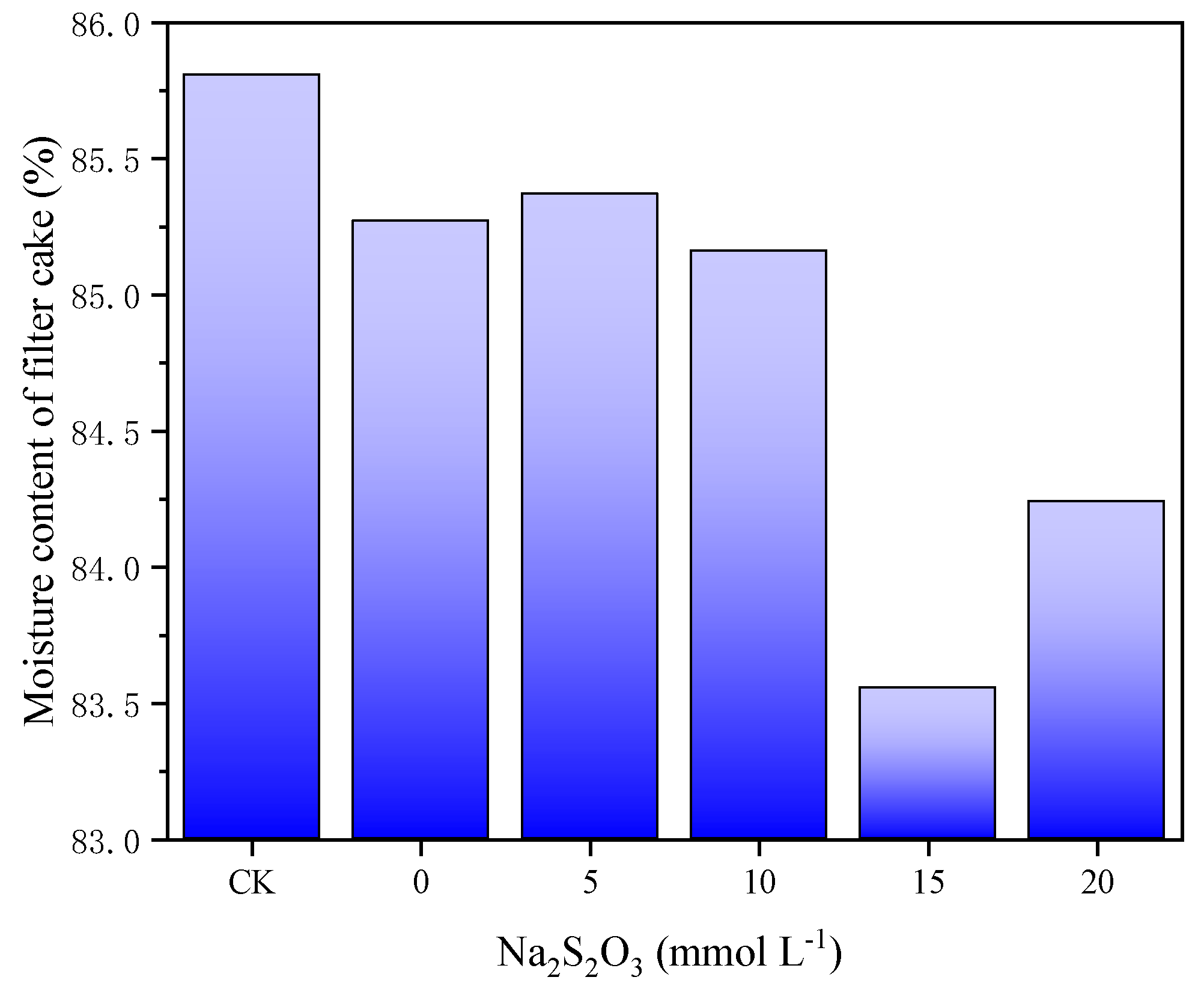1. Introduction
The development of human society leads to the production of more and more domestic sewage and industrial wastewater, which enters waste water treatment plants (WWTPs) [
1]. At present, a large amount of excess sludge has been produced, which poses a great threat to the environment and human health [
2]. It is estimated that more than 36 million tons of sewage sludge (approximately 80% moisture content) were produced in China in 2019. How to reasonably and safely dispose of these hazardous sludge has gained worldwide concerns [
3]. It is well known that the high moisture content and poor dewatering performance of sewage sludge are the main obstacles, which seriously increases the operating costs of WWTPs. Sewage sludge contains both free and bound water [
4], and the bound water is the most difficult to remove because it binds to sewage sludge components such as flocculants, especially extracellular polymers (EPS) [
5]. Therefore, the removal of the bound water is a major challenge.
Lots of sewage sludge processing techniques have been developed, such as mechanic and thermal treatment [
7,
8], chemical methods [
9,
10], microbiological treatment [
11], ultrasound treatment [
12], and microwave treatment [
13]. Most of these methods can effectively realize sewage sludge dewatering, but also have certain drawbacks, such as long processing times, high costs, low utilization rates. As the volume of the sewage sludge increases, more efficient and faster processing methods are needed. Advanced oxidation processes can produce strong oxidizing species (such as ·OH, ·O2-, and ·SO4-), which can effectively crack the sewage sludge cells and achieve dehydration [
14,
15,
16,
17]. Due to the advantages of high efficiency, straightforward equipment, and environmental friendliness, non-thermal discharge plasma has garnered significant interest [
18,
19]. The non-thermal discharge plasma has been widely used in the prevention and control of gaseous pollutants [
20], water pollution treatment [
15], sterilization and disinfection [
21]. Ozone is one of the main species produced in the non-thermal discharge plasma process [
22,
23], which has been reported to improve the dewaterability of the sewage sludge [
24]. Ozone is selective in the oxidation and degradation of organic matter. These drawbacks will reduce the efficiency of non-thermal discharge plasma treatment of the sewage sludge. It is important to improve the utilization of ozone in the non-thermal discharge plasma process.
Some reducing agents are generally used to catalyze ozone decomposition. Compared to nitrogen-containing reducing agents, sulfur-containing reducing agents do not generate toxic byproducts (e.g. N-nitrosodimethylamine, NDMA) [
25]. In ozone systems, thiosulfate is often used as an ozone terminator [
26,
27]. When the concentration of thiosulfate (TSA) is low, it can promote ozone to produce free radicals, and the reactions are shown as follows. It was reported that low concentration of TSA promoted ozone oxidation to achieve desulfurization and denitrification [
28]. Yang et al. discovered that oxidation of refractory contaminants was significantly boosted by the addition of TSA at relatively low concentrations during ozonation process. Information regarding the potentials of TSA on promoting the dewatering of the sewage sludge in the non-thermal discharge plasma process is still lacking.
Thus, in this study the excess sludge disintegration by non-thermal discharge plasma coupled with thiosulfate was investigated. The main objectives of this study are (1) to evaluate the effects of non-thermal discharge plasma coupled with thiosulfate on sewage sludge dewatering, (2) to explore the process of sewage sludge dewatering, and (3) to unravel the reaction mechanisms of non-thermal discharge plasma coupled with thiosulfate on sewage sludge dewatering.
2. Materials and Methods
2.1. Sludge collection and chemicals
All sludge used in this study was sampled diurnally from the active sludge return unit of a WWTP (anaerobic-anoxic-oxic process, treatment capacity 40,000 m3 d-1), which is located in Yangling, Shaanxi, China (location: 108°4′27.95′′E, 34°16′56.24′′N). The collected sewage sludge was immediately transferred to the lab and stored in the refrigerator at 4 °C. Prior to all experiments, the sewage sludge was shaken well and brought back to room temperature. Sodium thiosulfate (Na2S2O3) was purchased from Kermel Chemical Reagent Co., Ltd. (Tianjin, China). Terephthalic acid (C8H6O4) and p-benzoquinone (C6H4O2) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). All the chemicals were of analytical grade and used without further purification.
