Submitted:
15 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
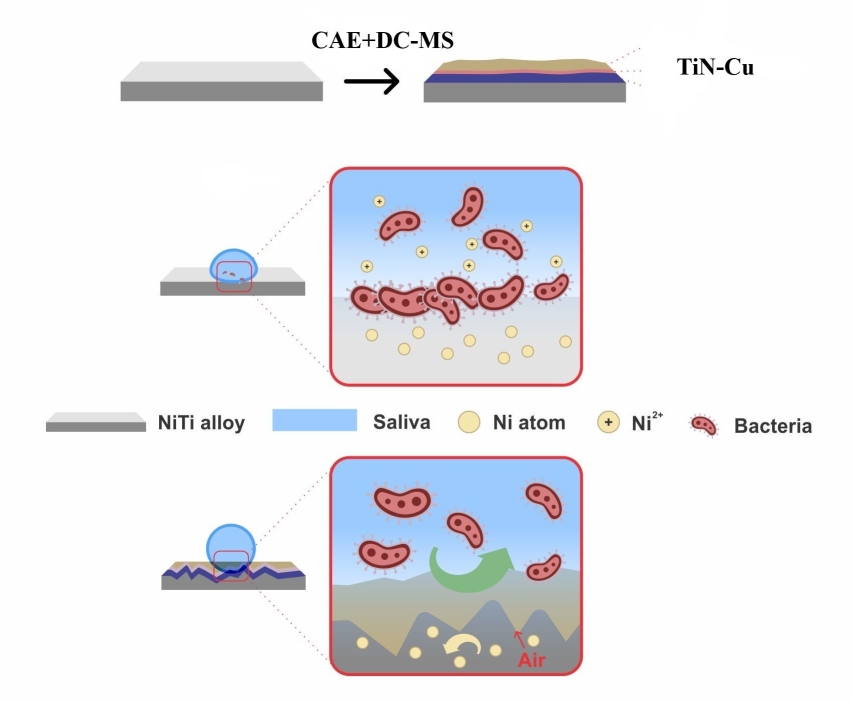
Keywords:
Introduction
Materials and Methods
Deposition of the films
Physico-chemical characterization of the films
Inductively Coupled Plasma- Optical Emission Spectroscopy
MTT Cytotoxicity Assay
Bacterial adhesion and biofilm formation
Statistical Analysis
Results
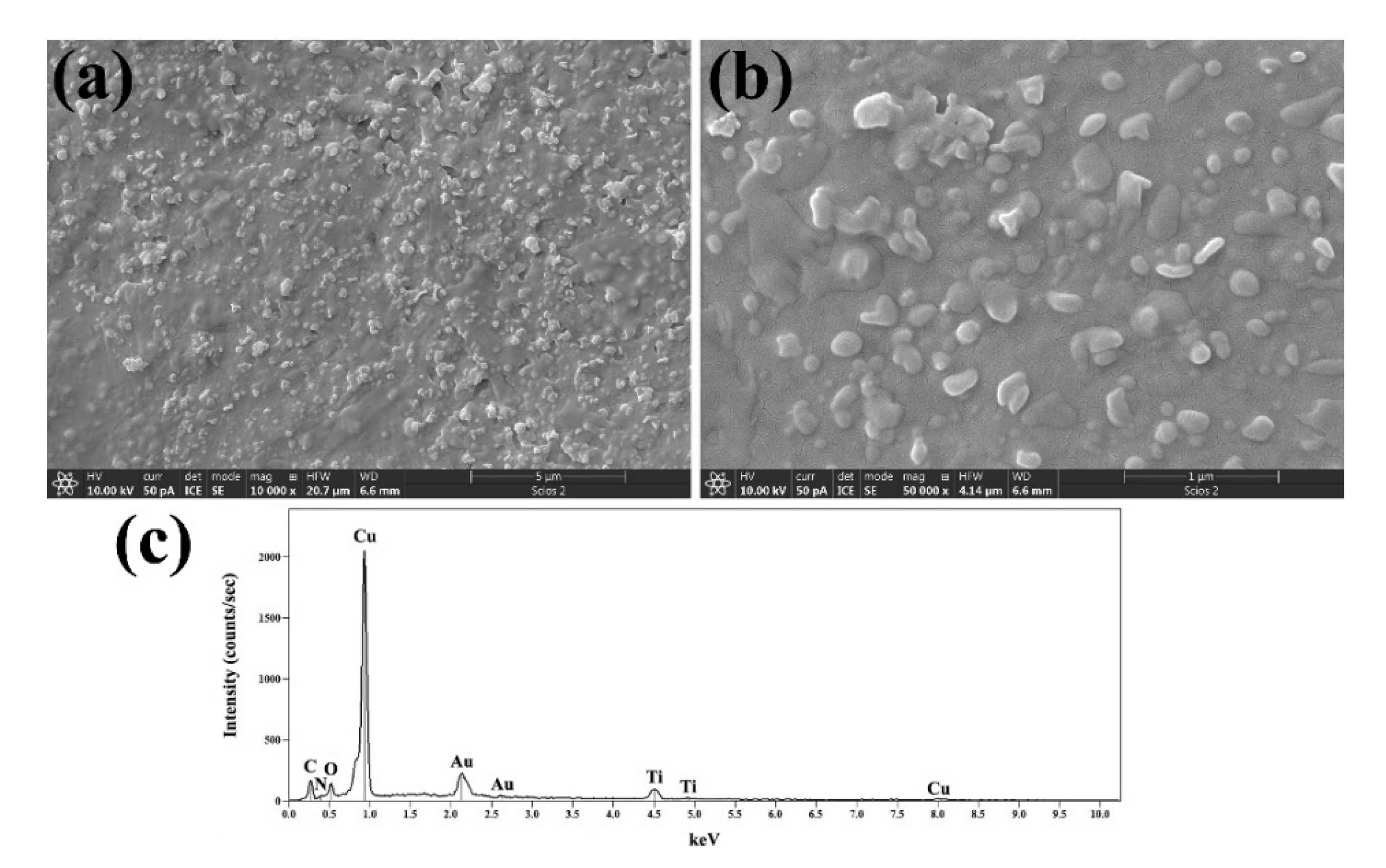
FESEM
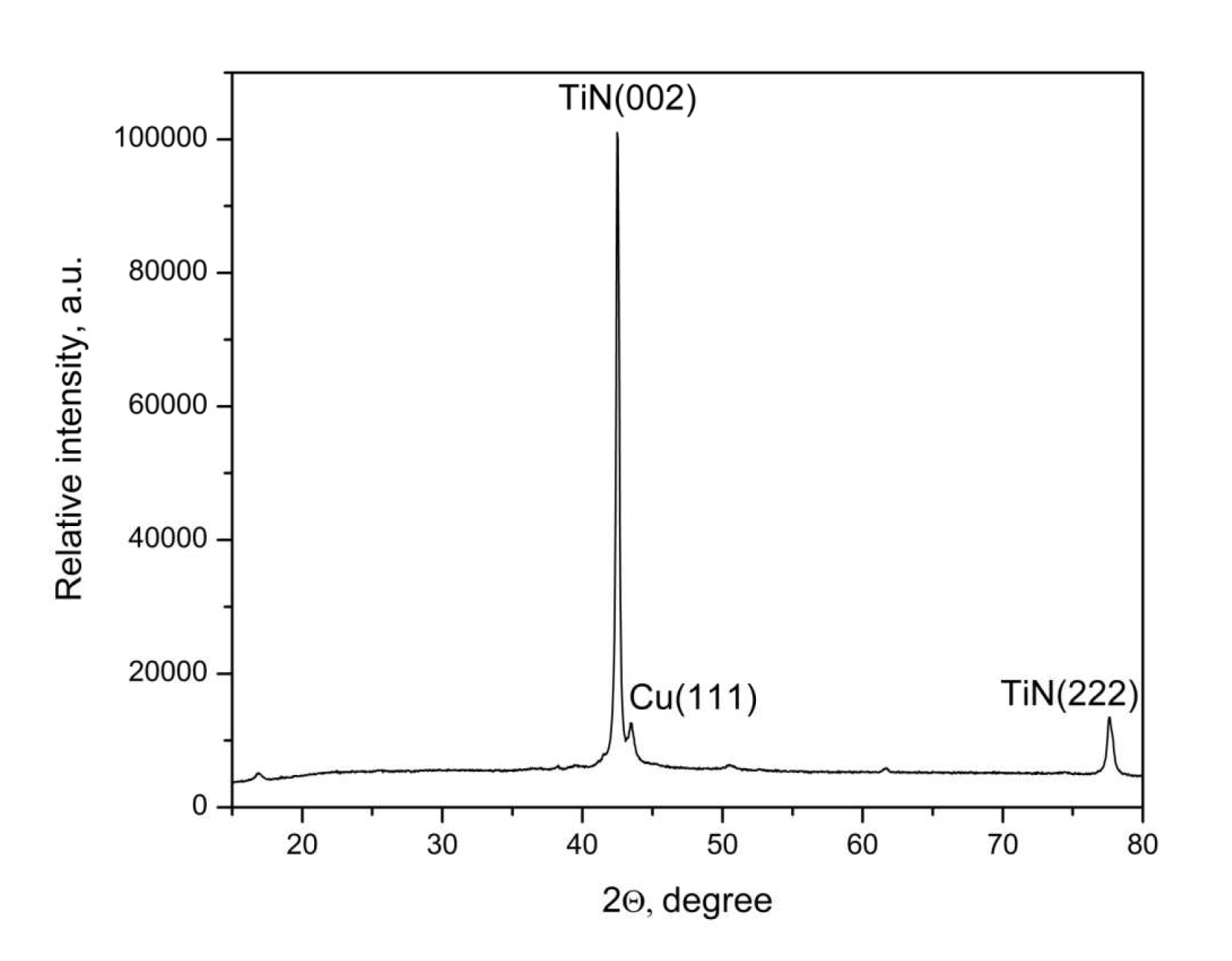
XRD
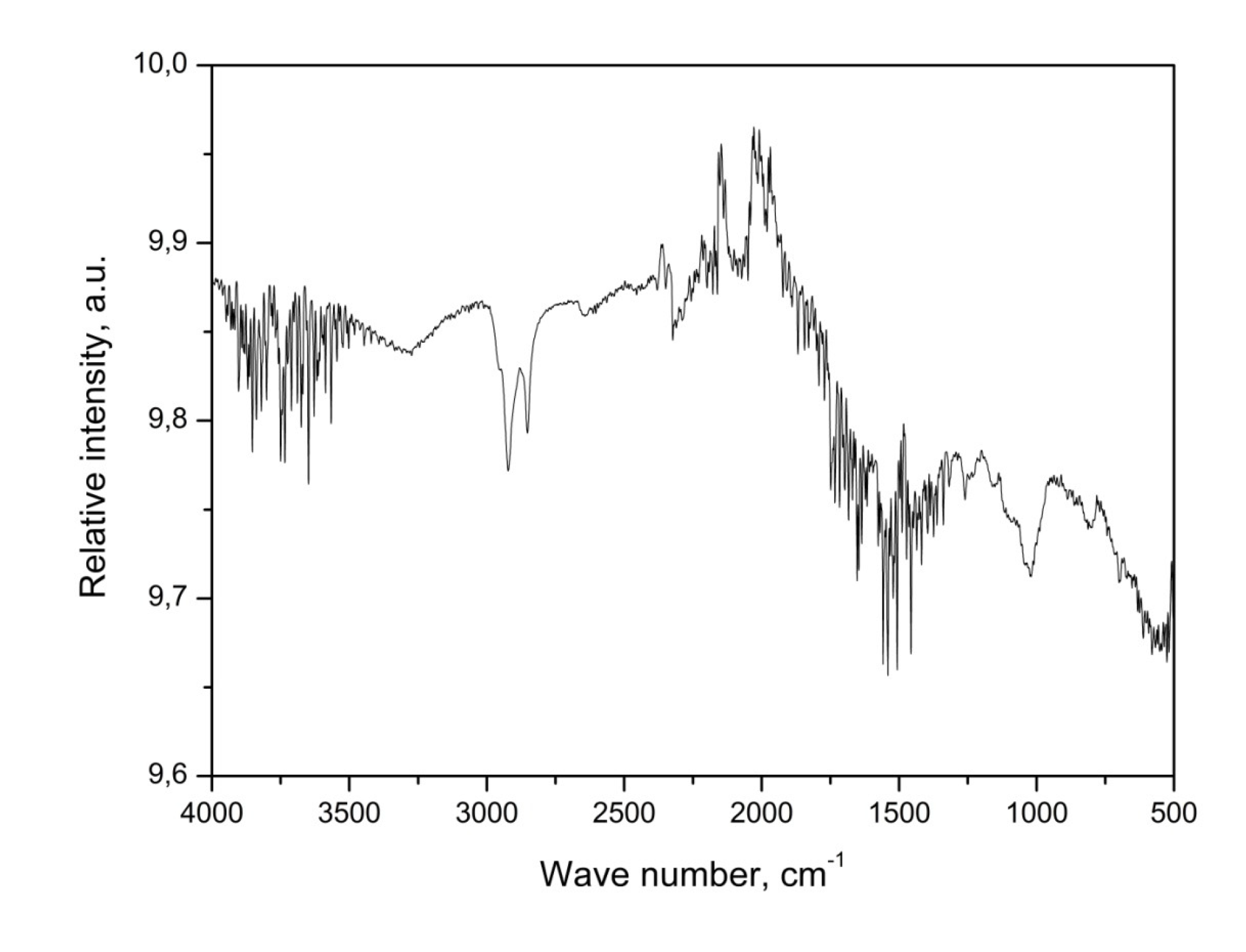
FTIR
Ion Release Measurements
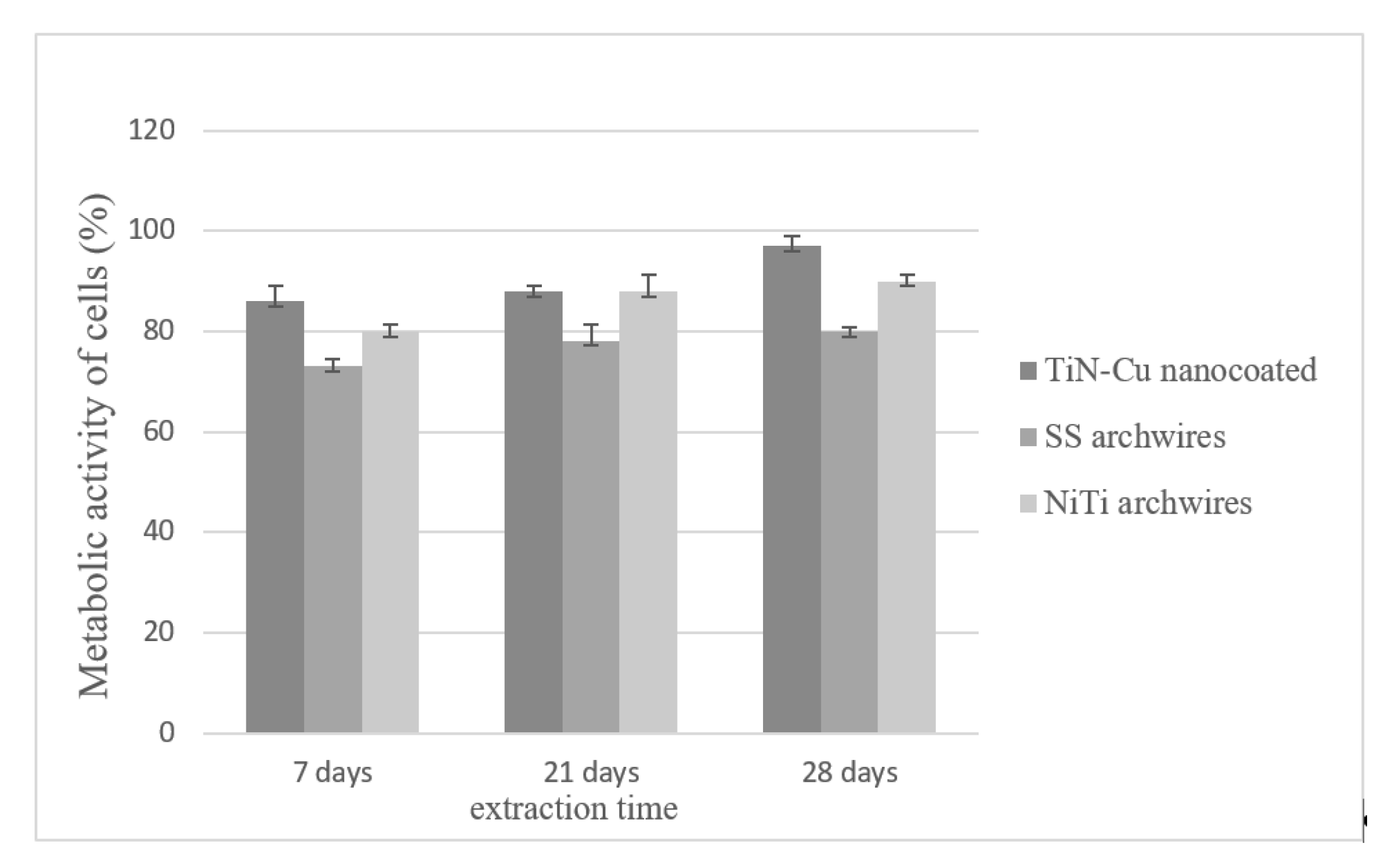
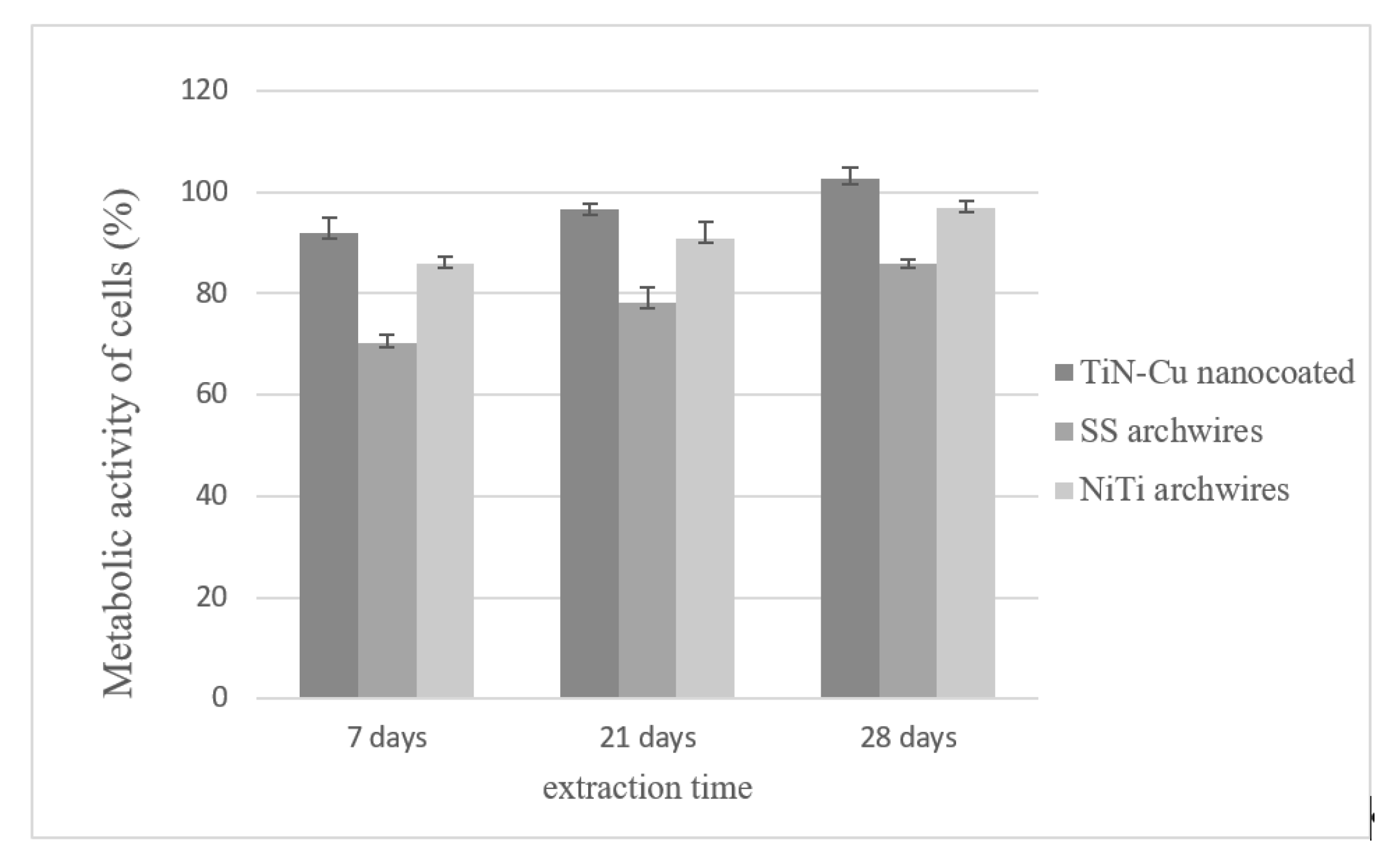
MTT Analysis
Antibacterial Analysis
Discussion
Conclusions
References
- ADA American Dental Association. Oral Health and Well-Being in the United States. Available at: https://www.ada.org/en/science-research/health-policy-institute/oral-health-and-well-being.
