Submitted:
14 August 2023
Posted:
15 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
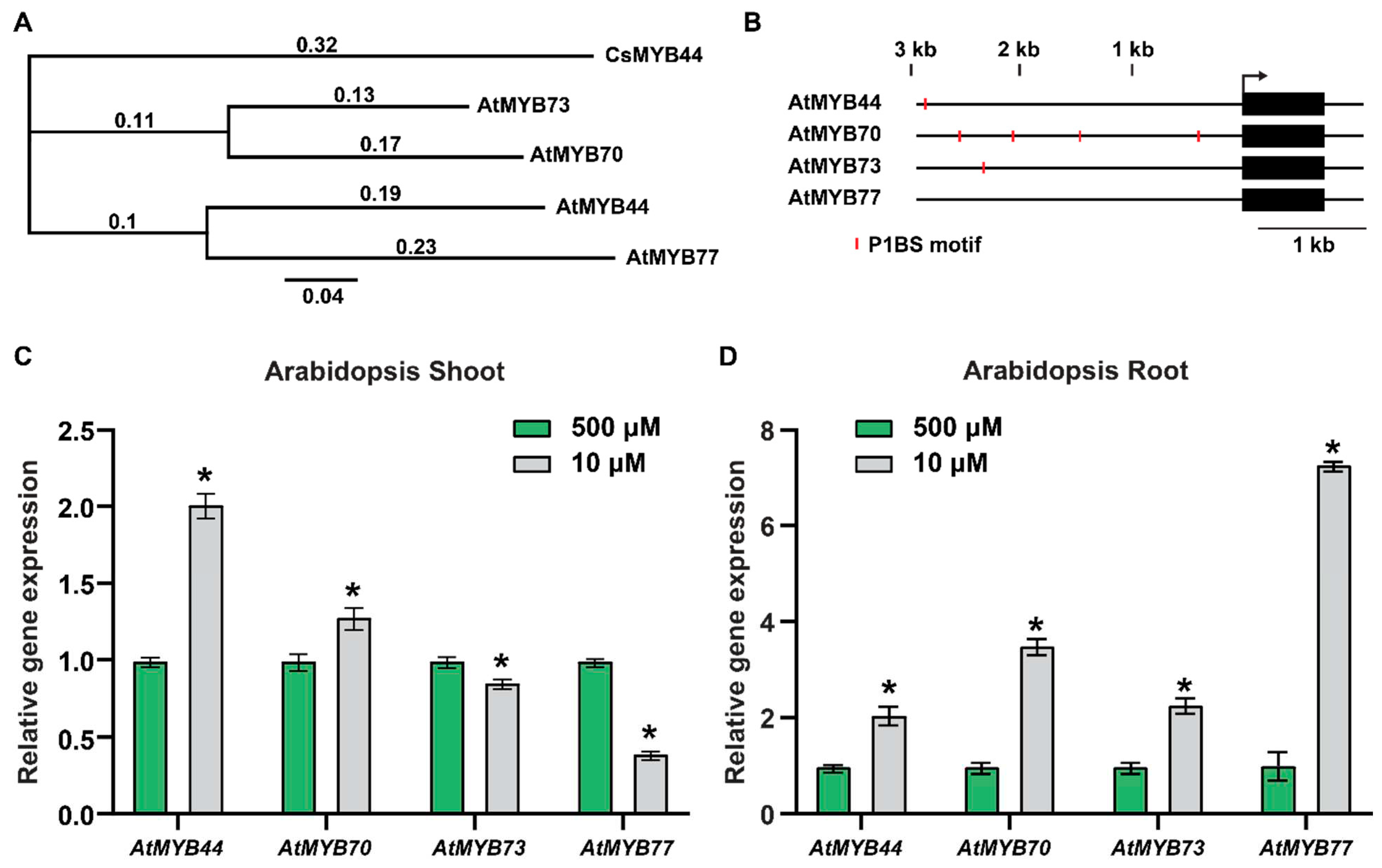
2.1. Identification of mobile CsMYB44 orthologs in Arabidopsis.
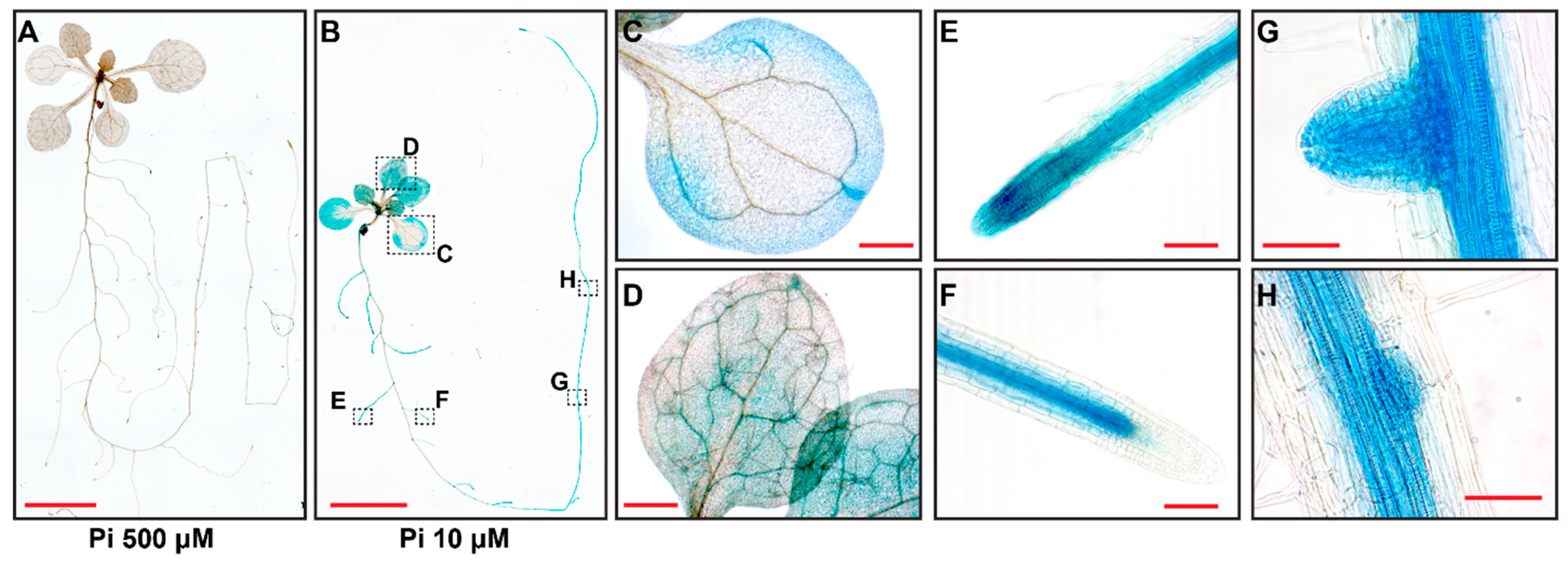
2.2. AtMYB44 is expressed in leaf and root vascular tissues under Pi-stress conditions.
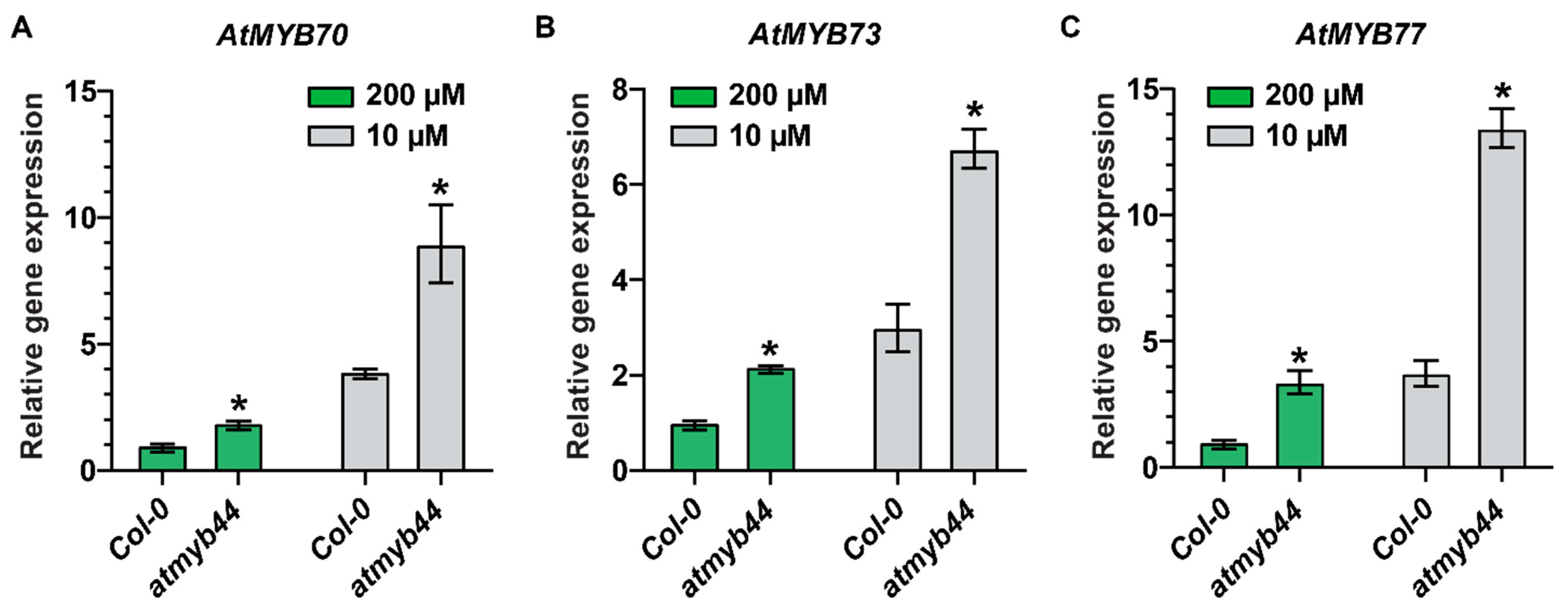
2.3. AtMYB70, AtMYB73 and AtMYB77 transcript levels were elevated in atmyb44 mutant, compared with Wild-type.
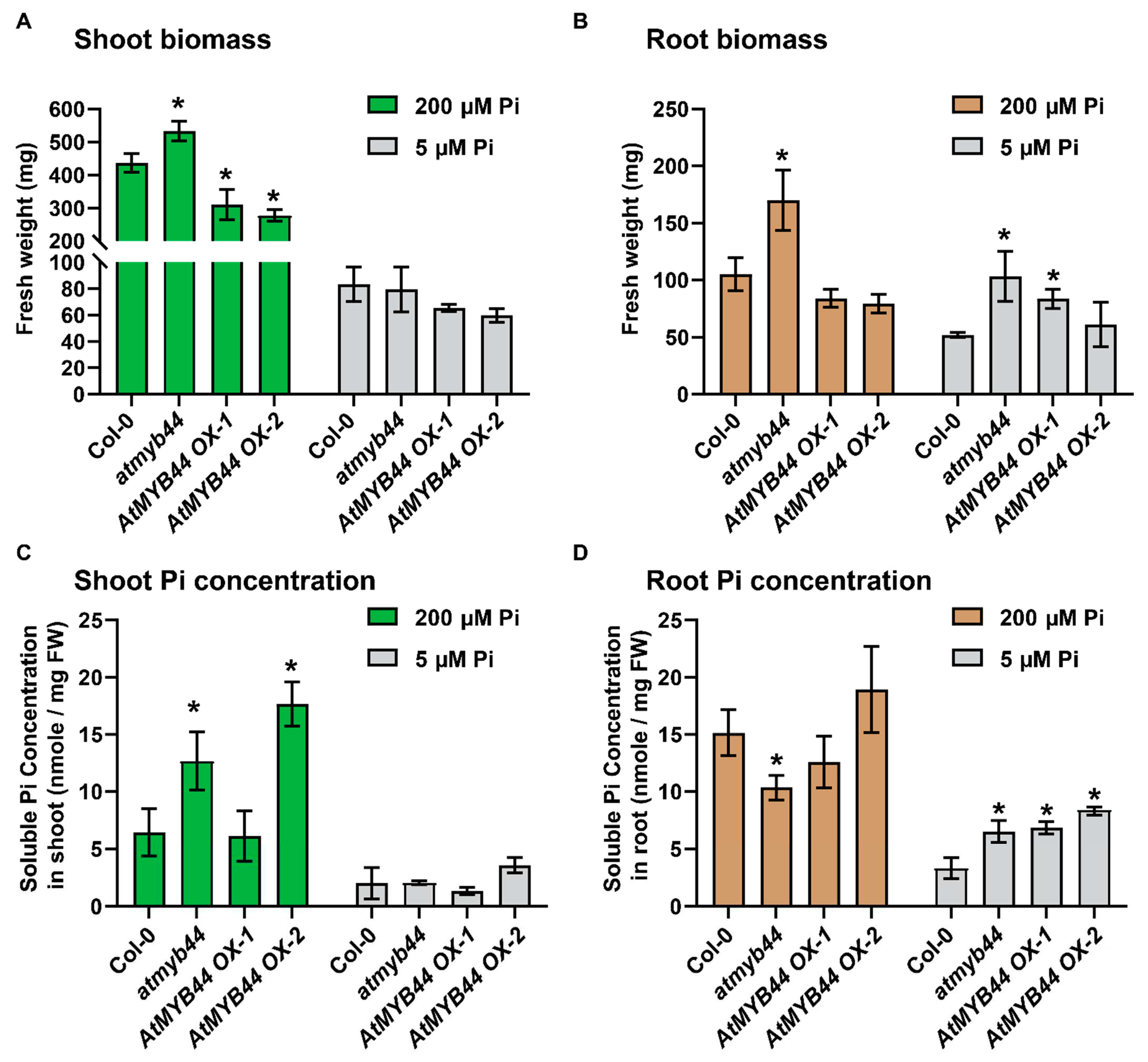
2.4. The atmyb44 root had an elevated Pi concentration under Pi-stress conditions.
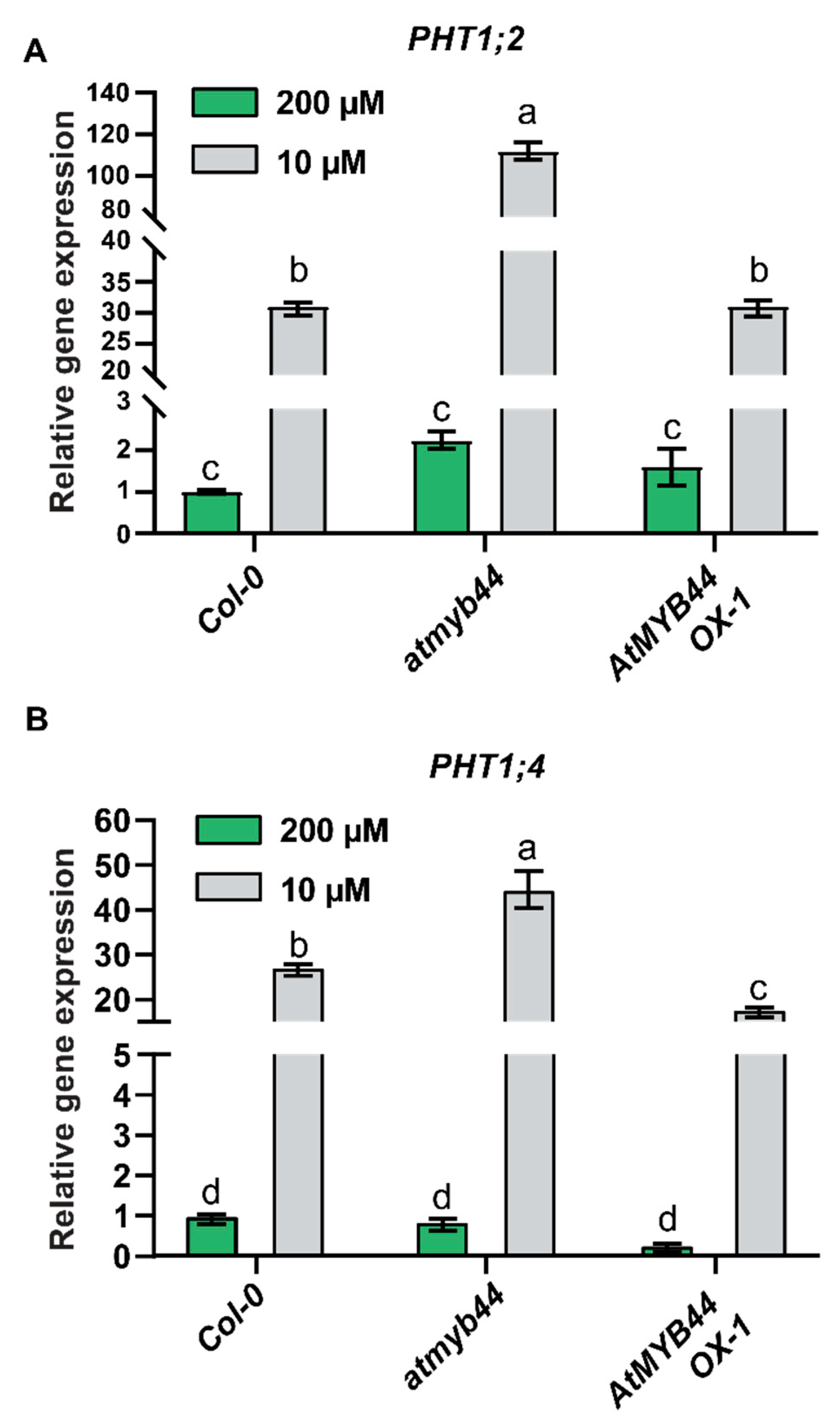
2.5. Expression of PHT1;2 and PHT1;4 is negatively regulated by AtMYB44 in roots.
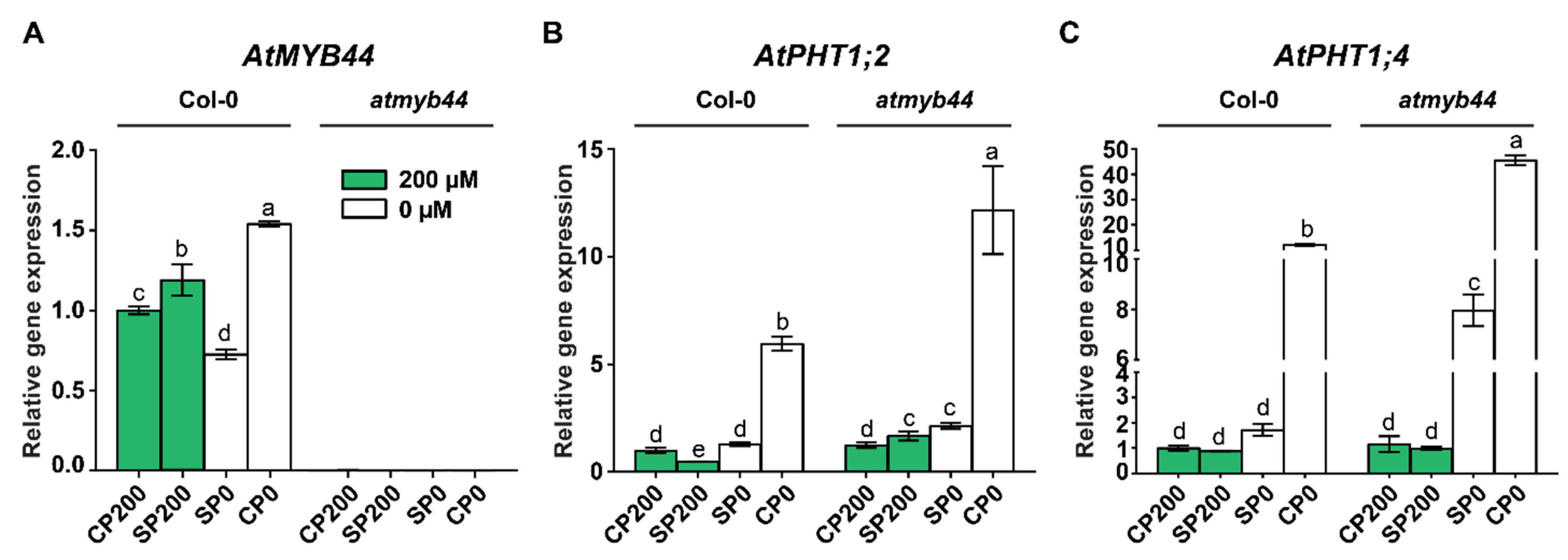
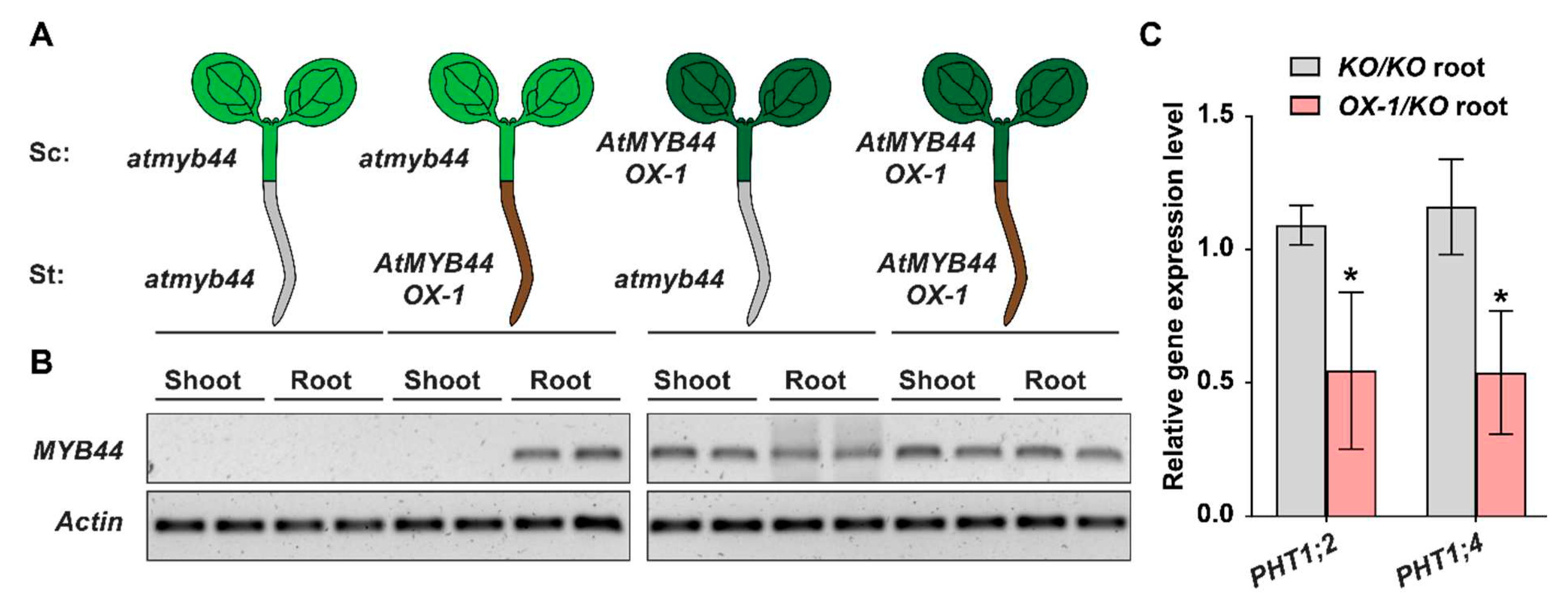
2.6. Mobile AtMYB44 mRNA functions as a negative regulator of PHT1;2 and PHT1;4 expression.
3. Discussion
3.1. AtMYB44 expression responds to an imposed Pi-stress.
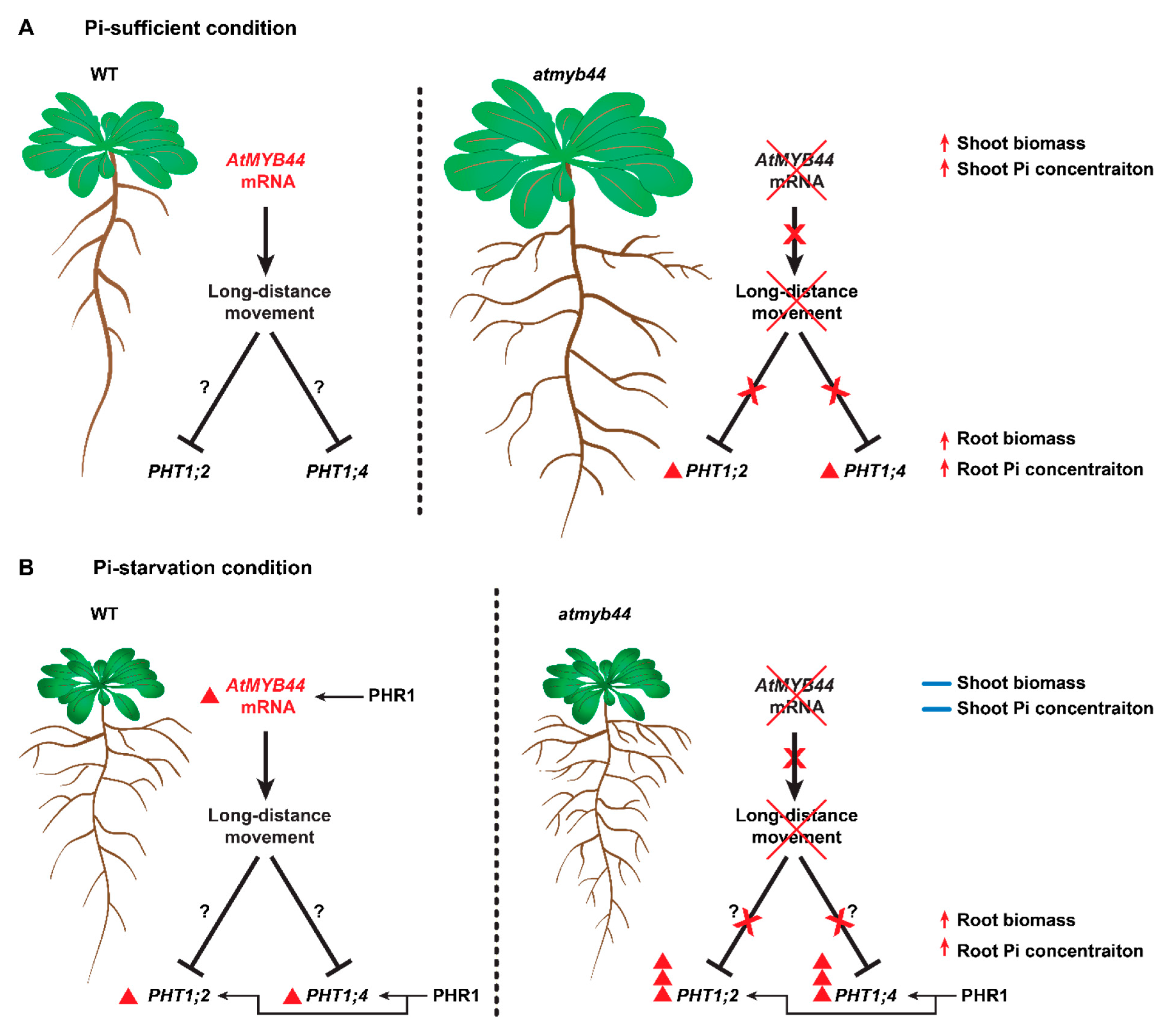
3.2. AtMYB44 mRNA acts as a systemic Pi signaling factor to negatively regulate root Pi transport systems.
4. Materials and Methods
4.1. Plant materials
4.2. Growth conditions and Pi-stress treatments
4.3. Phylogenetic analysis
4.5. Micrografting
4.6. RNA extraction and qRT-PCR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc Natl Acad Sci U S A 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.; Sylvester-Bradley, R.; Jones, D.L.; Healey, J.R.; Talboys, P.J. Feed the crop not the soil: rethinking phosphorus management in the food chain. Environ Sci Technol 2014, 48, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Arredondo, D.L.; Leyva-Gonzalez, M.A.; Gonzalez-Morales, S.I.; Lopez-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: improving low-phosphate tolerance in crops. Annu Rev Plant Biol 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu Rev Plant Biol 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.K.; Chen, J.Y.; Yan, Y.; Lucas, W.J. Insights into plant phosphate sensing and signaling. Curr Opin Biotechnol 2018, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Blasing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M.; et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 2007, 30, 85–112. [Google Scholar] [CrossRef]