2.2. Experimental section
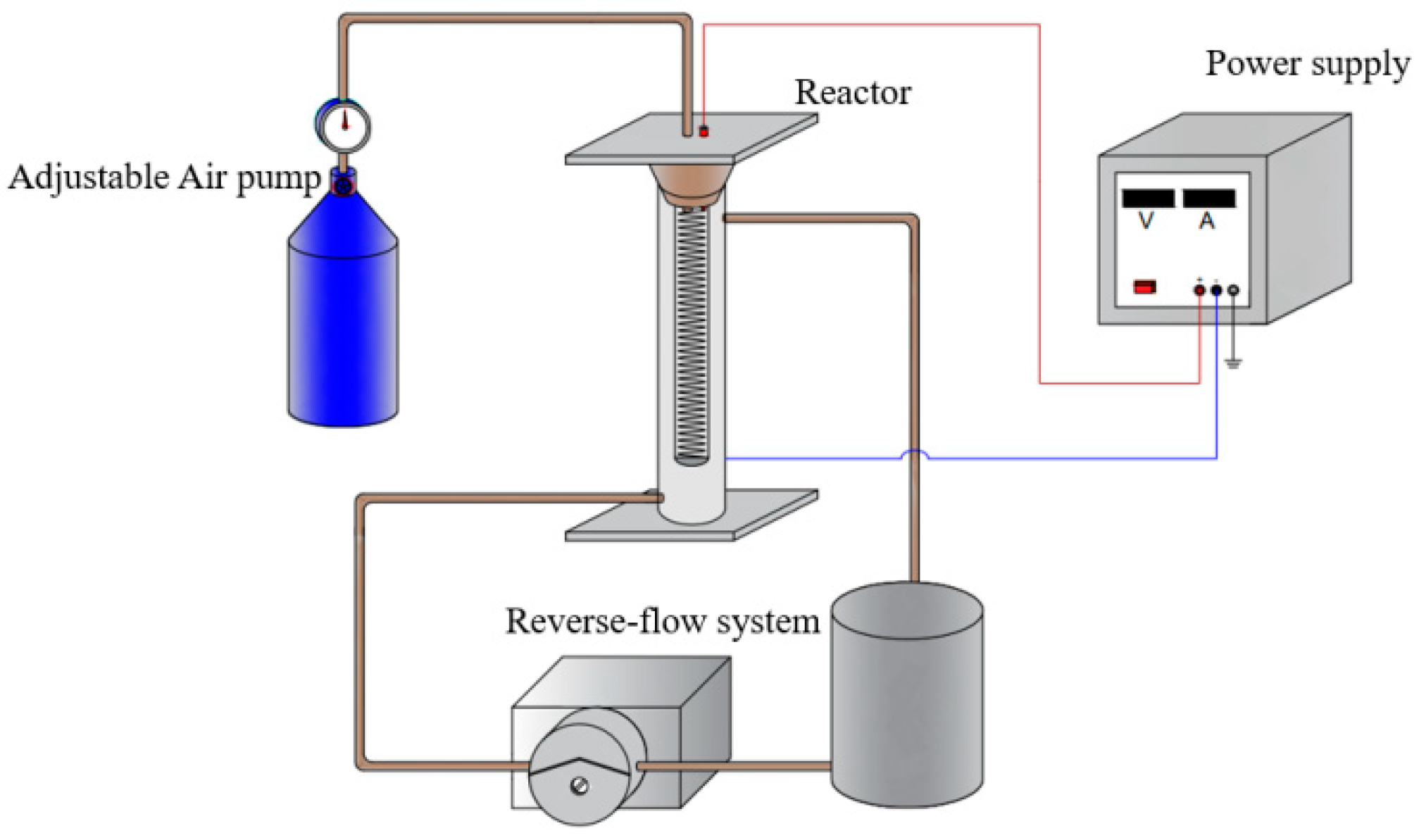
The system of sewage sludge disintegration consisted of the discharge plasma system, the reverse-flow system, and the reactor, as shown in
Figure 1. In the discharge plasma system, a stainless steel wire served as the discharge electrode (pitch 0.8 cm; length 10 cm), which was tightly placed into a quartz glass tube (inner diameter 1 cm; wall thickness 1 mm; length 15 cm). The quartz glass tube was used as the discharge barrier. The ground electrode consisted of the sewage sludge and the wire inserted into it. The air dried by a silica column was pumped into the quartz glass tube from the top, and the gas flow was controlled at 120 L h-1. An AC-stabilized power source (CTP-2000K) was used, which was purchased from Nanjing Suman Electronics Co., Ltd. (China). The discharge voltage was 3 kV and the discharge frequency was 7 kHz in this study. The reactor for sewage sludge disintegration was a plexiglass tube (inner diameter 4 cm; wall thickness 2 mm; length 25 cm). When the discharge plasma was triggered, the generated active species would enter the sewage sludge through an aerator at the bottom of the quartz glass tube.
In each batch experiment, 300 mL of the raw sludge suspension with different concentrations of TSA were mixed and then added to the reaction vessel. A peristaltic pump was utilized to circulate the sewage sludge suspension during the treatment. All the experiments were performed in triplicate.
2.3. Analytical methods
The particle size distribution of sewage sludge flocs was determined with a laser diffraction particle size analyzer (Mastersizer APA 2000, Malvern Instruments Ltd, UK). The sewage sludge collected from treatments were centrifuged at 3000 rpm for 10 min, and the supernatant was separated. Then, the sewage sludge was freeze-dried and sputtered with gold prior to scanning electron microscopy (SEM) analysis. SEM images were collected using a cold field emission scanning electron microscope (SEM, Hitachi S-4800, Japan) at an accelerating voltage of 10.0 kV.
The sewage sludge suspension was centrifuged in a 50 mL centrifuge tube at 3000 g for 10 min, and the supernatant was collected as soluble EPS (S-EPS). A heat extraction method was used to extract the loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS) [
30], as illustrated in
Text S1 in the Supporting Information. The dissolved organic carbon (DOC) in the EPS was detected with a total organic carbon analyzer (TOC MultiN /CUV, Analytic Jena, Germany). Polysaccharides (PS) in EPS was quantified with the phenol-sulfuric acid process [
31]. Protein (PN) in EPS was measured by the modified Lowry method [
32]. The three-dimensional fluorescence spectrum (3D-EEM) of the various EPS fractions was measured using a fluorescence spectrometer (F-4600, Hitachi, Japan). Fourier transform infrared (FT-IR) spectroscopy of the EPS was determined with a Fourier infrared spectrum instrument (Vetex 70, Bruck, Germany). Fluorescence intensity was analyzed with a fluorescence spectrometer (F-4600, Hitachi, Japan). The soluble chemical oxygen demand (SCOD), the settling velocity (SV30), and the sludge moisture content of filter cake (Wc%) of the sewage sludge were detected using the standard procedures [
33].