- Papadopoulou, K.; Eliades, T. Microbiologically-influenced corrosion of orthodontic alloys: a review of proposed mechanisms and effects. Aust. Orthod. J. 2009, 25, 63–75. [Google Scholar] [PubMed]
- Rincic Mlinaric, M.; Karlovic, S.; Ciganj, Z.; Pop Acev, D.; Pavlic, A.; Spalj, S. Oral antiseptics and nickel titanium alloys: mechanical and chemical effect of interaction. Odontology,.
- Kulkarni, P.; Agrawal, S.; Bansal, A.; Jain, A.; Tiwari, U; Anand, A. Assessment of nickel release from various dental appliances used routinely in pediatric dentistry. Indian. J. Dent. 2016, 7, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, UK.; Sokucu, O.; Uitto, VJ.; Aydin, A.; Demirer, S.; Toker, H.; Erdem, O.; Sayalet, A. The role of nickel accumulation and epithelial cell proliferation in orthodontic treatment- induced gingival overgrowth. Eur. J. Orthod. 2007, 29, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Amauri, JP.; Hwang, G.; Koo, H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 2020, 11, 1354. [Google Scholar]
- Lucchese, A.; Gherlone, E. Prevalence of white-spot lesions before and during orthodontic treatment with fixed appliances. Eur. J. Orthod. 2012, 35, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Morán-Martínez, J.; Monreal-de Luna, KD.; Betancourt-Martínez, ND.; Carranza-Rosales, P.; Contreras-Martínez, JG.; López-Meza, MC. ; Rodríguez-Villarreal.; O. Genotoxicity in oral epithelial cells in children caused by nickel in metal crowns. Genet, 2013; 12. [Google Scholar]
- Alp, G.; Çakmak, G.; Sert, M.; Burgaz, Y. Corrosion potential in artificial saliva and possible genotoxic and cytotoxic damage in buccal epithelial cells of patients who underwent Ni-Cr based porcelain-fused-to-metal fixed dental prostheses. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 19–26. [Google Scholar] [CrossRef]
- Sfondrini, MF.; Cacciafiesta, V.; Maffia, E.; Scribante, A.; Alberti, G.; Biesuz, R.; Klersy, C. Nickel release form new conventional stainless steel, recycled, and nickel free orthodontic brackets: a in vitro study. Am. J. Orthod. Dentofac. Orthop. 