1. Introduction
The consumption of milk and its derivatives dates back thousands of years, and it was already considered a food par excellence, even without scientific evidence to support it as such [
1]. In the world, more than 6 billion people are active consumers of milk and its derivatives, of which the majority reside in developing countries [
2]. World milk production has increased by 75% in the last 33 years, from 530 in 1988 to almost 928 million tons in 2021 [
3]. For example, in the year 2021, it exceeded the production of the year 2020 by 1.3%. For its part, in Peru as of October 2022, 1.9 million tons of raw milk have been produced, with the participation of more than 2 million producers, of which 65% are farmers and ranchers, and only 13% It is dedicated solely to livestock [
4].
The dairy production chain has as a priority to obtain at the end of the process products with a high level of nutritional quality and innocuous for the health of the consumer [
5]. The physicochemical parameters, technological and sanitary characteristics of milk play a very important role as indicators of excellence for its processing and transformation [
6]. The quality of the milk and the safety during processing is an indicator of the health status of the farm at the group or individual level [
5]. These indicators are mainly based on nutritional evaluation parameters, somatic cell count, microbiological and physicochemical analyzes [
7,
8].
The evaluation of microbiological parameters allows to verify the hygienic quality of the milk, which can be affected by contamination factors such as poor hygiene in the equipment, collection and transfer center, handling of the operators or failures in the storage process. Certain methodologies, such as the count of coliforms or mesophilic aerobics, allow evaluating the degree of impact of these bad practices on the quality and safety of milk [
9].
Somatic cells are white blood cells, typical of the immune system and are involved in the protection of the mammary gland, which increase and travel through the blood to neutralize bacteria that cause diseases or intramammary infections [
10]. There is evidence of a trend towards a directly proportional behavior of the somatic cell count with the quality of milk and dairy products, whose elevated results have been associated with changes in milk composition [
11,
12]. There is a negative correlation between the number of somatic cells with the percent of lactose and positive with the percent of total solids, fat and protein [
13]. These variations described above negatively affect the safety, useful life and organoleptic characteristics of the final product [
14].
In most countries, maximum limits allowed for the number of somatic cells are established. In Peru, Supreme Decree N° 007-2017-MINAGRI [
15] approved the regulation of milk and dairy products. In this regulation it is established that raw milk to be admissible for human consumption, the somatic cell count must be less than 500000 cells/ml and the maximum permissible limits of aerobic mesophilic and coliforms totals must be 1000000 CFU/ml and
1000 MPN/ml, respectively.
The main physicochemical parameters of milk (solids not fat, protein, fat, density and pH) are characteristics that depend on the stage of lactation, milking routine, diet, breed, as well as storage conditions and their study allow monitoring the quality of milk [
16]. In this context and the importance of dairy production and its derivatives in food safety, the objective of this research was to evaluate the physicochemical and sanitary parameters of bovine milk collected in 31 collection centers and dairy processors in the Amazon region.
2. Materials and Methods
2.1. Sample and Sampling
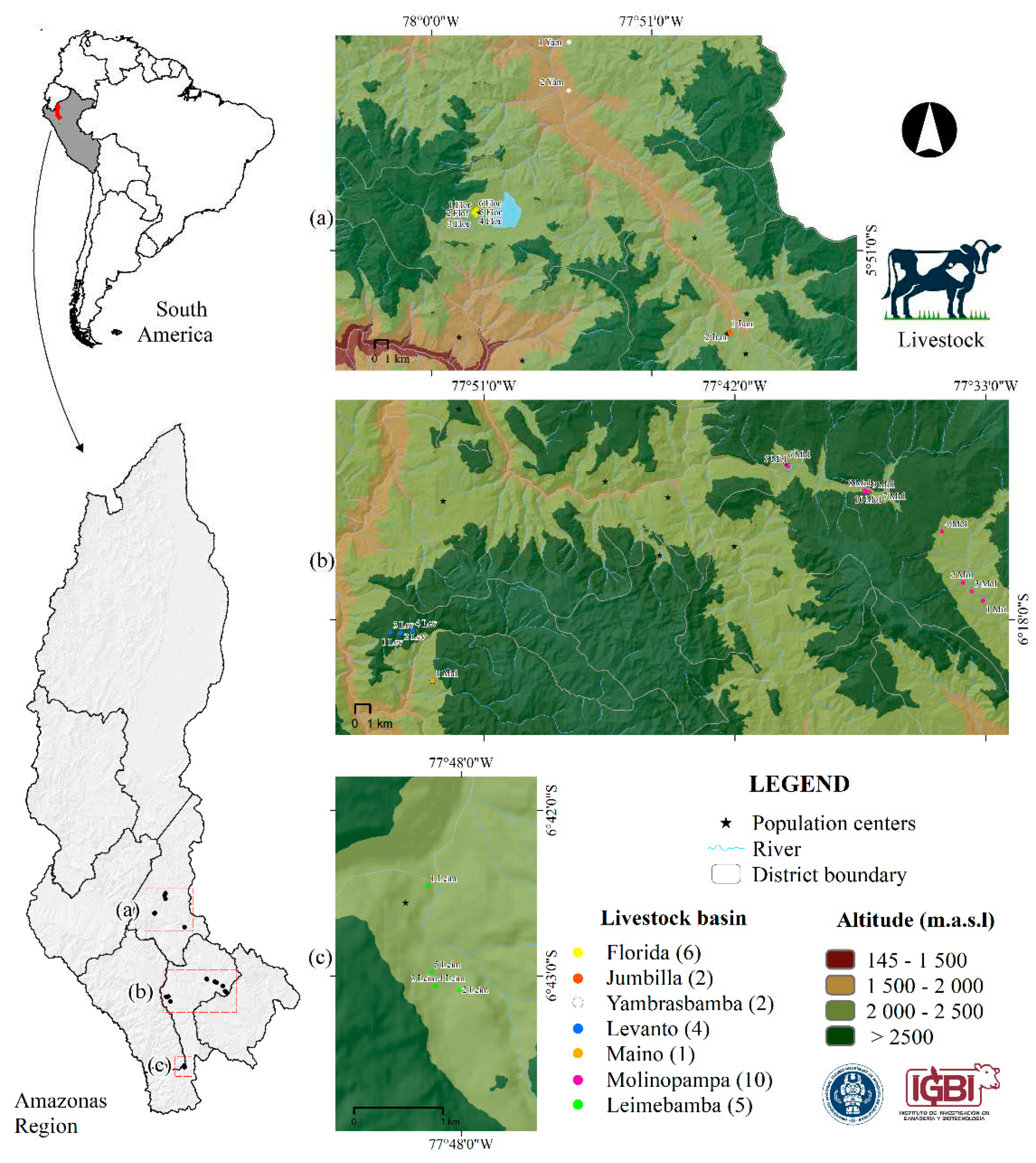
Thirty-one collection centers and dairy processors from the livestock basins of Molinopampa (10), Florida-Pomacochas (06), Yambrasbamba (03), Jumbilla (02), Leymebamba (05), Levanto (04), and San Isidro del Maino (01) belonging to the Amazonas region, Peru (
Figure 1). Four replicates of 100 ml of milk were obtained for each collection center evaluated (n=124). The milk was extracted directly from the tanks and placed in sterile vials. Each vial was labeled with the following information: collection center number, place, date and time of sampling. All samples were transported at 4°C to the Laboratory of Infectious and Parasitic Diseases of Domestic Animals of the National University Toribio Rodríguez of Mendoza from Amazonas for processing.