3. Results and discussion
3.1. Sewage sludge disintegration performance
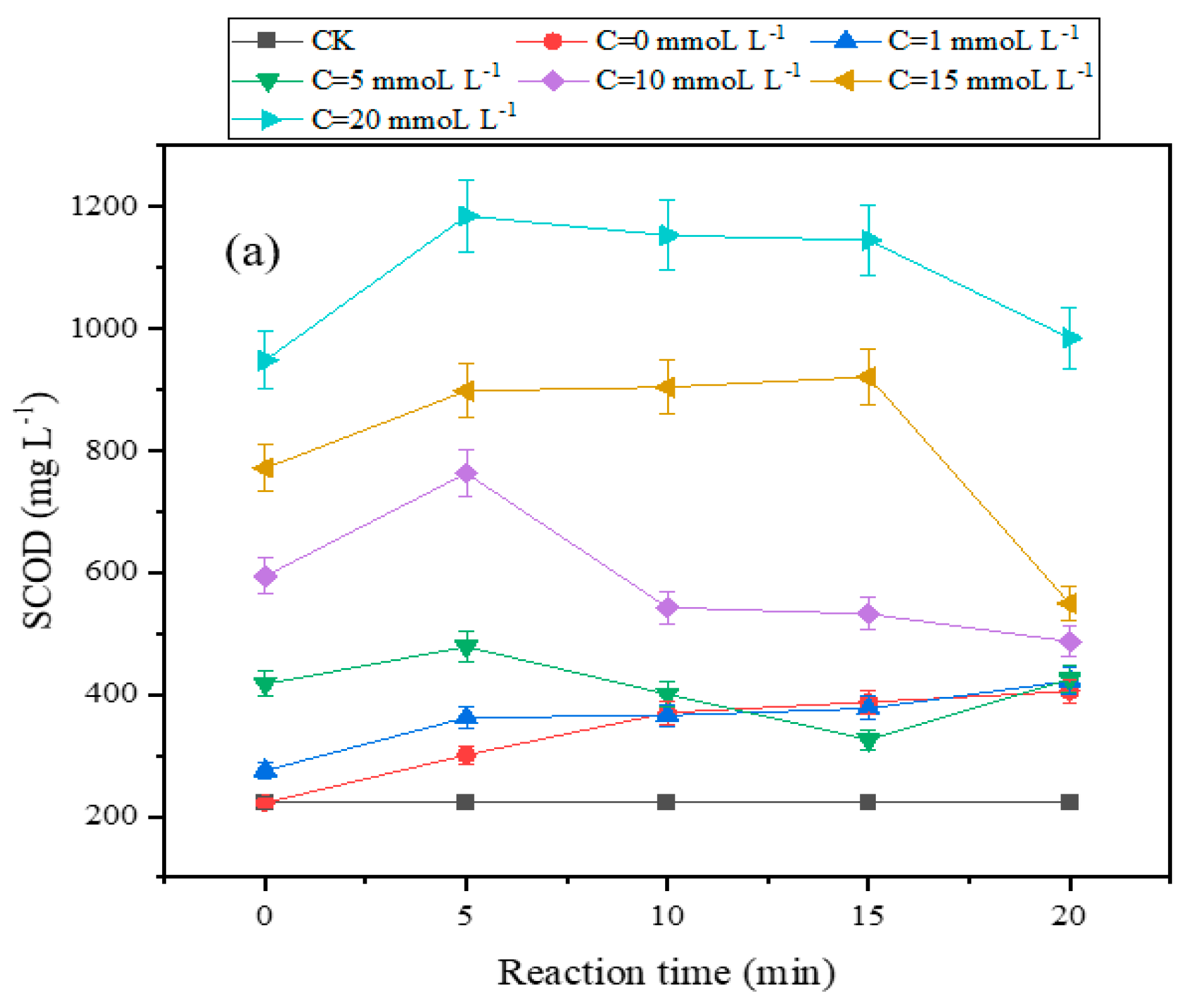
The change in SCOD in the sewage sludge can be used to gauge the effectiveness of sewage sludge disintegration [
34]. As shown in
Figure 2, when the TSA concentration was low (0 mmol L-1, 1 mmol L-1, and 5 mmol L-1), the SCOD increased with the increase in the treatment time. When the concentration of TSA was high (10-20 mmol L-1), the SCOD decreased and had an inflection point. The inflection point of SCOD occurred after 15 min of reaction when the concentration of TSA was 15 mmol L-1, whereas there was not an inflection point at the concentration of TSA 20 mmol L-1. 15 mmol L-1 was the optimal amount of TSA in this system. At the low concentration of TSA, ozone can accelerate the production of free radicals, whereas high concentration of TSA will prevent the reactions [
28,
29]. With the raising of TSA dosage, the SCOD increased in general after 20 min of processing. The SCOD of the untreated sludge was 222.38 mg L-1. The SCOD increased to 404.93 mg L-1 after 20 min of single discharge plasma treatment, and it further increased to 549.08 mg L-1 after adding 15 mmol L-1 of TSA. In the presence of low concentration of TSA, more amounts of free radicals would be generated, and the free radicals oxidized parts of organic matter in sludge into solution [
34,
35].
As shown in
Figure 3, the moisture content of the filter cake decreased first and then increased with the TSA dosages. When the concentration of TSA was 15 mmol L-1, the water content of the filter cake was the lowest, which corroborated the results of SCOD. The moisture content of the filter cake after 20 min of discharge plasma treatment with 15 mmol L-1 of TSA was 83.55%. This was similar to the result of previous study [
36]. These results also demonstrated that TSA was beneficial to improve the sludge dewatering in the discharge plasma process.
3.2. Changes in sludge particle size and morphology during dewatering process
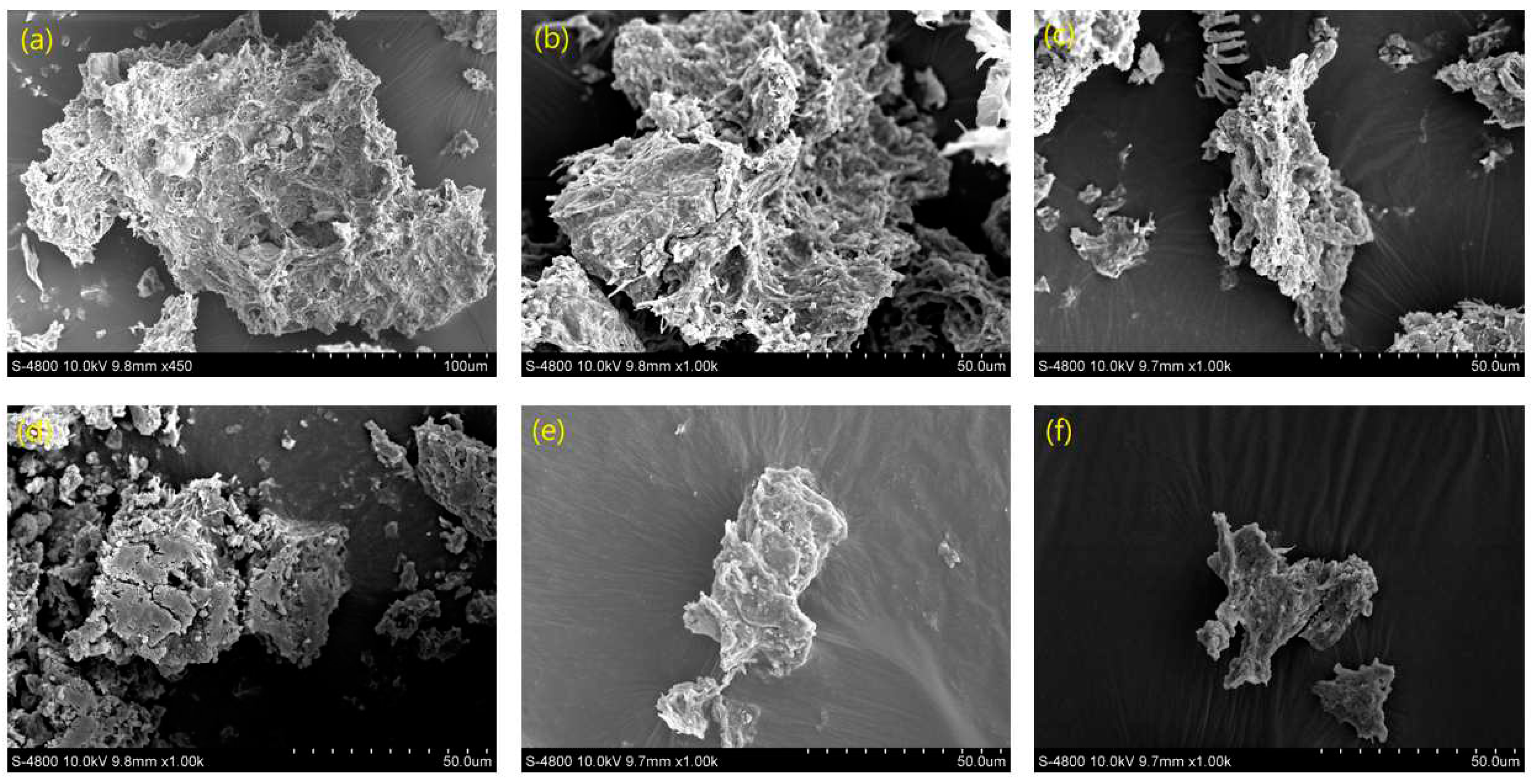
As shown in
Figure 4, the surface of the initial sewage sludge without treatment was compact and had a complete structure, which enhanced the ability of the sludge floc to bind water, thus making it difficult to dehydrate. After the discharge plasma treatment, the sludge floc became looser and finer, and the surface also became coarser, especially in the presence of TSA. The strong oxidizing free radicals generated by the discharge plasma system destroyed the microbial cells and the massive floc structure, and promoted the release of organic matter and water in the floc from the mud phase to the water phase [
36]. On the other hand, the EPS wrapped on the surface of the floc was destroyed by the oxidation of free radicals, which led to it become rough and affected the spatial distribution of hydrophilic functional groups [
37]. These phenomena were consistent with previous studies [
36,
38].