- Lei, M.G.; Zhu, C.M.; Liu, Y.D.; Karthikeyan, A.S.; Bressan, R.A.; Raghothama, K.G.; Liu, D. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytologist 2011, 189, 1084–1095. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: facts and promises. Curr Opin Plant Biol 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Li, L.; Liu, K.H.; Sheen, J. Dynamic Nutrient Signaling Networks in Plants. Annu Rev of Cell Dev Biol 2021, 37, 341–367. [Google Scholar] [CrossRef]
- Thibaud, M.C.; Arrighi, J.F.; Bayle, V.; Chiarenza, S.; Creff, A.; Bustos, R.; Paz-Ares, J.; Poirier, Y.; Nussaume, L. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J 2010, 64, 775–789. [Google Scholar] [CrossRef]
- Chen, J.; Ham, B.K. Systemic Signaling: A Role in Propelling Crop Yield. Plants 2022, 11. [Google Scholar] [CrossRef]
- Aung, K.; Lin, S.I.; Wu, C.C.; Huang, Y.T.; Su, C.L.; Chiou, T.J. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a MicroRNA399 target gene. Plant Physiol 2006, 141, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Buhtz, A.; Kehr, J.; Scheible, W.R. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 2008, 53, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 2002, 31, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.I.; Chiang, S.F.; Lin, W.Y.; Chen, J.W.; Tseng, C.Y.; Wu, P.C.; Chiou, T.J. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol 2008, 147, 732–746. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Jain, A.; Poling, M.D.; Lewis, A.J.; Raghothama, K.G.; Smith, A.P. Arabidopsis Pht1;5 Mobilizes Phosphate between Source and Sink Organs and Influences the Interaction between Phosphate Homeostasis and Ethylene Signaling. Plant Physiol 2011, 156, 1149–1163. [Google Scholar] [CrossRef]
- Huang, T.K.; Han, C.L.; Lin, S.I.; Chen, Y.J.; Tsai, Y.C.; Chen, Y.R.; Chen, J.W.; Lin, W.Y.; Chen, P.M.; Liu, T.Y.; et al. Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 2013, 25, 4044–4060. [Google Scholar] [CrossRef]
- Park, B.S.; Seo, J.S.; Chua, N.H. NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 2014, 26, 454–464. [Google Scholar] [CrossRef]
- Martin, C.; Paz-Ares, J. MYB transcription factors in plants. Trends Genet 1997, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Seo, J.S.; Han, S.W.; Koo, Y.J.; Kim, C.H.; Song, S.I.; Nahm, B.H.; Choi, Y.D.; Cheong, J.J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 2008, 146, 623–635. [Google Scholar] [CrossRef]