2.2. Parameter Evaluation
The physicochemical parameters evaluated were fat (%), solids not fat (%), protein (%), lactose (%), pH and density (g/ml) using a Lactoscan equipment (Ultrasonic Milk Analyzer SP50, Milkotronic, Bulgaria). Somatic cell counting was performed following the automatic somatic cell counter protocol (DeLaval, De Laval Cell Counter DCC 10435, Sweden) using single-use cassettes coded N° 92865880. The microbiological analysis of viable mesophilic aerobic bacteria (Colony Forming Unit - CFU/ml) and total coliforms (Most Probable Number - MPN/mL) was carried out by the dilution method.
2.3. Data Analysis
Assumptions such as normality and homogeneity of variances of the data set were verified. The variance analysis procedure was applied using the mixed general linear model (MGLM) and for the comparison between the collection centers the Di Rienzo, Guzman and Casanoves (DGC) test was performed. For the variable protein in the collection center, a statistical model adjustment was made applying heteroskedasticity using a vardent g(d)=d model, which achieved an AIC= 10.20 and BIC= 167.22 lower in regard to other models for compliance with data normality. For the variable somatic cells, total coliforms and mesophilic aerobic bacteria, normality was measured using the Wilk Shapiro test. In addition, we performed a multivariate test, specifically, cluster analysis with the Ward method and Euclidean distance to identify groups that share similar characteristics, and an analysis of variance with (MGLM) was applied to determine the variables that significantly contribute to the conformation of groups and to the explanation of the phenomenon under study. Finally, we applied the multivariate principal component test and a Pearson correlation analysis (p<0.05). The data were processed with the statistical software InfoStat/Professional version 2018p.
3. Results
3.1. Raw Milk Parameters by Collection Center
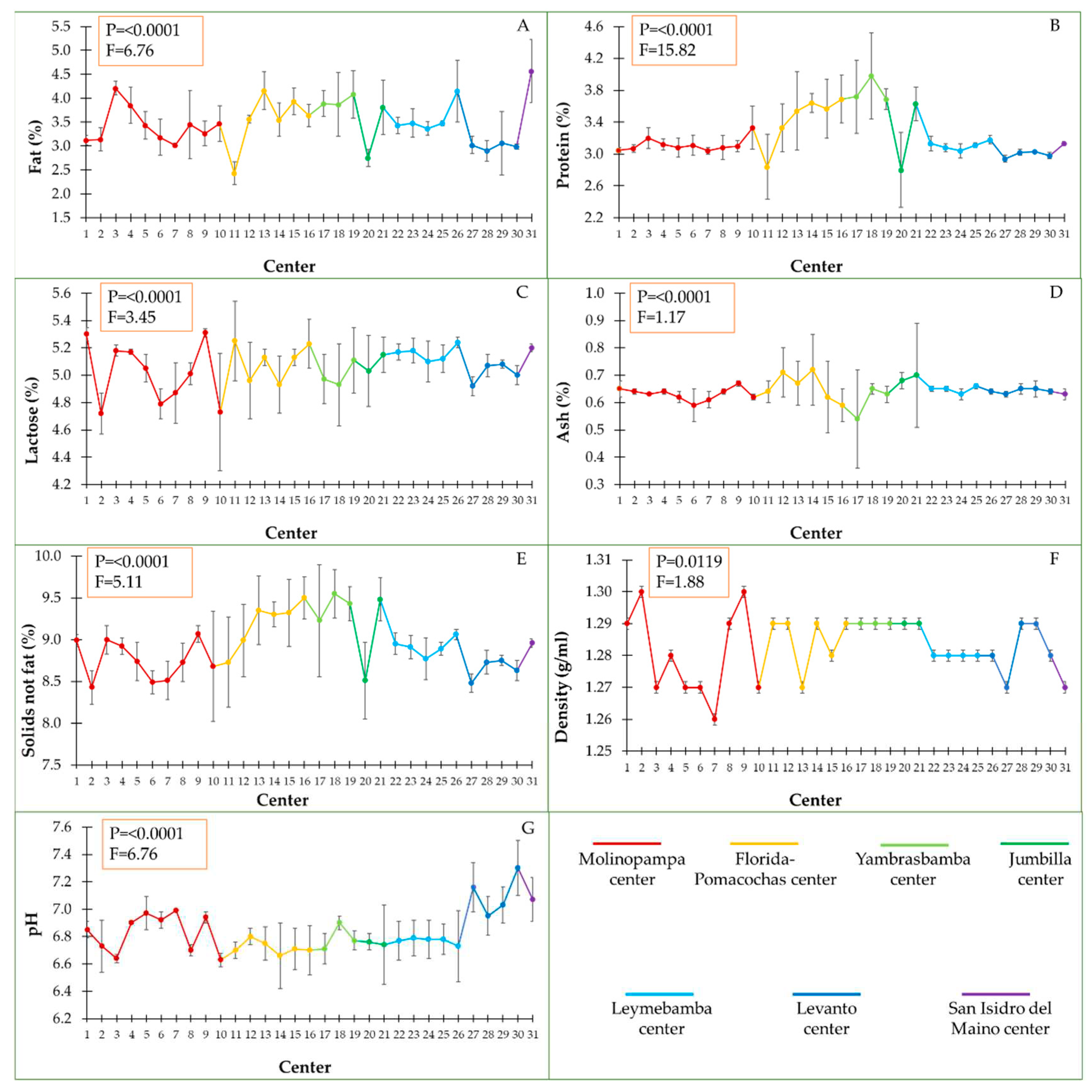
According to the collection center, we found significant differences for the physicochemical parameters (
Figure 2). The fat percent varied significantly between the collection centers, in the collection center eleven corresponding to Florida-Pomacochas, the milk contained 2.43% fat and the center twenty corresponding to Jumbilla, the milk contained 2.75%. However, in collection center three, corresponding to Molinopampa and center thirty-one corresponding to San Isidro del Maino, the milk fat percent was higher (4.21 and 4.57%, respectively) (
Figure 2A).