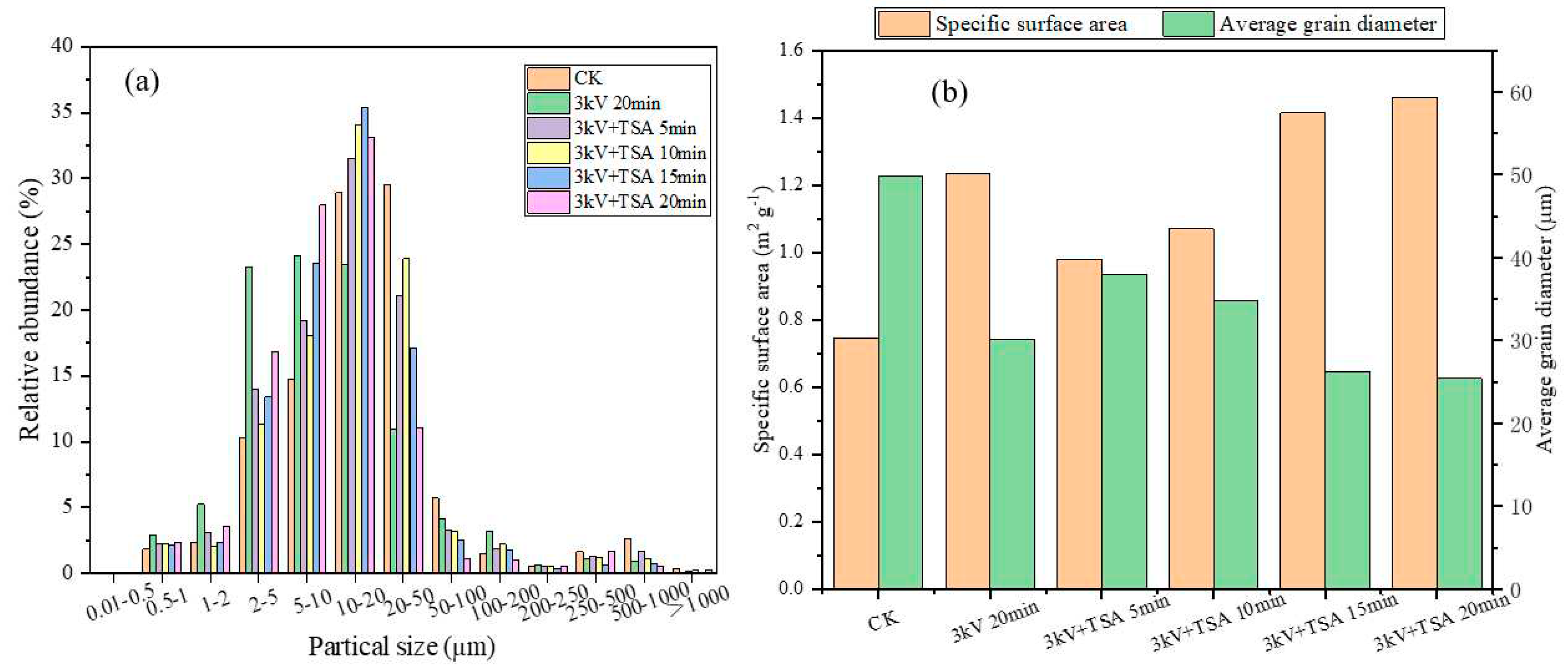
According to
Figure 5a, the discharge plasma coupled with TSA treatment increased the proportion of small particle size (0.01-0.5, 0.5-1, 1-2, 2-5, 5-10, and 10-20 µm) of sludge, and the proportion was getting higher and higher in general with the processing. However, the proportion of large particle size (20-50, 50-100, 100-200, 200-250, 250-50, 500-1000, and >1000 µm) showed a gradually decreasing trend. This was consistent with the results of the sludge morphology. The changes in average particle size and specific surface area of sludge particles under different treatments are visible in
Figure 5b. After 5, 10, 15, and 20 min of treatment, the average particle size of the sludge gradually decreased from the initial 49.8 µm to 37.9, 34.7, 26.3, and 25.5 µm, while the specific surface area gradually increased from 0.746 m2/g to 0.978, 1.07, 1.41, and 1.46 m2/g, respectively. These were in line with previous studies [
34,
36]. The oxidizing species could destroyed the structure and integrity of the sludge floc, causing the floc to break into smaller particle-size fragments.
