuidae) on genetically-modified soybean expressing Cry1Ac and Cry1F proteins. Pest Manag. Sci. 2020, 76, 4029–4035. [Google Scholar] [CrossRef]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; Santos, A.C.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 64,150–158. [Google Scholar] [CrossRef]
- Horikoshi, R.J.; Bernardi, D.; Bernardi, O.; Malaquias, J.B.; Okuma, D.M.; Miraldo, L.L.; Amaral, F.S.A.; Omoto, C. Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: implications for resistance management. Sci. Rep. 2016, 6, 34864. [Google Scholar] [CrossRef] [PubMed]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, J.R.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, Samuel.; Head, G. P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [CrossRef]
- Machado, E.P.; Rodrigues Junior, G.L.S.; Somavilla, J.C.; Führ, F.M.; Zago, S.L.; Marques, L.H.; Santos, A.C.; Nowatzki, T.; Dahmer, M.L.; Omoto, C.; et al. Cross-crop resistance of Spodoptera frugiperda selected on Bt maize to genetically-modified soybean expressing Cry1Ac and Cry1F proteins in Brazil. Sci. Rep. 2020, 10, 10080. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Fabrick, J.A. and Yves Carrière. Global patterns of insect resistance to transgenic Bt crops: The first 25 years. J. Econ. Entomol. 2023, 116, 297–309. [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Saber, M.; Parsaeyan, E.; Vojoudi, S.; Bagheri, M.; Mehrvar, A.; Kamita, S.G. Acute toxicity and sublethal effects of methoxyfenozide and thiodicarb on survival, development and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Crop Prot. 2013, 43, 14–17. [Google Scholar] [CrossRef]
- De França, S.M.; Breda, M.O.; Barbosa, D.R.; Araujo, A.M.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; Intech Open: London, UK, 2017; pp. 23–39. [Google Scholar]
- Barros, E. M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Ramalho, F.S.; Azeredo, T.L.; Nascimento, A.R.B.; Fernandes, F.S.; Nascimento Júnior, J.L.; Malaquias, J.B.; da Silva, C.A.D.; Zanuncio, J.C. Feeding of fall armyworm, Spodoptera frugiperda, on Bt transgenic cotton and its isoline. Entomol. Exp. Appl. 2011, 134, 207–214. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Matos, J.M.L.; Orozco-Restrepo, S.M.; Paula-Moraes, S.V.; Pereira, E.J.G. Like parents, like offspring? Susceptibility to Bt toxins, development on dual-gene Bt cotton, and parental effect of Cry1Ac on a nontarget lepidopteran pest. J. Econ. Entomol. 2020, 113, 1234–1242. [Google Scholar] [CrossRef]
- Greene, D.F.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 488–497. [Google Scholar] [CrossRef]
- Farias, J.R.B.; Nepomuceno, A.L.; Neumaier, N.; Ecofisiologia da soja. Circular Técnica Embrapa Soja. 2007, 48, 1–9. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/470308 (accessed on 20 May 2023).
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–266. [Google Scholar] [CrossRef]
- SAS Institute. Statistical Analysis System: Getting Started with the SAS Learning; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- Bernardi, D.; Salmeron, E.; Horikoshi, R.J.; Bernardi, O.; Dourado, P.M.; Carvalho, R.A.; Martinelli, S.; Head, G.P.; Omoto, C. Cross-resistance between Cry1 proteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PLoS ONE 2015, 10, e0140130. [Google Scholar] [CrossRef] [PubMed]
- Bueno, R.C.O.F.; Bueno, A.F.; Moscardi, F.; Parra, J.R.P.; Hoffmann-Campo, C.B. , Lepidopteran larva consumption of soybean foliage: basis for developing multiple-species economic thresholds for pest management decisions. Pest. Manag. Sci. 2010, 67, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.M.; Paula-Moraes, S.V.; Pasini, A.; Hoback, W.W.; Hayashida, R.; Bueno, A.F. Simulated soybean pod and flower injuries and economic thresholds for Spodoptera eridania (Lepidoptera: Noctuidae) management decisions. Crop Prot. 2022, 155, 105936. [Google Scholar] [CrossRef]
- Wu, H.-M.; Feng, H.-L.; Wang, G.-D.; Zhang, L.-L.; Zulu, L.; Liu, Y.-H.; et al. Sublethal effects of three insecticides on development and reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae). Agronomy 2022, 12, 1334. [Google Scholar] [CrossRef]
- Lutz, A.L.; Bertolaccini, I.; Scotta, R.R.; Curis, M.C.; Favaro, M.A.; Fernandez, L.N.; Sánchez, D.E. Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae). Pest Manag. Sci. 2018, 74, 2817–2821. [Google Scholar] [CrossRef]
- Kong, F.; Song, Y.; Zhang, Q.; Wang, Z.; Liu, Y. Sublethal effects of chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) moth: Implication for attract-and-kill strategy. Toxics 2021, 9, 20. [Google Scholar] [CrossRef]
- Teng, H.; Yuan, Y.; Zhang, T.; Chang, X.; Wang, D. Evaluation of the sublethal effect of tetrachlorantraniliprole on Spodoptera exigua and its potential toxicity to two non-target organisms. PLoS ONE 2020, 15, e0242052. [Google Scholar] [CrossRef]
- Jameel, M.; Jamal, K. Efficacy of sub lethal concentration of flubendiamide against larvae of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). J. Entomol. Zool. Stud. 2017,, 5, 670–674. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).