2009, 137, 809–815. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, MA.; Wen, C. A newlook at biomedical Ti-based shape memory alloy. Acta. Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Das, KK.; Reddy, R.; Bagoji, IB.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, JP.; Biradar, MS. Primary concept of nickel toxicity – an overview. J. Basic. Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- Ikeda, D.; Ogawa, M.; Hara, Y.; Nishimura, Y.; Odusanya, O.; Azuma, K.; Satoshi Matsuda, S.; Yatsuzuka, M.; Murakami, A. Effect of nitrogen plasma-based ion implantation on joint prosthetic material. Surf. Coat. Technol. 2002, 156, 301–305. [Google Scholar] [CrossRef]
- Weng, K.; Chen, Y.; Lin, T.; Wang, D. Characterization of Titanium Nitride Coatings Deposited by Metal Plasma Ion Pre-Implantation and Cathodic Arc Evaporation. J. Nannosci. Nanotechnol. 2009, 9, 1127–1132. [Google Scholar] [CrossRef]
- Gill, P.; Musaramthota, V.; Munroe, N.; Datye, A.; Dua, R.; Haider, W.; McGoron, A.; Rokicki, R. Surface modification of Ni–Ti alloys for stent application after magnetoelectropolishing. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 50, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rau, JV.; Wu, VM.; Graziani, V.; Fadeeva, IV.; Fomin, AS.; Fosca, M.; Uskokovic, V. The Bone Building Blues: Self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. C. 2017, 79, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; Chai, Z.; Zhu, C.; Fang, X; , Ma, B. ; Wan, L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Tudose, IV.; Comanescu, F.; Pascariu, P.; Bucur, S.; Rusen, L.; Iacomi, F.; Koudoumas, E.; Suchea, M. Chapter 2 - Chemical and physical methods for multifunctional nanostructured interface fabrication, Editor(s): Valentina Dinca, Mirela Petruta Suchea, In Micro and Nano Technologies, Functional Nanostructured Interfaces for Environmental and Biomedical Applications, Elsevier, 2019, pp. 15-26.