3.3. Changes in EPS distribution
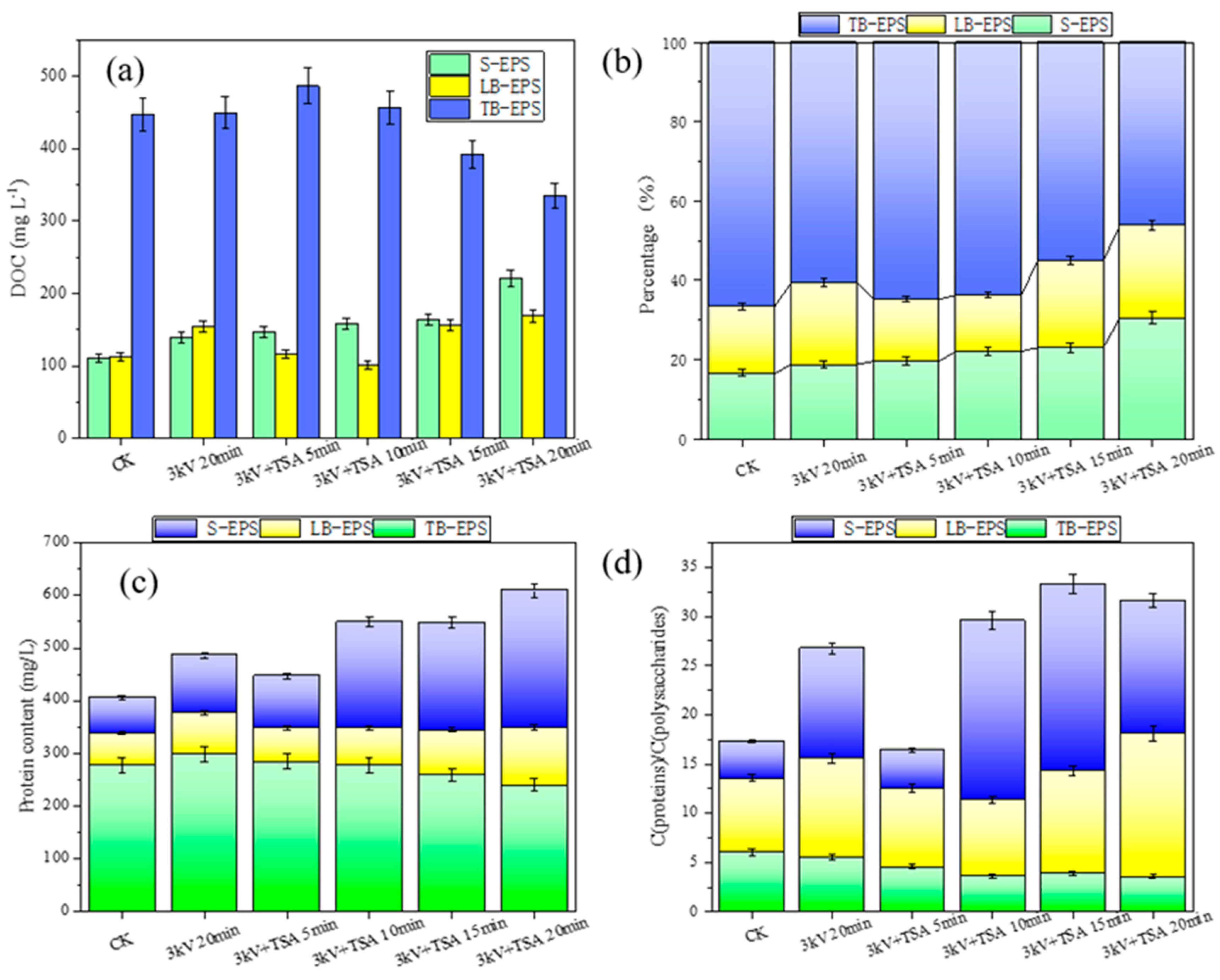
EPS is an important component of sludge, and it can affect the physical and chemical properties of sludge [
3]. As illustrated in
Figure 6a, the content of TB-EPS in the raw sludge (447.0 DOC mg L-1) was much higher than the content of SPES (111.3 DOC mg L-1) and LB-EPS (112.8 DOC mg L-1) [
39]. After discharge plasma coupled with TSA treatment, the contents of S-EPS and LB-EPS showed increasing trends, while the content of TB-EPS showed a decreasing trend. As observed from
Figure 6b, after 5, 10, 15, and 20 min of treatment, the proportion of TB-EPS gradually decreased from 66.6% to 64.8%, 63.8%, 55.1%, and 46.2%; S-EPS gradually increased from 16.6% to 19.6%, 22.1%, 23.0%, and 30.5%. Protein and polysaccharide are considered to be the two most important components of EPS, and the ratio of protein to polysaccharide content is considered as an important indicator to characterize the dewatering performance of sludge [
40]. In
Figure 6c-6d, the changes in protein contents and ratio of protein to polysaccharide were consistent with the changes of EPS, which were consistent with previous studies [
39,
41].
These results indicated that the discharge plasma coupled with TSA treatment could affect the evolution of different types of EPS. On the one hand, the free radicals generated in the discharge plasma coupled with TSA system could directly destroy the free microbial cells or penetrate into the interior of the sludge floc, resulting in the release of a large amount of intracellular organic matter, which was manifested by the increased EPS contents [
34,
42]. On the other hand, due to the fragmentation of floc and the redistribution of organic matter wrapped in floc during the cracking process of sludge, the internal EPS was continuously transformed into the external EPS [
43].
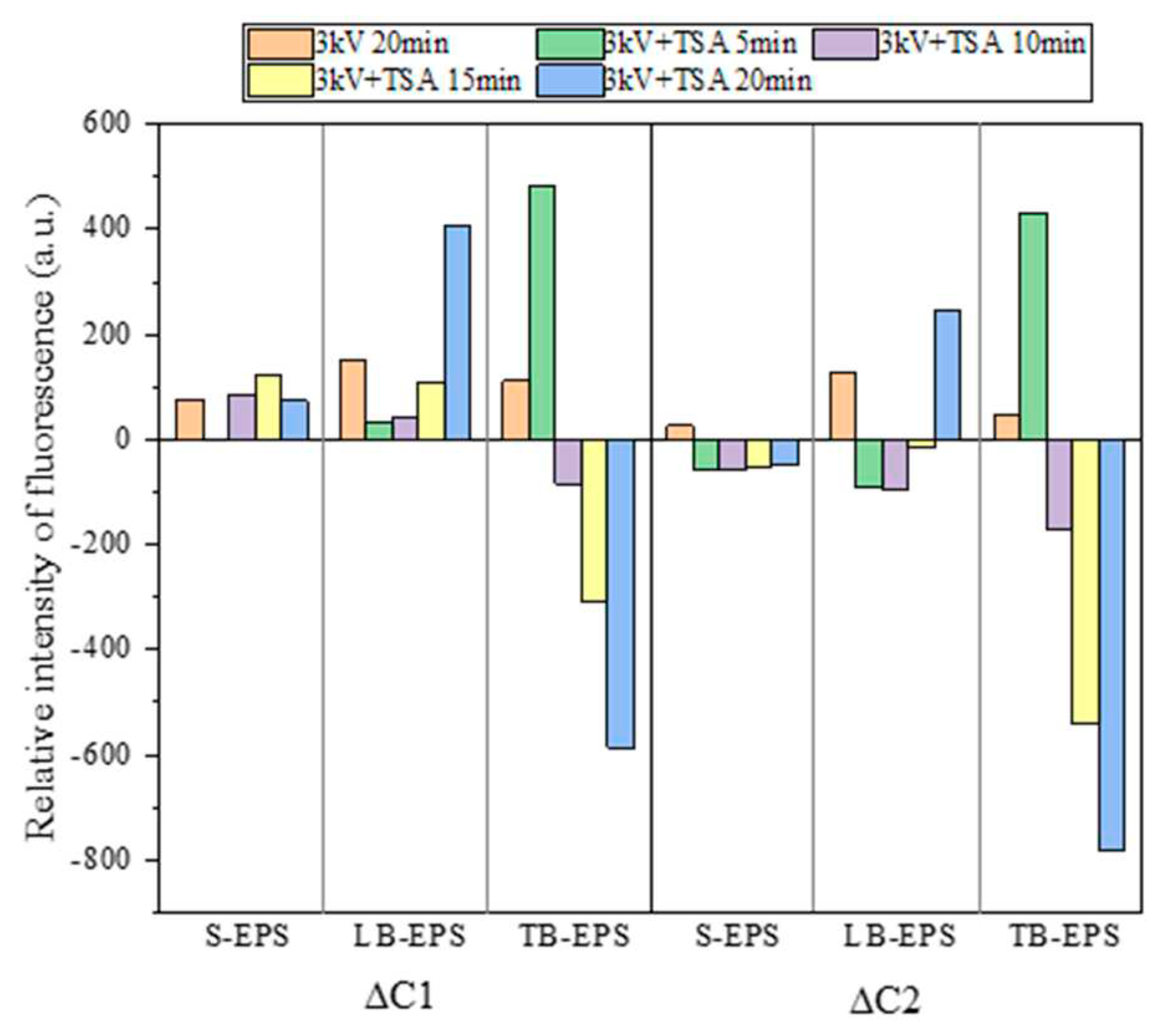
3D-EEM is often used to characterize the changes of organic components in EPS [
44,
45]. As shown in
Figures S1-S3, the 3D-EEM spectra of EPS of the initial sludge samples showed two fluorescence peaks (A and B), and their specific locations (EX/EM) were roughly 280/330 nm (peak A) and 225/325 nm (peak B), corresponding to soluble microbial by-products and aromatic proteins, respectively [
44,
45]. Two main fluorescence components (C1 and C2) in the 3D-EEM spectra of EPS were diagnosed, and their changes are shown in
Figure 7. The C1 and C2 corresponded to aromatic proteins and soluble microbial by-products, respectively. The relative fluorescence intensities of C1 and C2 gradually increased in S-EPS and LB-EPS. These could be due to the combined actions of the lysing of microbial cells (including free microorganisms in the water phase and encapsulated microorganisms in the mud phase), the transfer of organic components (from TB-EPS to S-EPS and LB-EPS), and the oxidative degradation of organic matter by free radicals. The relative fluorescence intensities of C1 and C2 gradually decreased in TB-EPS, which was mainly caused by the transfer of organic components in TB-EPS and the lysis of microorganisms in the mud phase. TB-EPS was initially dominated by the lysis of microorganisms and then mainly by the transfer of organic components. These findings were similar to those previous studies [
35,
46].