- Jaradat, M.R.; Feurtado, J.A.; Huang, D.; Lu, Y.; Cutler, A.J. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol 2013, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Persak, H.; Pitzschke, A. Dominant repression by Arabidopsis transcription factor MYB44 causes oxidative damage and hypersensitivity to abiotic stress. Int J Mol Sci 2014, 15, 2517–2537. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, M.; Jia, Z.; Liu, F.; Ma, H.; Huang, Y.; Song, S. AtMYB44 Positively Regulates the Enhanced Elongation of Primary Roots Induced by N-3-Oxo-Hexanoyl-Homoserine Lactone in Arabidopsis thaliana. Mol Plant Microbe Interact 2016, 29, 774–785. [Google Scholar] [CrossRef]
- Zhou, X.; Zha, M.; Huang, J.; Li, L.; Imran, M.; Zhang, C. StMYB44 negatively regulates phosphate transport by suppressing expression of PHOSPHATE1 in potato. J Exp Bot 2017, 68, 1265–1281. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zheng, Y.; Ham, B.K.; Chen, J.Y.; Yoshida, A.; Kochian, L.V.; Fei, Z.J.; Lucas, W.J. Vascular-mediated signalling involved in early phosphate stress response in plants. Nature Plants 2016, 2. [Google Scholar] [CrossRef]
- Müller, R.; Morant, M.; Jarmer, H.; Nilsson, L.; Nielsen, T.H. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol 2007, 143, 156–171. [Google Scholar] [CrossRef]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Perez-Perez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 2010, 6, e1001102. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, L.; Zhang, Y.; Zheng, Z.; Liu, D. Arabidopsis PHL2 and PHR1 Act Redundantly as the Key Components of the Central Regulatory System Controlling Transcriptional Responses to Phosphate Starvation. Plant Physiol 2016, 170, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Jaradat, M.R.; Wu, W.; Ambrose, S.J.; Ross, A.R.; Abrams, S.R.; Cutler, A.J. Structural analogs of ABA reveal novel features of ABA perception and signaling in Arabidopsis. Plant J 2007, 50, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Ni, J.; Wang, Y.; Bai, Y.; Shi, J.; Gan, J.; Wu, Z.; Wu, P. OsPHF1 regulates the plasma membrane localization of low- and high-affinity inorganic phosphate transporters and determines inorganic phosphate uptake and translocation in rice. Plant Physiol 2011, 157, 269–278. [Google Scholar] [CrossRef]