The protein percent varied significantly between the collection centers, registering 2.8% as a minimum and 3.98% as a maximum. A similar effect to the percent of fat was observed in center eleven corresponding to Florida-Pomacochas, the milk contained 2.84% protein and center twenty corresponding to Jumbilla, the milk contained 2.80% protein. In the eighteenth collection center corresponding to Yambrasbamba, the maximum value of 3.98% protein was recorded and, in the centers belonging to Molinopampa they presented less variation in protein percent (
Figure 2B).
The percent of lactose was highly variable among the milk from the evaluated centers, centers two, six and ten corresponding to Molinopampa registered the lowest percent of lactose (
Figure 2C). However, the percent of ash, the variability was lower with respect to lactose (
Figure 2D). In the fourteenth collection center corresponding to Florida-Pomacochas, the highest percent of ash was obtained (0.72%).
The solids not fat of the milk according to the collection center were variable, the minimum percent of these solids was registered in the collection center two of Molinopampa (8.43%) and the maximum was in the center eighteen corresponding to Yambrasbamba (9.55%) (
Figure 2E). The highest density variability was observed in the Molinopampa collection centers and the lowest variability was in the Yambrasbamba, Jumbilla, and Leymebamba centers (
Figure 2F). The pH varied of 6.6 to 7.3, being significantly between collection centers. The maximum pH value was recorded in the milk that came from the Levanto collection center and the lowest pH was in the milk from the Molinopampa collection center (
Figure 2G).
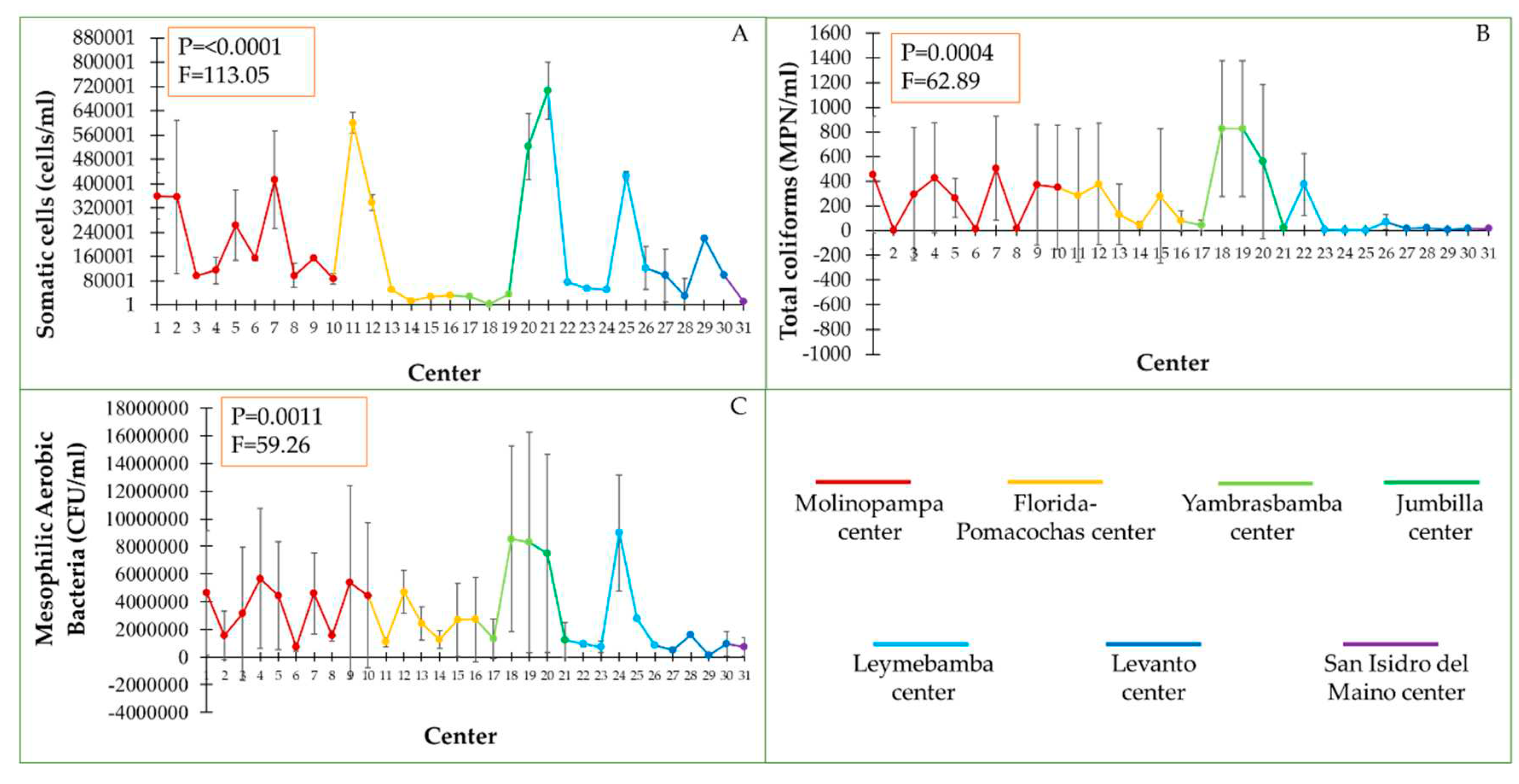
According to the collection center, we found significant differences for somatic cell count and microbiological analysis (
Figure 3). The somatic cell count according to the collection center varied significantly, the highest value of cells was registered in the twenty-first collection center corresponding to Jumbilla, exceeding 700 thousand cells/ml of milk, followed by the eleventh collection center corresponding to Florida- Pomacochas, exceeding 600000 cell/ml of milk and the collection center thirty corresponding to San Isidro del Maino registered the lowest number of cells with 13000 cells/ml (
Figure 3A).