- Nickel - Registration Dossier - ECHA. Available online: Registration Dossier - ECHA (europa.eu) (accessed on 9 June 2023).
- Sugisawa, H.; Kitaura, H.; Ueda, K.; Kimura, K.; Ishida, M.; Ochi, Y.; Kishikawa, A.; Ogawa, S.; Takano-Yamamoto, T. Corrosion resistance and mechanical properties of titanium nitride plating on orthodontic wires. Dent. Mater. J. 2018, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kitaura, H.; Sugisawa, H.; Noguchi, T.; Ohori, F.; Mizoguchi, I. Titanium nitride plating reduces nickel ion release from orthodontic wire. Appl. Sci. 2021, 11. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.d.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic contact dermatitis caused by titanium screws and dental implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef]
- Azizi, A.; Jamilian, A.; Nucci, F.; Kamali, Z.; Hosseinikhoo, N.; Perillo, L. Release of metal ions from round and rectangular NiTi wires. Prog. Orthod. 2016, 17, 10. [Google Scholar] [CrossRef]
- Taylor, AA.; Tsuji, JS.; Garry, MR.; McArdle, ME. ; Goodfellow, WL Jr.; Adams, WJ.; Menzie, CA. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manage. 2020; 65, 131–159. [Google Scholar]
- Jenko, M.; Godec, M.; Kocijan, A.; Rudolf, R.; Dolinar, D.; Ovsenik, M.; Gorensek, M.; Zaplotnik, R.; Mozetic, M. A new route to biocompatible Nitinol based on a rapid treatment with H2/O2 gaseous plasma. Appl. Surf. Sci. 2019, 473, 976–984. [Google Scholar] [CrossRef]
- Rongo, R.; Valletta, R.; Bucci, R.; Rivieccio, V.; Galeotti, A.; Michelotti, A.; D'Antò, V. In vitro biocompatibility of nickel-titanium esthetic orthodontic archwires. Angle. Orthod. 2016, 86, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, LM.; de Roo Puente, YJD.; Hoyos-Nogués, M.; Gil, FJ.; Perez, RA. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470-4490.