- Yan, J.; Chia, J.C.; Sheng, H.; Jung, H.I.; Zavodna, T.O.; Zhang, L.; Huang, R.; Jiao, C.; Craft, E.J.; Fei, Z.; et al. Arabidopsis Pollen Fertility Requires the Transcription Factors CITF1 and SPL7 That Regulate Copper Delivery to Anthers and Jasmonic Acid Synthesis. Plant Cell 2017, 29, 3012–3029. [Google Scholar] [CrossRef]
- Brooks, M.D.; Juang, C.L.; Katari, M.S.; Alvarez, J.M.; Pasquino, A.; Shih, H.J.; Huang, J.; Shanks, C.; Cirrone, J.; Coruzzi, G.M. ConnecTF: A platform to integrate transcription factor-gene interactions and validate regulatory networks. Plant Physiol 2021, 185, 49–66. [Google Scholar] [CrossRef]
- Daram, P.; Brunner, S.; Persson, B.L.; Amrhein, N.; Bucher, M. Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta 1998, 206, 225–233. [Google Scholar] [CrossRef]
- Muchhal, U.S.; Pardo, J.M.; Raghothama, K.G. Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci U S A 1996, 93, 10519–10523. [Google Scholar] [CrossRef]
- Okumura, S.; Mitsukawa, N.; Shirano, Y.; Shibata, D. Phosphate transporter gene family of Arabidopsis thaliana. DNA Res 1998, 5, 261–269. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate transport and signaling. Curr Opin Plant Biol 2000, 3, 182–187. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Pochon, N.; Ayadi, A.; Nakanishi, T.M.; Thibaud, M.C. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front Plant Sci 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Bayle, V.; Arrighi, J.F.; Creff, A.; Nespoulous, C.; Vialaret, J.; Rossignol, M.; Gonzalez, E.; Paz-Ares, J.; Nussaume, L. Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 2011, 23, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Wang, F.; Yang, J.; Gao, M.; Li, C.; Liu, Y.; Liu, Y.; Yamaji, N.; Ma, J.F.; et al. The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell 2015, 27, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; Linhares, F.; Solano, R.; Martin, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 2001, 15, 2122–2133. [Google Scholar] [CrossRef]
- Hamburger, D.; Rezzonico, E.; Petetot, J.M.C.; Somerville, C.; Poirier, Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 2002, 14, 889–902. [Google Scholar] [CrossRef]