The microbiological analysis (total coliforms and mesophilic aerobic bacteria) was highly variable in all collection centers (
Figure 3B). In two collection centers in Yambrasbamba (collection center 18 and 19) and one in Jumbilla (collection center 20), they registered the highest number of total coliforms. However, in two collection centers in Leymebamba (collection center 24 and 25) and one in Molinopampa (collection center 2) there was a low count of total coliforms (from 1 to 5 MPN/ml). The highest amount of mesophilic aerobic bacteria was recorded in milk from the collection centers of Yambrasbamba (8562500 CFU/ml) and Leymebamba (8985000 CFU/ml). Milk with less mesophilic aerobic bacteria were from the collection centers of Levanto (135000 CFU/ml) and Molinopampa (750000 CFU/ml) (
Figure 3C).
3.2. Raw Milk Quality Parameters by Livestock Basin
The number of somatic cells, fat, protein, solids not fat, and pH varied by basin (
Table 1). The Jumbilla basin presented a lower number of somatic cells in regard to the other basins. The milk fat percent was higher in basins such as Florida-Pomacochas, Jumbilla, Leymebamba, Molinopampa, Yambrasbamba and San Isidro del Maino in regard to milk from the Levanto basin. The protein percent in milk was higher in the Yambrasbamba basin in regard to milk from the other basins. The percent of solids not fat was higher in the milk from the Florida-Pomacochas and Yambrasbamba basins in regard to the milk from the other basins. The milk with the most alkaline pH was recorded in the Levanto and San Isidro del Maino basins. Lactose, ash, density, total coliforms and mesophilic aerobic bacteria did not vary by basin.
3.3. Classification of Raw Milk from Different Livestock Basins and Grouped by Similar Characteristics
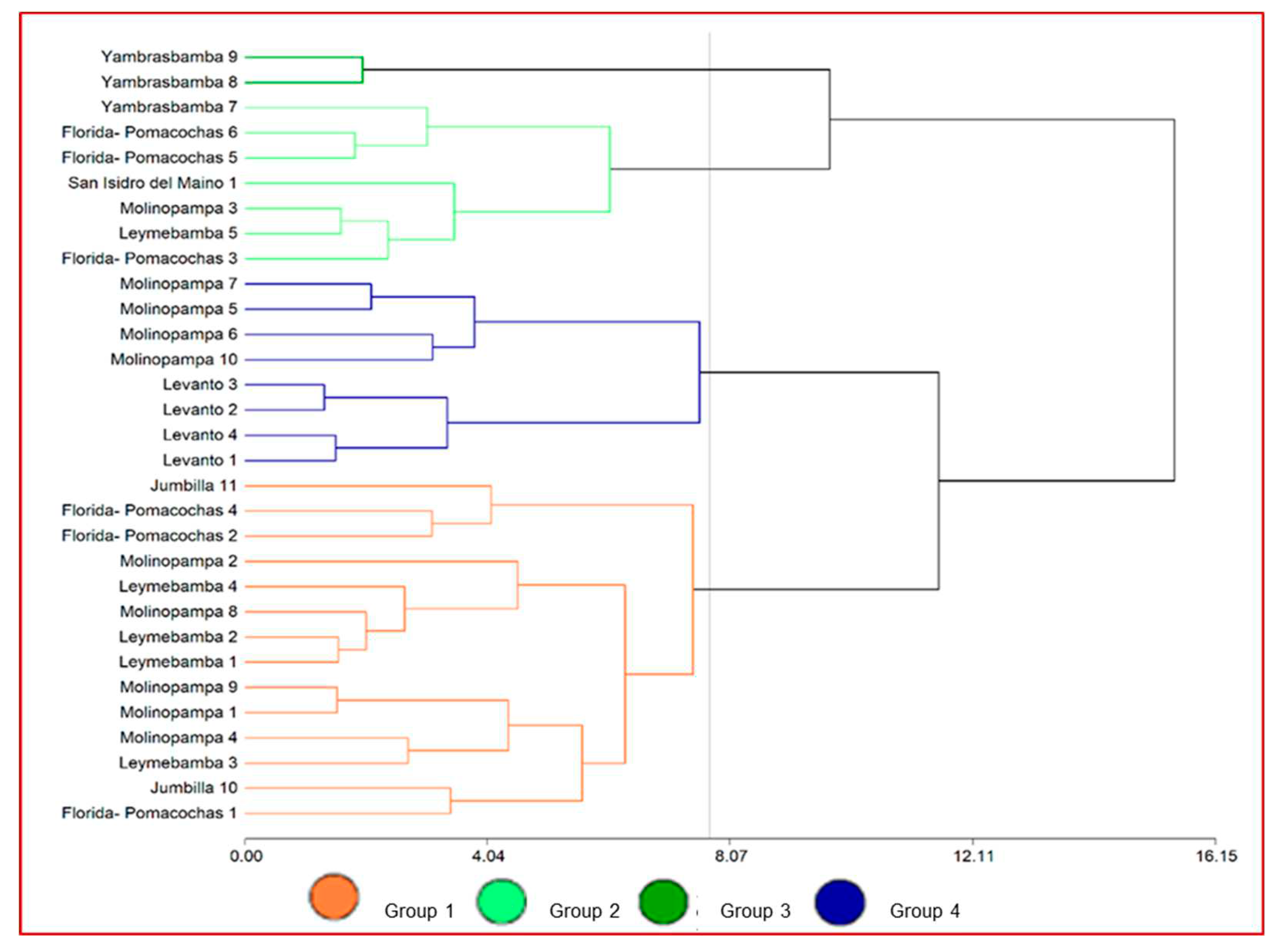
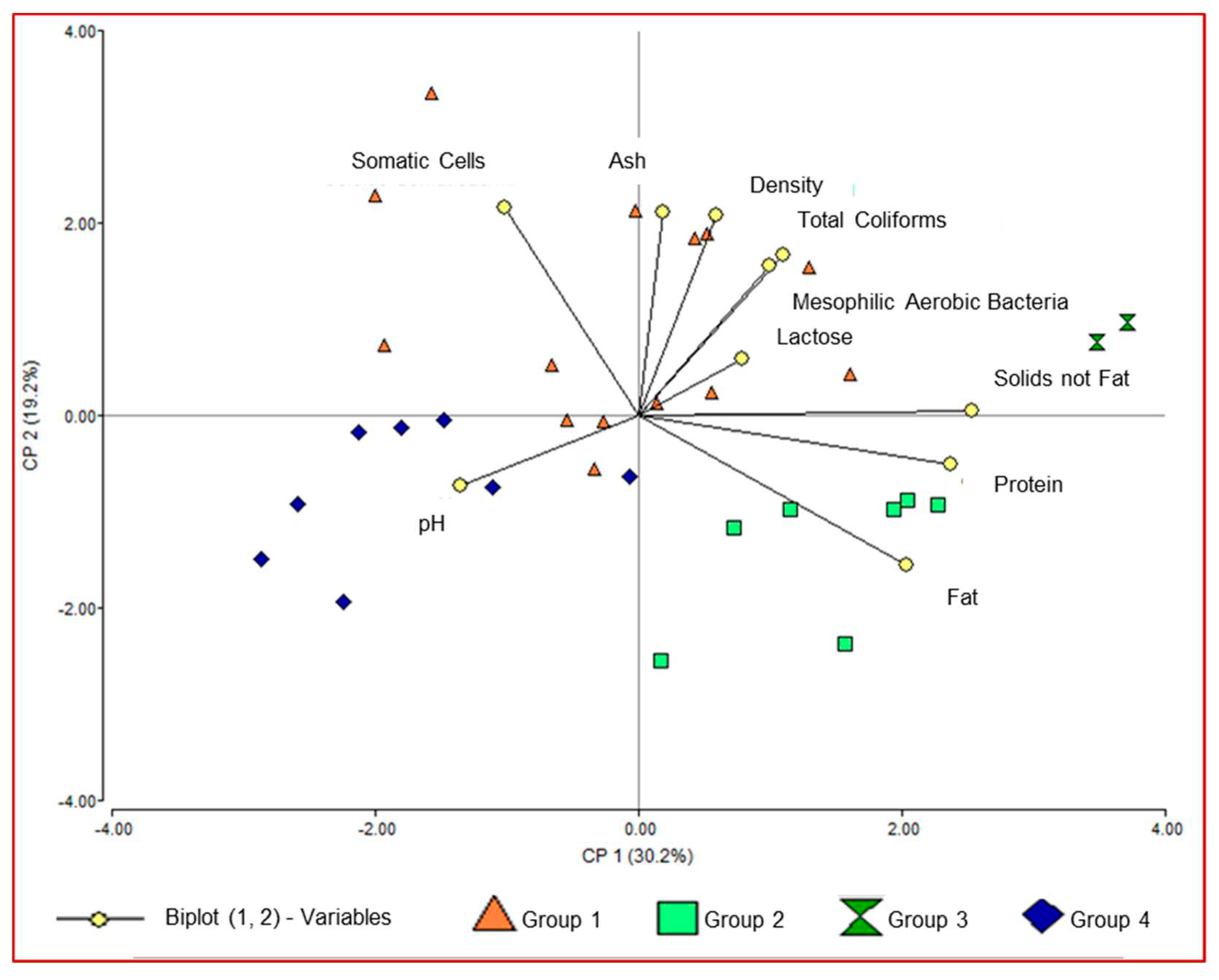
According to the multivariate test (cluster analysis with the Ward method and Euclidean distance), we were able to identify four groups that share similar characteristics (
Figure 4). Group 1 was conformed of two collection centers, Group 2 was conformed of seven collection centers, Group 3 conformed of eight collection centers and Group 4 conformed of the largest number of collection centers (fourteen) that show similarity in its physicochemical characteristics, somatic cell count and milk microbiology.
3.3. Characteristics of Raw Milk According to Group
We found highly significant differences between the groups. Group 1 differs from Groups 2, 3 and 4 in the percent of ash and milk density. Groups 2 and 3 are characterized by having a higher percent of fat in milk in regard to the other groups. Group 3 was characterized by the highest protein percent in milk, however, in this group the highest number of total coliforms and mesophilic aerobic bacteria was recorded. Regarding Group 4, the highest pH and the lowest protein percent were recorded. The groups with the lowest somatic cell counts were Groups 2, 3 and 4 (
Table 2).
The principal component analysis for the 10 variables evaluated explained 49.4% of the variance with their values. The first component managed to explain 30.2% and the second component explained 19.2% of the variance (