- Wang, N.; Yu, J.; Yan, J.; Hua, F. Recent advances in antibacterial coatings for orthodontic appliances. Front. Bioeng. Biotechnol. 2023, 11, 1093926. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, LM.; Libedinsky, A.; Elorza, AA. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Ren, Y.; Jongsma, MA.; Mei, L.; Van Der Mei, HC.; Busscher, HJ. Orthodontic treatment with fixed appliances and biofilm formation--a potential public health threat? Clin. Oral. Investig. 2014, 18, 1711–1718. [Google Scholar] [CrossRef]
- Kolenbrander, PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 2000, 54, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, LP.; Gontijo, LC.; Franco Junior, AR.; Pereira, MF.; Schuenck, RP,, Malacarne-Zanon, J. Evaluation of antimicrobial potential and surface morphology in thin films of titanium nitride and calcium phosphate on orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 209–214. [Google Scholar] [CrossRef]
- Jabbari, YSA. ; Fehrman, J.; Barnes, AC.; Zapf, AM.; Zinelis, S.; Berzins, DW. Titanium nitride and nitrogen ion implanted coated dental materials. Coatings. 2012, 2, 160–178. [Google Scholar] [CrossRef]
| TiN-Cu coated archwire | NiTi archwire | SS archwire | |||||||
| Ion | 7-day | 21-day | 28-day | 7-day | 21-day | 28-day | 7-day | 21-day | 28-day |
| Ti water | 2.8±0.2 | 3.8±0.1 | 4.1±0.1 | 3.1±0.2 | 3.0±0.1 | 3.1±0.1 | 1.6±.1 | 1.8±0.2 | 1.7±0.2 |
| Ti acid | 929±12 | 958±20 | 963±19 | 354±9 | 1144±23 | 1398±31 | 6.9±0.2 | 2.9±1.0 | 7.4±2.9 |
| Ni water | 6.1±0.1 | 6.1±0.1 | 1.7±0.1 | 33.9±0.1 | 21.0±0.1 | 19.7±0.2 | 3.5±0.1 | 3.7±0.3 | 3.7±0.2 |
| Ni acid | 59.9±0.8 | 1429±29 | 1443±21 | 555±5 | 1917±15 | 2129±20 | 49±2 | 75.7±3 | 111±2 |
| Cu water | 1210±0.8 | 1010±0.2 | 956±0.4 | 49.8±0.2 | 71.6±0.8 | 119±1.2 | 46.8±0.5 | 30.3±0.8 | 13±1.2 |
| Cu acid | 1020±1.2 | 961±0.4 | 879±0.7 | 59.3±0.4 | 78.2±0.2 | 128.1±0.1 | 82.4±0.2 | 40.1±0.1 | 25.6±1.0 |
| TiN-Cu coated archwires | NiTi archwires | SS archwires | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Mean(cfu/mL) | SD | log | Mean(cfu/mL) | SD | log | Mean(cfu/mL) | SD | log |
| S.mutans | 1.55x104 | 1.6x103 | 4.19 | 2.20x104 | 1.06x104 | 4.29 | 7.39x105 | 3.1x104 | 5.87 |
| S.mitis | 1.3x103 | 1.2x102 | 3.08 | 1.07x104 | 1.87x103 | 3.75 | 3.49x105 | 2.8x104 | 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