- Rouached, H.; Arpat, A.B.; Poirier, Y. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol Plant 2010, 3, 288–299. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chang, C.Y.; Chiou, T.J. The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol 2009, 12, 312–319. [Google Scholar] [CrossRef]
- Ham, B.K.; Lucas, W.J. Phloem-Mobile RNAs as Systemic Signaling Agents. Annu Rev Plant Biol 2017, 68, 173–195. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zheng, Y.; Ham, B.K.; Zhang, S.P.; Fei, Z.J.; Lucas, W.J. Plant lncRNAs are enriched in and move systemically through the phloem in response to phosphate deficiency. J Integr Plant Biol 2019, 61, 492–508. [Google Scholar] [CrossRef]
- Yan, Y.; Ham, B.K. The Mobile Small RNAs: Important Messengers for Long-Distance Communication in Plants. Front Plant Sci 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, C.A.; Lopez-Bucio, J.; Cruz-Ramirez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Ma, G.J.; Zhang, M.L.; Xiong, H.; Wu, H.; Zhao, C.Z.; Liu, C.S.; Jia, H.X.; Chen, L.; Kjorven, J.O.; et al. The ARF7 and ARF19 Transcription Factors Positively Regulate PHOSPHATE STARVATION RESPONSE1 in Arabidopsis Roots. Plant Physiol 2018, 178, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, Smeekens S, Tonelli C, Paz-Ares J, Weisshaar B. Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J 1998, 16, 263–276. [CrossRef]
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.J.; Gao, J.; Wang, P.; Duan, C.G.; Zhu, X.; Zhu, J.K. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci Signal 2014, 7, ra53. [Google Scholar] [CrossRef]
- Wan, J.; Wang, R.; Zhang, P.; Sun, L.; Ju, Q.; Huang, H.; Lu, S.; Tran, L.S.; Xu, J. MYB70 modulates seed germination and root system development in Arabidopsis. iScience 2021, 24, 103228. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu Rev Plant Biol 2012, 63, 563–590. [Google Scholar] [CrossRef]
- Belin, C.; Megies, C.; Hauserova, E.; Lopez-Molina, L. Abscisic Acid Represses Growth of the Arabidopsis Embryonic Axis after Germination by Enhancing Auxin Signaling. Plant Cell 2009, 21, 2253–2268. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.T.; Wang, L.F.; Guo, J.X.; Lu, K.K.; Song, R.F.; Zuo, J.X.; Chen, H.H.; Liu, W.C. Abscisic acid facilitates phosphate acquisition through the transcription factor ABA INSENSITIVE5 in Arabidopsis. Plant J 2022, 111, 269–281. [Google Scholar] [CrossRef]