Figure 5). In addition, we can observe significant positive correlations between the percent of fat with the percent of protein (p<0.001*; r=0.62) and the percent of solids not fat (p=0.001*; r=0.66), which means that the higher the protein levels, the higher the concentration of fats and solids not fat. There is also a significant correlation between the percent of lactose with the percent of solids not fat (p<0.02*; r=0.43), indicating that the higher the lactose concentration there is a higher concentration of solids not fat. In addition, a positive correlation between ash and density was entered (p<0.04; r= 0.37), demonstrating that the higher the ash concentration, the higher the milk density. On the other hand, the significant negative correlations found between somatic cells and the fat percent (p<0.01*; r=-0.46), indicate that the higher the somatic cell count, the lower the fat percent and the higher the protein and solids not fat percent, pH decreases (p<0.04*; r=-0.37 and p<0.04*; r=-0.38 respectively).
4. Discussion
In general, the physicochemical and sanitary parameters of raw milk, corresponding to 31 collection centers and dairy processors in the Amazonas region, show us that certain parameters such as fat (average value of 3.2%), somatic cell count (maximum of 500000 cells/ml), the density (between 1.029 and 1.034 g/ml) are within the technical specifications of Supreme Decree N° 007-2017-MINAGRI [
15]. The percent of solids not fat (minimum of 8.2%) and the parameter of viable aerobic mesophilic bacteria (maximum of 1000000 UCF/ml) do not reach the standards indicated in Supreme Decree N° 007-2017-MINAGRI [
15]. In this sense, the milk produced in this region does not meet all the nutritional and sanitary specifications according to Peruvian regulations. The percent of fat (around 3%), protein (2.9% to 3.9%), lactose (approximately 4.84%) and pH (approximately 6.61) are among the normal values for bovine milk [
17,
18].
In this investigation we found that, at a low somatic cell count in milk, the fat percent is higher, the pH level is higher, and the solids not fat is lower (
Figure 5). The effect of somatic cells on the amount of fat is contradictory, while some authors describe a decrease in the percent of fat as the number of somatic cells increases [
19], the results of other investigations show an opposite relationship [
20,
21]. Possibly, the release of lipolytic enzymes by certain somatic cells is the reason why there is a decrease in milk fat with high somatic cell counts [
10]. On the other hand, the percent of solids is significantly higher in milk with a high somatic cell count. Our results are similar to those described by Silva et al. [
22] and Cinar et al. [
13], who find a direct relationship between the somatic cell count and the number of solids. However, the increase in solids is not necessarily favorable, since there may be a reduction in milk production [
22]. Another factor that presented changes according to the somatic cell count category was the pH, being this higher in milk with low somatic cell count. This is due to a decrease in the secretory activities of mammary cells [
23].