3.4. Mechanisms of sludge cracking
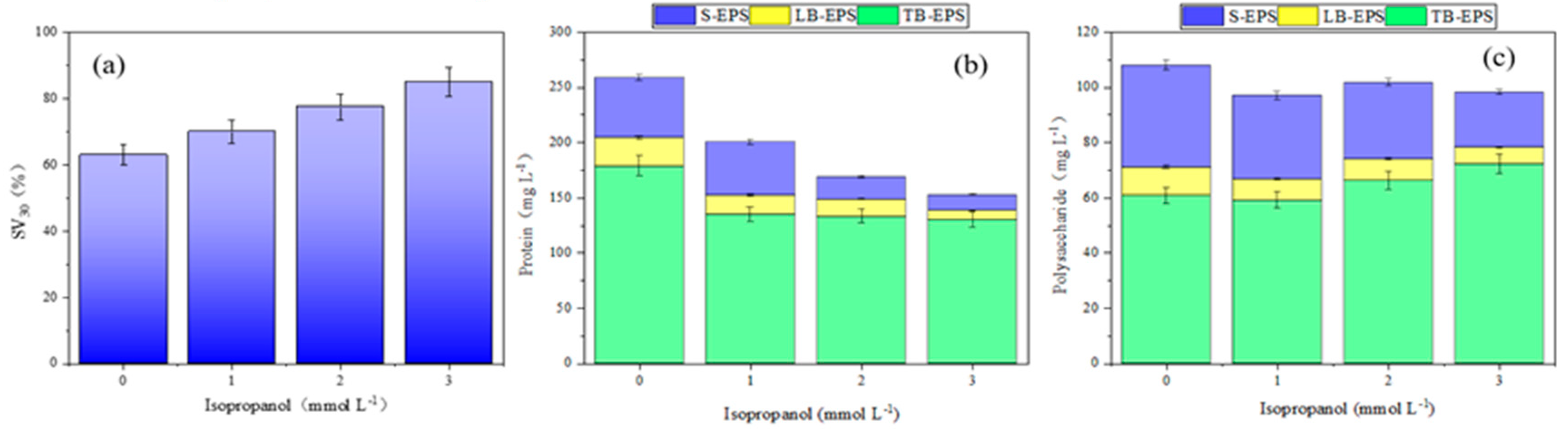
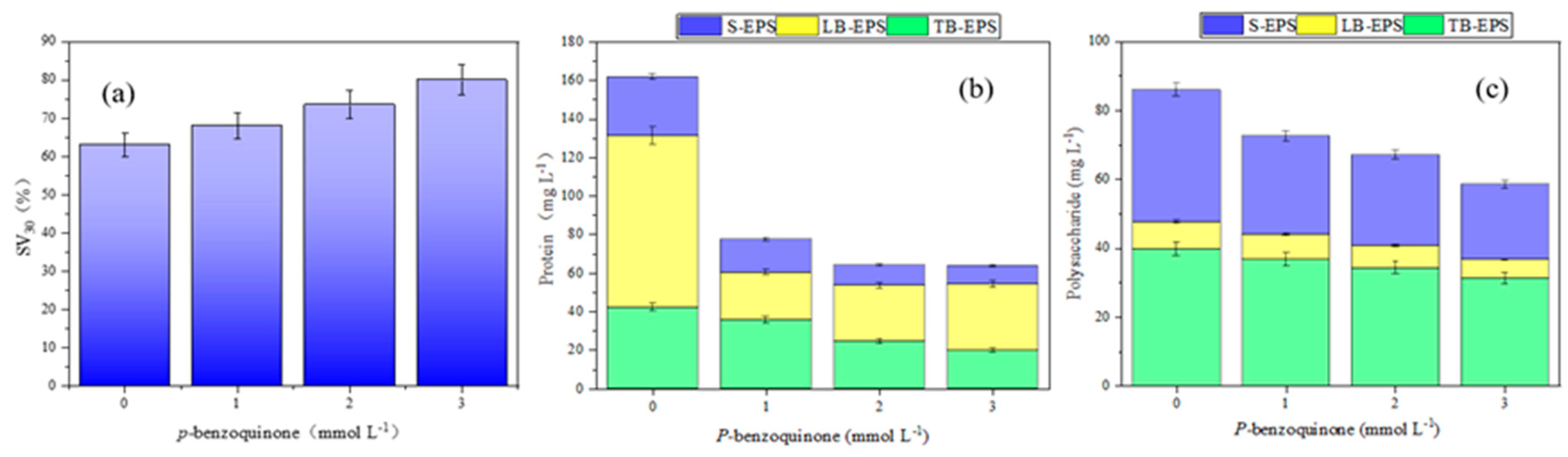
To analyze the mechanisms of cracking sludge in the discharge plasma coupled with TSA system, radical quenching experiments were conducted by adding the trapping agents. ·OH and ·O2- can be inhibited by isopropyl alcohol and p-benzoquinone, respectively [
25]. As shown in
Figure 8, with the increase in p-benzoquinone concentration, the settling velocity (SV30) of sludge increased, and the contents of protein and polysaccharide in EPS showed obvious decreasing trends. Analogously, the addition of isopropanol also exhibited the similar effects, as shown in
Figure 9. These results showed that ·OH and ·O2- played important roles in sludge cracking process. As shown in
Figure 8 and
Figure 9, the influence of ·OH on EPS components was greater than that of ·O2-. Therefore, ·OH played more important roles.