- Nilsson, L.; Muller, R.; Nielsen, T.H. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Envir 2007, 30, 1499–1512. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Madhuvanthi, R.; Karthikeyan, A.S.; Raghothama, K.G. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2009, 2, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Wang, Y.Y.; Yang, A.; Zhang, W.H. OsMYB2P-1, an R2R3 MYB Transcription Factor, Is Involved in the Regulation of Phosphate-Starvation Responses and Root Architecture in Rice. Plant Physiol 2012, 159, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Guo, M.; Xu, L.; Wang, X.; Zhao, H.; Wang, J.; Yi, K. An SPX-RLI1 Module Regulates Leaf Inclination in Response to Phosphate Availability in Rice. Plant Cell 2018, 30, 853–870. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Jia, X.; Wang, X.; Zhang, Y.; Liu, J.; Yang, Q.; Ruan, W.; Yi, K. Alternative splicing of REGULATOR OF LEAF INCLINATION 1 modulates phosphate starvation signaling and growth in plants. Plant Cell 2022, 34, 3319–3338. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.C.; Lin, S.I.; Shih, A.C.; Chen, J.W.; Lin, W.Y.; Tseng, C.Y.; Li, W.H.; Chiou, T.J. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 2009, 151, 2120–2132. [Google Scholar] [CrossRef]
- Buhtz, A.; Pieritz, J.; Springer, F.; Kehr, J. Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol 2010, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Rojas-Triana, M.; Stecyk, E.; Schudoma, C.; Zhang, W.; Yang, L.; Minambres, M.; Walther, D.; Schulze, W.X.; Paz-Ares, J.; et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat Plants 2015, 1, 15025. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Y.; Wang, S.; Zhang, W.; Wang, S.; Xu, C.; Yu, Y.; Li, T.; Jiang, F.; Li, W. A constitutive and drought-responsive mRNA undergoes long-distance transport in pear (Pyrus betulaefolia) phloem. Plant Sci 2020, 293, 110419. [Google Scholar] [CrossRef]
- Xia, C.; Huang, J.; Lan, H.; Zhang, C. Long-Distance Movement of Mineral Deficiency-Responsive mRNAs in Nicotiana Benthamiana/Tomato Heterografts. Plants 2020, 9. [Google Scholar] [CrossRef]