The somatic cell count is an indicator that makes it possible to study the hygienic quality of milk. This indicator reflects the health of the udder of a herd and is related to the quality of the milk produced [
12]. Several countries have set a limit that guarantees the hygienic quality of milk, for example the maximum allowed value of somatic cells for Australia, Norway, New Zealand, Switzerland and in general the European Union is 400000 cells/ml of milk [
24,
25], for Canada it is 500000 cells/ml of milk [
26] and for the United States it is 750000 cells/ml of milk [
27]. In our research, on average we recorded 182623 cells/ml of milk, these values are within the maximum limit allowed at the level of the standards of the aforementioned countries. However, in a collection center in the Florida-Pomacochas livestock basin (600100 cells/ml) and Jumbilla (705823 cells/ml) they exceeded the maximum permissible values of the European Union but not of the United States. These are indications that management at milking time needs to be improved, proper udder cleaning and the use of teat dips are needed.
Regarding total coliforms, it ranged between 339.98 and 481.43 NMP/ml, these values of our research are lower than those found in tank milk [
28]. The number of coliforms in milk is related to hygiene habits during milking and contact with feces [
29]. The high microbiological load in the samples means that there may not be adequate milking, handling or storage of milk by the producer and processing centers in the Amazonas region. However, in two collection centers in Leymebamba and one in Molinopampa there was a low count of total coliforms (from 1 to 5 MPN/ml). These centers are within the limits of coliforms in raw milk, whose internationally accepted value is less than 100 MPN/ml [
30]. In addition, in other investigations on the coliform count in informally sold milk, the number of coliforms was higher than 103 MPN/ml in 66% of the samples analyzed [
31]. Ghali-Mohammed et al. [
32] indicate that the number of coliforms varies according to handling practices and season of the year, finding a higher number of coliforms in milk produced in the dry season (November to March). However, Celano et al. [
33] mention that they did not find differences in the number of coliforms in raw milk according to the season (winter in regard to summer). The wholesomeness of milk is a concern for consumer food safety, since safety remains a public health challenge and 81% of milk samples exceed international limits for total coliforms in raw milk [
34].
In relation to viable mesophilic aerobic bacteria, the values found in this study are below those described by Albuja et al. [
35] (between 8070000 to 122000000 UCF/ml) and above the permissible limits (UCF/ml < 1000000) of Supreme Decree N° 007-2017-MINAGRI of Peru [
15]. When the sample exceeds 100000 UCF/ml it is an indicator of poor hygiene practices during milking and milk production [
34]. Cases have been seen that more than 96% of raw milk samples exceed the permissible limits according to international standards [
34]. In addition, other investigations have reported values of 3.15 Log CFU/ml of raw milk at the level of raw tanker milk [
36], in milk from 33 herds in the state of New York 3.8 Log CFU/ ml of mesophilic aerobic bacteria has been reported [
37], in 45 herds in Norway 4.27 Log CFU/ml has been reported [
38], in 23 herds in Northern Italy 4.09 Log CFU/ml has been reported [
39], in 2 herds in southern Germany 4.5 Log CFU/ml has been reported [
40], in a herd in Croatia it has been reported from 3 to 5.17 Log CFU/ml [
41] and in 20 herds in the Czech Republic, 4.18 Log CFU/ml has been reported [
42]. These values indicate that raw milk contains mesophilic aerobic bacteria that could be affecting public health.
In the analysis of the correlation of the physicochemical and health parameters of all the samples, no clear association between the somatic cell count and the physicochemical parameters was observed. The significant correlation between somatic cell count and percent protein, as well as the correlation between total coliforms and lactose, is due to the fact that when examining bulk milk, the total volume of milk dilutes the effects of affected cows with mastitis and the inflammatory response because it is highly variable between individuals of the same herd [
43,
44]. However, the presence of fungi, bacteria and other microorganisms that produce alterations in the nutritional and organoleptic properties of milk will always be present [
45]. Actions such as training for producers, hygiene in the utensils used in milk storage, milking practices, cold rooms, water quality, among others, could help reduce the presence of these microorganisms to maintain the useful life of the milk [
37,
39].
5. Conclusions
Collection centers showed differences in all physicochemical parameters, somatic cell count and milk microbiology. According to the livestock basins, there is evidence of differences for somatic cells, fat, protein, solids not fat, pH, coliforms and bacteria, being the Jumbilla basin the one that presented the least amount of somatic cells, the Levanto basin the lowest fat percent and the Yambrasbamba basin higher protein, while the other parameters evaluated did not vary.
The cluster analysis generated four groups with similar behavior, Group 1 is higher in ash and density, Group 2 and 3 higher fat percent, Group 3 with a higher amount of protein, but a higher number of total coliforms and mesophilic aerobic bacteria. Group 4 registered the highest pH and the lowest protein percent. There is a positive correlation between fat with protein and solids not fat, lactose with solids not fat, ashes with density, which indicates a tendency to a directly proportional behavior. In addition, there are negative correlations between somatic cells and fat, and protein and solids not fat with pH, which translates into an inversely proportional behavior.
The somatic cell count is within the maximum permissible limits of international standards, however, the levels of total coliforms and mesophilic aerobic bacteria in some collection centers exceed these permissible limits. Local governments must include policies that allow regulating the consumption of milk that exceeds the maximum permissible levels to guarantee the food security of the population. Likewise, producers need more training in milk production and management.
Author Contributions
Conceptualization, H.F., Ll.S.P., H.D.E. and J.C.V.; methodology, L.M., S.M.P.V., M.A.A.R. and C.M.; software, M.E.M.P. and J.A.S.U.; validation, M.E.M.P., G.T.S.P. and J.A.S.U.; formal analysis, M.E.M.P. and J.A.S.U.; investigation, H.F., Ll.S.P., H.D.E., W.A.Ch. and J.C.V.; resources, L.M., W.A.Ch. and W.B.; data curation, M.E.M.P. and W.B.; writing—original draft preparation, H.F., Ll.S.P., H.D.E., G.T.S.P. and J.C.V.; writing—review and editing, W.A.Ch., J.A.S.U., G.T.S.P. and S.M.P.V.; visualization, M.E.M.P. and J.A.S.U.; supervision, L.M., H.F. and W.B.; project administration, L.M., W.A.Ch. and W.B.; funding acquisition, W.A.Ch., H.F., L.M. and W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by proyecto SNIP N° 352068 “Creación del Banco de Recursos Zoogenéticos de la Universidad Nacional Toribio Rodríguez de Mendoza, Sede Chachapoyas, Provincia Chachapoyas - Región Amazonas”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank the producers and owners of the raw milk collection centers for providing us with the samples for this research. Likewise, we thank the Office of the Vicerrectorado de Investigación de la Universidad Nacional Toribio Rodríguez de Mendoza, Provincia Chachapoyas - Región Amazonas”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition–a review. Lipids in Health and Disease 2007, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization). Milk and milk products. 2019. Available online: https://www.fao.org/3/cb4479en/cb4479en_milk.pdf (accessed on 01 August 2023).