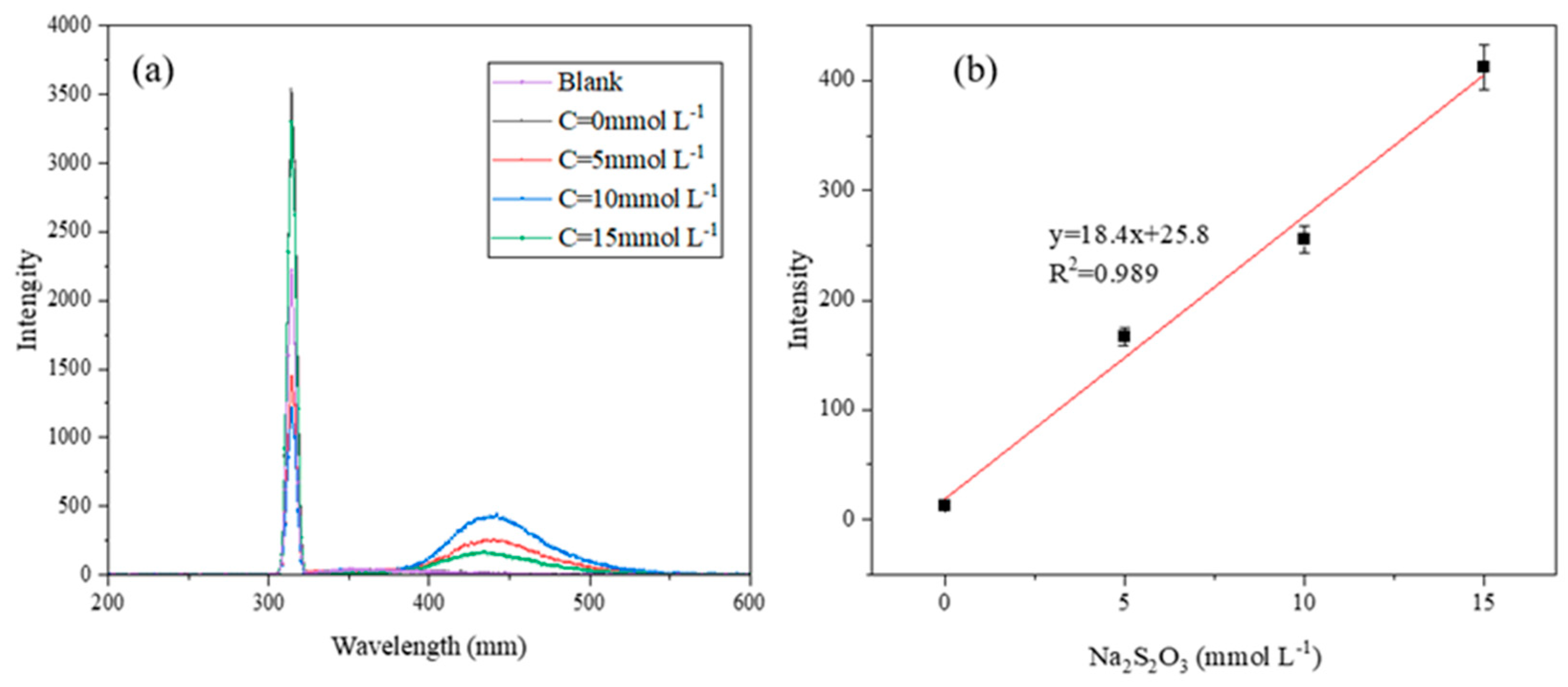
To further verify the role of free radicals, the ·OH produced by the discharge plasma coupled with TSA system was analyzed by fluorescence analysis, and terephthalic acid (PTA) was used as an indicator of ·OH [
47,
48]. As shown in
Figure 10, with the increase in TSA concentration, the content of hydroxy-terephthalic acid (HTA) produced by reactions of PTA with ·OH increased, which proved the existence and roles of ·OH in the system. To better study the relationship between the concentration of ·OH and the amount of TSA, a semi-quantitative analysis of the fluorescence intensity of HTA under different amounts of TSA was carried out. As shown in
Figure 10b, with the increase in TSA concentration, the fluorescence intensity of HTA increased almost linearly. That is, the ·OH produced by the discharge plasma also increased linearly with TSA addition. This was in line with a previous study [
29].
4. Conclusions
In this study, the effect of discharge plasma coupled with thiosulfate on sludge disintegration was evaluated. Discharge plasma oxidation coupled with thiosulfate promoted the sludge disintegration under the optimal dosage. During the treatment, reactive oxygen species (·OH and ·O2-) oxidation could cause cell lysis and destruction of sludge flocs, which together induced the enhancement of EPS content and redistribution between different EPS fractions. 3D-EEM and PARAFC modeling analysis of EPS concluded that the migration and decomposition of EPS were the main processes for the EPS changing. The intact and dense surface morphology of the sludge flocs was destroyed into more fragmented ones, accompanied with increased a large number of small particles. Meanwhile, the intracellular bound water in the sludge flocs was released outside as free water. On the basis of this study, further studies can be conducted on carrying out batch tests outside the lab.
Supplementary Materials
Figures S1-S3 represent 3D-EEM of EPS. Text S1 includes extraction of various EPS.
Acknowledgments
The authors thank the National Natural Science Foundation of China (21976143), the National Key R&D Program of China (2018YFC1802004, 2018YFC1801003).
References
- Xu, Q.; Li, X.; Ding, R.; Wang, D.; Liu, Y.; Wang, Q.; Zhao, J.; Chen, F.; Zeng, G.; Yang, Q.; Li, H. Understanding and mitigating the toxicity of cadmium to the anaerobic fermentation of waste activated sludge. Water Res. 2017, 124, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.C.; Serrano, A.; Siles, J.A.; Chica, A.F.; Martin, M.A. Centralized management of sewage sludge and agro-industrial waste through co-composting. J. Environ. Manage. 2017, 196, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Wang, D.; Ma, T.; Bai, R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Wang, B.; Zhao, Y.; Chai, X.; Niu, D.; Zhao, T. Enhanced dewatering characteristics of waste activated sludge with Fenton pretreatment: Effectiveness and statistical optimization. Fron. Environ. Sci. Eng. 2014, 8, 267–276. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J.; Dewil, R.; De heyder, B. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Walczak, J. Enhancing combined biological nitrogen and phosphorus removal from wastewater by applying mechanically disintegrated excess sludge. Water Res. 2015, 76, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Deng, J.; Lei, H.; Bai, T.; Fan, Q.; Li, Z. Dewaterability of waste activated sludge with ultrasound conditioning. Bioresource Technol. 2009, 100, 1074–1081. [Google Scholar] [CrossRef]
- Ye, F.; Liu, X.; Li, Y. Effects of potassium ferrate on extracellular polymeric substances (EPS) and physicochemical properties of excess activated sludge. J. Hazard. Mater. 2012, 199, 158–163. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, W.; Wang, Q.; Huang, Y.; Meng, C.; Wang, D. Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: Role of aluminum speciation. Water Res. 2016, 105, 615–624. [Google Scholar] [CrossRef]
- Zahedi, S.; Icaran, P.; Yuan, Z.; Pijuan, M. Effect of free nitrous acid pre-treatment on primary sludge at low exposure times. Bioresource Technol. 2017, 228, 272–278. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Zheng, G.; Zhou, L. Improving the compression dewatering of sewage sludge through bioacidification conditioning driven by Acidithiobacillus ferrooxidans: Dewatering rate vs. dewatering extent. Environ. Technol. 2019, 40, 3176–3189. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.-a.; Chen, H.; Wu, J.; Wang, Y.; Liu, J.; Lin, M. Effects of ultrasound assisted Fenton treatment on textile dyeing sludge structure and dewaterability. Chem. Eng. J. 2014, 242, 102–108. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Talhi, H.A.; Mohamed, S.A.; Kumar, R.; Barakat, M.A. Visible light photocatalytic disintegration of waste activated sludge for enhancing biogas production. J. Environ. Manage. 2018, 216, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Feki, E.; Khoufi, S.; Loukil, S.; Sayadi, S. Improvement of anaerobic digestion of waste-activated sludge by using H2O2 oxidation, electrolysis, electro-oxidation and thermo-alkaline pretreatments. Environ. Sci. Pollut. Res. 2015, 22, 14717–14726. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Zhu, S.; Zhu, M.; Li, D.; Xu, S. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange II from water. J. Hazard. Mater. 2012, 229, 331–339. [Google Scholar] [CrossRef]
- Yuan, H.-p.; Yan, X.-f.; Yang, C.-f.; Zhu, N.-w. Enhancement of waste activated sludge dewaterability by electro-chemical pretreatment. J. Hazard. Mater. 2011, 187, 82–88. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.M.; Senthilnathan, J. An innate quinone functionalized electrochemically exfoliated graphene/Fe3O4 composite electrode for the continuous generation of reactive oxygen species. Chem. Eng. J. 2017, 316, 964–977. [Google Scholar] [CrossRef]