- Kitagawa, M.; Wu, P.; Balkunde, R.; Cunniff, P.; Jackson, D. An RNA exosome subunit mediates cell-to-cell trafficking of a homeobox mRNA via plasmodesmata. Science 2022, 375, 177–182. [Google Scholar] [CrossRef]
- Kehr, J.; Morris, R.J.; Kragler, F. Long-Distance Transported RNAs: From Identity to Function. Annu Rev Plant Biol 2022, 73, 457–474. [Google Scholar] [CrossRef]
- Haywood, V.; Yu, T.S.; Huang, N.C.; Lucas, W.J. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J 2005, 42, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Yu, T.S. The sequences of Arabidopsis GA-INSENSITIVE RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J 2009, 59, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.G.; Miller, W.A.; Hannapel, D.J. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 2006, 18, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Hannapel, D.J.; Banerjee, A.K. Multiple Mobile mRNA Signals Regulate Tuber Development in Potato. Plants 2017, 6. [Google Scholar] [CrossRef]
- Notaguchi, M.; Wolf, S.; Lucas, W.J. Phloem-mobile Aux/IAA transcripts target to the root tip and modify root architecture. J Integr Plant Biol 2012, 54, 760–772. [Google Scholar] [CrossRef]
- Toscano-Morales, R.; Xoconostle-Cazares, B.; Martinez-Navarro, A.C.; Ruiz-Medrano, R. Long distance movement of an Arabidopsis Translationally Controlled Tumor Protein (AtTCTP2) mRNA and protein in tobacco. Front Plant Sci 2014, 5, 705. [Google Scholar] [CrossRef]
- Branco, R.; Masle, J. Systemic signalling through translationally controlled tumour protein controls lateral root formation in Arabidopsis. J Exp Bot 2019, 70, 3927–3940. [Google Scholar] [CrossRef]
- Ham, B.K.; Brandom, J.L.; Xoconostle-Cazares, B.; Ringgold, V.; Lough, T.J.; Lucas, W.J. A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 2009, 21, 197–215. [Google Scholar] [CrossRef]
- Zhang, W.N.; Thieme, C.J.; Kollwig, G.; Apelt, F.; Yang, L.; Winter, N.; Andresen, N.; Walther, D.; Kragler, F. tRNA-Related Sequences Trigger Systemic mRNA Transport in Plants. Plant Cell 2016, 28, 1237–1249. [Google Scholar] [CrossRef]