- FAO (Food and Agriculture Organization). Milk production. 2022. Available online: https://www.fao.org/dairy-production-products/production/en/#:~:text= (accessed on 24 July 2023).
- MIDAGRI (Ministerio de Desarrollo Agrario y Riego). Valor Bruto de la Producción Agropecuaria enero-noviembre del 2022. 2022. Available online: https://www.gob.pe/institucion/midagri/informes-publicaciones/2994756-informe-mensual-del-valor-bruto-de-la-produccion-agropecuaria-2022 (accessed on 10 July 2023).
- Jurado-Gámez, H.A.; Solarte-Portilla, C.E.; Burgos-Arcos, Á.J.; González-Rodríguez, A.; Rosero-Galindo, C. Relationship of the compositional content and sanitary quality of Holstein cows’ milk of the high tropic of Nariño. Revista Mexicana de Ciencias Pecuarias 2020, 11, 421–434. [Google Scholar] [CrossRef]
- Pretto, D.; De Marchi, M.; Penasa, M.; Cassandro, M. Effect of milk composition and coagulation traits on Grana Padano cheese yield under field conditions. The Journal of Dairy Research 2013, 80, 1. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.O.; Martínez, M.I.; Rodríguez, H.E. Calidad sanitaria y composicional de la leche cruda producida en la vereda Quebrada Vieja, Soracá (Boyacá). Conexión Agropecuaria JDC 2016, 6, 87–93. [Google Scholar]
- Naing, Y.W.; Wai, S.S.; Lin, T.N.; Thu, W.P.; Htun, L.L.; Bawm, S.; Myaing, T.T. Bacterial content and associated risk factors influencing the quality of bulk tank milk collected from dairy cattle farms in Mandalay Region. Food Science & Nutrition 2019, 7, 1063–1071. [Google Scholar]
- Ndahetuye, J.B.; Artursson, K.; Båge, R.; Ingabire, A.; Karege, C.; Djangwani, J.; Nyman, A.K.; Ongol, M.P.; Tukei, M.; Persson, Y. MILK Symposium review: Microbiological quality and safety of milk from farm to milk collection centers in Rwanda. Journal of Dairy Science 2020, 103, 9730–9739. [Google Scholar] [CrossRef]
- Talukder, M.; Ahmed, H. Effect of somatic cell count on dairy products: a review. Asian Journal of Medical and Biological Research 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Malik, T.A.; Mohini, M.; Mir, S.H.; Ganaie, B.A.; Singh, D.; Varun, T.K.; Howal, S.; Thakur, S. Somatic cells in relation to udder health and milk quality-a review. Journal of Animal Health and Production 2018, 6, 18–26. [Google Scholar] [CrossRef]
- Bobbo, T.; Penasa, M.; Cassandro, M. Combining total and differential somatic cell count to better assess the association of udder health status with milk yield, composition and coagulation properties in cattle. Italian Journal of Animal Science 2020, 19, 697–703. [Google Scholar] [CrossRef]
- Cinar, M.; Serbester, U.; Ceyhan, A.; Gorgulu, M. Effect of somatic cell count on milk yield and composition of first and second lactation dairy cows. Italian Journal of Animal Science 2015, 14, 3646. [Google Scholar] [CrossRef]
- Kul, E.; Şahin, A.; Atasever, S.; Uğurlutepe, E.; Soydaner, M. The effects of somatic cell count on milk yield and milk composition in Holstein cows. Veterinarski Arhiv 2019, 89, 143–154. [Google Scholar] [CrossRef]
- MIDAGRI (Ministerio de Desarrollo Agrario y Riego) Decreto Supremo Nº, 0.0.7.-2.0.1.7.-M.I.N.A.G.R.I. Decreto Supremo que aprueba el Reglamento de la Leche y Productos Lácteos. 2017. Available online: https://www.midagri.gob.pe/portal/decreto-supremo/ds-2017/19598-decreto-supremo-n-007-2017-minagri (accessed on 22 May 2023).
- Asefa, Z.; Teshome, G. Physical properties and chemical compositions of raw cow milk in milk shades around addis ababa, Ethiopia. Journal of Natural Sciences Research 2019, 9, 33–37. [Google Scholar]
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Veterinary World 2018, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.A. Mejía O.B. Composición nutricional de la leche de ganado vacuno. Revista Lasallista de Investigación 2005, 2, 38–42. [Google Scholar]
- El-Tahawy, A.S.; El-Far, A.H. Influences of somatic cell count on milk composition and dairy farm profitability. International Journal of Dairy Technology 2010, 63, 463–469. [Google Scholar] [CrossRef]
- de Macedo, S.N.; Gonçalves, J.L.; Cortinhas, C.S.; de Freitas Leite, R.; dos Santos, M.V. Effect of somatic cell count on composition and hygiene indicators of bulk tank milk. Brazilian Journal of Veterinary Research and Animal Science 2018, 55, 1–11. [Google Scholar] [CrossRef]
- Forsbäck, L.; Lindmark-Månsson, H.; Andrén, A.; Åkerstedt, M.; Svennersten-Sjaunja, K. Udder quarter milk composition at different levels of somatic cell count in cow composite milk. Animal 2009, 3, 710–717. [Google Scholar] [CrossRef]
- Silva, J.E.; Barbosa, S.B.; Abreu, B.D.; Santoro, K.R.; Silva, E.C.; Batista, Â.M.; Martinez, R.L. Effect of somatic cell count on milk yield and milk components in Holstein cows in a semi-arid climate in Brazil. Revista Brasileira de Saúde e Produção Animal 2018, 19, 391–402. [Google Scholar] [CrossRef]
- Atasever, S.; Erdem, H.; Altop, A. Relationships between milk somatic cell count and pH in dairy cows. Journal of Animal and Veterinary Advances 2010, 9, 1575–1577. [Google Scholar] [CrossRef]
- Norman, H.D.; Miller, R.H.; Wright, J.R.; Wiggans, G.R. Herd and state means for somatic cell count from dairy herd improvement. Journal of Dairy Science 2000, 83, 2782–2788. [Google Scholar] [CrossRef]
- Van Schaik, G.; Lotem, M.; Schukken, Y.H. Trends in somatic cell counts, bacterial counts, and antibiotic residue violations in New York State during 1999–2000. Journal of Dairy Science 2002, 85, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Elmoslemany, A.M.; Keefe, G.P.; Dohoo, I.R.; Dingwell, R.T. Microbiological quality of bulk tank raw milk in Prince Edward Island dairy herds. Journal of Dairy Science 2009, 92, 4239–4248. [Google Scholar] [CrossRef]
- FDA (Food Drug Administration) Grade “A” Pasteurized Milk Ordinance 2007 Revision, U.S. Department of Health and Human Services. Public Health Service. 2007. Available online: https://www.fda.gov/media/140394/download (accessed on 12 April 2023).