- Ching, W.K.; Colussi, A.J.; Sun, H.J.; Nealson, K.H.; Hoffmann, M.R. Escherichia coli disinfection by electrohydraulic discharges. Environ. Sci. Technol. 2001, 35, 4139–4144. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Lv, L.; Wang, J. Degradation of caffeic acid by dielectric barrier discharge plasma combined with Ce doped CoOOH catalyst. J. Hazard. Mater. 2021, 402, 123772. [Google Scholar] [CrossRef]
- Mok, Y.S.; Nam, C.M.; Cho, M.H.; Nam, I.S. Decomposition of volatile organic compounds and nitric oxide by nonthermal plasma discharge processes. Ieee Trans. Plasma Sci. 2002, 30, 408–416. [Google Scholar]
- Larouss, M. The biomedical applications of plasma: A brief history of the development of a new field of research. Ieee Trans. Plasma Sci. 2008, 36, 1612–1614. [Google Scholar] [CrossRef]
- He, Y.; Sang, W.; Lu, W.; Zhang, W.; Zhan, C.; Jia, D. Recent Advances of Emerging Organic Pollutants Degradation in Environment by Non-Thermal Plasma Technology: A Review. Water 2022, 14, 1351. [Google Scholar] [CrossRef]
- Ye, Q.; Wan, H.; Lei, Y.; Zhang, J.; Li, J. Study on the technique of water-treatment by discharge plasma. High Voltage Eng. 2003, 29, 32–34. [Google Scholar]
- Nilsson, F.; Davidsson, A.; Falas, P.; Bengtsson, S.; Bester, K.; Jonsson, K. Impact of activated sludge ozonation on filamentous bacteria viability and possible added benefits. Environ. Technol. 2019, 40, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Gan, J.Y.; Papiernik, S.K.; Yates, S.R. Isomeric effects on thiosulfate transformation and detoxification of 1,3-dichloropropene. Environ. Toxicol. Chem. 2001, 20, 960–964. [Google Scholar] [CrossRef]
- Hemdal, J.F. Reduction of Ozone Oxidants in Synthetic Seawater by Use of Sodium Thiosulfate. Progressive Fish-Culturist 1992, 54, 54–56. [Google Scholar] [CrossRef]
- Pollmann, J.; Ortega, J.; Helmig, D. Analysis of atmospheric sesquiterpenes: Sampling losses and mitigation of ozone interferences. Environ. Sci. Technol. 2005, 39, 9620–9629. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.; Zhu, Y.; Lin, F.; Ma, Q.; Wang, Z.; Cen, K. Simultaneous removal of SO2 and NOx by combination of ozone oxidation and Na2S2O3 solution spray. CIESC J. 2016, 67, 2041–2047. [Google Scholar]
- Yang, J.; Luo, C.; Li, T.; Cao, J.; Dong, W.; Li, J.; Ma, J. Superfast degradation of refractory organic contaminants by ozone activated with thiosulfate: Efficiency and mechanisms. Water Res. 2020, 176, 115751. [Google Scholar] [CrossRef]
- Ge, L.; Wang, H.; Ma, L.; Li, X. Optimization of extraction process of extracellular substances by physic method. Environ. Chem. 2006, 25, 722–725. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Bureau, N.E. Methods for Monitoring and Analysis of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Zhang, J.; Tian, Y.; Li, N.; Kong, L.C.; Sun, L.; Yu, M.; Zuo, W. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability. Chem. Eng. J. 2016, 301, 238–248. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; He, S.; Zhou, J.; Wang, T.; Zhu, L. Efficient degradation of antibiotics by non-thermal discharge plasma: Highlight the impacts of molecular structures and degradation pathways. Chem. Eng. J. 2020, 395, 125091. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Li, J.; Xiao, L. Rapid and efficient activated sludge treatment by electro-Fenton oxidation. Water Res. 2019, 152, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.Y.; Wang, J.H.; Lu, X.Q.; Su, L.H.; Zhu, X.F.; Zhou, T.; Zhao, Y.C. Effective gel-like floc matrix destruction and water seepage for enhancing waste activated sludge dewaterability under hybrid microwave-initiated Fe(II)-persulfate oxidation process. Chemosphere 2019, 221, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ni, B.J.; Horvat, K.; Song, L.; Chai, X.; Dai, X.; Mahajan, D. Occurrence State and Molecular Structure Analysis of Extracellular Proteins with Implications on the Dewaterability of Waste-Activated Sludge. Environ. Sci. Technol. 2017, 51, 9235–9243. [Google Scholar] [CrossRef]
- You, G.; Wang, P.; Hou, J.; Wang, C.; Xu, Y.; Miao, L.; Lv, B.; Yang, Y.; Liu, Z.; Zhang, F. Insights into the short-term effects of CeO2 nanoparticles on sludge dewatering and related mechanism. Water Res. 2017, 118, 93–103. [Google Scholar] [CrossRef]
- Peipei, H.; Guanghui, Y.; Liming, S.; Pinjing, H. Effects of protein and polysaccharide distribution in sludge on dewatering performance. Environ. Sci. 2008, 3457–3461. [Google Scholar]
- Niu, M.; Zhang, W.; Wang, D.; Chen, Y.; Chen, R. Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants. Bioresource Technol. 2013, 144, 337–343. [Google Scholar] [CrossRef]
- Niu, T.; Zhou, Z.; Ren, W.; Jiang, L.-M.; Li, B.; Wei, H.; Li, J.; Wang, L. Effects of potassium peroxymonosulfate on disintegration of waste sludge and properties of extracellular polymeric substances. Int. Biod. Biodegrad. 2016, 106, 170–177. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Yang, Q.; Xu, Q.; Tao, Z.; Huang, X.; Wu, Y.; Tao, L.; Pi, Z.; Chen, Z.; Wang, D. Effect of citric acid on extracellular polymeric substances disruption and cell lysis in the waste activated sludge by pH regulation. Bioresource Technol. 2020, 302, 122859. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, A.; Jiang, G.; Li, Q. Transformation and degradation mechanism of landfill leachates in a combined process of SAARB and ozonation. Waste Manage. 2019, 85, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gholikandi, G.B.; Zakizadeh, N.; Masihi, H. Application of peroxymonosulfate-ozone advanced oxidation process for simultaneous waste-activated sludge stabilization and dewatering purposes: A comparative study. J. Environ. Manage. 2018, 206, 523–531. [Google Scholar] [CrossRef]
- Li, L.X.; Abe, Y.; Nagasawa, Y.; Kudo, R.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. An HPLC assay of hydroxyl radicals by the hydroxylation reaction of terephthalic acid. Biomed. Chrom. 2004, 18, 470–474. [Google Scholar]
- Ding, Q.; Wang, J.; Wang, Y. Detection of hydroxyl radicals in MIL-88A photoFenton catalytic system. Anal. Inst. 2021, 132–138. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).