- Yang, L.; Perrera, V.; Saplaoura, E.; Apelt, F.; Bahin, M.; Kramdi, A.; Olas, J.; Mueller-Roeber, B.; Sokolowska, E.; Zhang, W.; et al. m(5)C Methylation Guides Systemic Transport of Messenger RNA over Graft Junctions in Plants. Curr Biol 2019, 29, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Guelette, B.S.; Benning, U.F.; Hoffmann-Benning, S. Identification of lipids and lipid-binding proteins in phloem exudates from Arabidopsis thaliana. J Exp Bot 2012, 63, 3603–3616. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Ham, B.K.; El-Shabrawi, H.M.; Alexander, D.; Zhang, D.B.; Ryals, J.; Lucas, W.J. Proteomics and metabolomics analyses reveal the cucurbit sieve tube system as a complex metabolic space. Plant J 2016, 87, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.K.; Li, G.; Kang, B.H.; Zeng, F.; Lucas, W.J. Overexpression of Arabidopsis plasmodesmata germin-like proteins disrupts root growth and development. Plant Cell 2012, 24, 3630–3648. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ham, B.K.; Chong, Y.H.; Yeh, S.D.; Lucas, W.J. A Plant SMALL RNA-BINDING PROTEIN 1 Family Mediates Cell-to-Cell Trafficking of RNAi Signals. Mol Plant 2020, 13, 321–335. [Google Scholar] [CrossRef]
- Duan, K.; Yi, K.; Dang, L.; Huang, H.; Wu, W.; Wu, P. Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J 2008, 54, 965–975. [Google Scholar] [CrossRef]
- Yan, J.; Chia, J.C.; Sheng, H.; Jung, H.I.; Zavodna, T.O.; Zhang, L.; Huang, R.; Jiao, C.; Craft, E.J.; Fei, Z.; et al. Arabidopsis Pollen Fertility Requires the Transcription Factors CITF1 and SPL7 That Regulate Copper Delivery to Anthers and Jasmonic Acid Synthesis. Plant Cell 2017, 29, 3012–3029. [Google Scholar] [CrossRef]
- Hoekenga, O.A.; Maron, L.G.; Pineros, M.A.; Cancado, G.M.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A 2006, 103, 9738–9743. [Google Scholar] [CrossRef]
- Gansel, X.; Munos, S.; Tillard, P.; Gojon, A. Differential regulation of the NO3- and NH4+ transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. Plant J 2001, 26, 143–155. [Google Scholar] [CrossRef]
- Melnyk, C.W. Grafting with Arabidopsis thaliana. Methods Mol Biol 2017, 1497, 9–18. [Google Scholar] [CrossRef]
- Ham, B.K.; Wang, X.; Toscano-Morales, R.; Lin, J.; Lucas, W.J. Plasmodesmal endoplasmic reticulum proteins regulate intercellular trafficking of Cucumber mosaic virus in Arabidopsis. J Exp Bot 2023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