- Fuentes-Coto, G.; Ruiz-Romero, R.A.; Sánchez-Gómez, J.I.; Ávila-Ramírez, D.N.; Escutia-Sánchez, J. Análisis microbiológico de leche de origen orgánico: atributos deseables para su transformación. Agricultura, Sociedad y Desarrollo 2013, 10, 419–432. [Google Scholar] [CrossRef]
- Brousett-Minaya, M.; Torres-Jiménez, A.; Chambi-Rodríguez, A.; Mamani-Villalba, B.; Gutiérrez-Samata, H. Calidad fisicoquímica, microbiológica y toxicológica de leche cruda en las cuencas ganaderas de la región Puno-Perú. Scientia Agropecuaria 2015, 6, 165–176. [Google Scholar] [CrossRef]
- Salman, A.M.; Hamad, I.M. Enumeration and identification of coliform bacteria from raw milk in Khartoum State, Sudan. Journal of cell and Animal Biology 2011, 5, 121–128. [Google Scholar]
- Addo, K.K.; Mensah, G.I.; Aning, K.G.; Nartey, N.; Nipah, G.K.; Bonsu, C.; Smits, H.L. Microbiological quality and antibiotic residues in informally marketed raw cow milk within the coastal savannah zone of Ghana. Tropical Medicine & International Health 2011, 16, 227–232. [Google Scholar]
- Ghali-Mohammed, I.; Odetokun, I.A.; Raufu, I.A.; Adetunji, V.O. Handling practices and contamination of raw milk sold for consumption in markets of Kwara State, Nigeria. Sokoto Journal of Veterinary Sciences 2022, 20, 50–58. [Google Scholar] [CrossRef]
- Celano, G.; Calasso, M.; Costantino, G.; Vacca, M.; Ressa, A.; Nikoloudaki, O.; De Angelis, M. Effect of Seasonality on Microbiological Variability of Raw Cow Milk from Apulian Dairy Farms in Italy. Microbiology Spectrum 2022, 10, e00514-22. [Google Scholar] [CrossRef]
- Joubrane, K.; Jammoul, A.; Daher, R.; Ayoub, S.; El Jed, M.; Hneino, M.; Daher, Z. Microbiological contamination, antimicrobial residues, and antimicrobial resistance in raw bovine milk in Lebanon. International Dairy Journal 2022, 134, 105455. [Google Scholar] [CrossRef]
- Albuja Landi, A.K.; Escobar Arrieta, S.N.; Andueza Leal, F.D. Calidad bacteriológica de la leche cruda bovina almacenada en el centro de acopio Mocha. Tungurahua. Ecuador. Siembra 2021, 8, 2. [Google Scholar] [CrossRef]
- Kable, M.E.; Srisengfa, Y.; Laird, M.; Zaragoza, J.; McLeod, J.; Heidenreich, J.; Marco, M.L. The core and seasonal microbiota of raw bovine milk in tanker trucks and the impact of transfer to a milk processing facility. MBio 2016, 7, e00836-16. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Kent, D.J.; Boor, K.J.; Martin, N.H.; Wiedmann, M. Different management practices are associated with mesophilic and thermophilic spore levels in bulk tank raw milk. Journal of Dairy Science 2015, 98, 4338–4351. [Google Scholar] [CrossRef] [PubMed]
- Skeie, S.B.; Håland, M.; Thorsen, I.M.; Narvhus, J.; Porcellato, D. Bulk tank raw milk microbiota differs within and between farms: A moving goalpost challenging quality control. Journal of Dairy Science 2019, 102, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Zucali, M.; Bava, L.; Colombini, S.; Brasca, M.; Decimo, M.; Morandi, S.; Crovetto, G.M. Management practices and forage quality affecting the contamination of milk with anaerobic spore-forming bacteria. Journal of the Science of Food and Agriculture 2015, 95, 1294–1302. [Google Scholar] [CrossRef]
- Breitenwieser, F.; Doll, E.V.; Clavel, T.; Scherer, S.; Wenning, M. Complementary use of cultivation and high-throughput amplicon sequencing reveals high biodiversity within raw milk microbiota. Frontiers in Microbiology 2020, 11, 1557. [Google Scholar] [CrossRef]
- Dobranić, V.; Kazazić, S.; Filipović, I.; Mikulec, N.; Zdolec, N. Composition of raw cow’s milk microbiota and identification of enterococci by MALDI-TOF MS-short communication. Veterinarski Arhiv 2016, 86, 581–590. [Google Scholar]
- Mikulová, M. Content of free fatty acids, lipolytic bacteria and somatic cells in relation to milking technology. Journal of Agrobiology 2011, 28, 49. [Google Scholar] [CrossRef]
- Wickström, E.; Persson-Waller, K.; Lindmark-Månsson, H.; Östensson, K.; Sternesjö, Å. Relationship between somatic cell count, polymorphonuclear leucocyte count and quality parameters in bovine bulk tank milk. Journal of Dairy Research 2009, 76, 195–201. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring udder health and milk quality using somatic cell counts. Veterinary Research 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Rojas-Ronquillo, R.; Cruz Bautista, E.; Daniel Rentería, I.C.; Lammoglia, M.A. Determinación de la calidad microbiológica de la leche cruda de vaca durante la temporada invernal en Tuxpan, Veracruz. Casos y Experiencias Compartidas en las Ciencias 2014, 1107–1111. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).