Submitted:
15 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
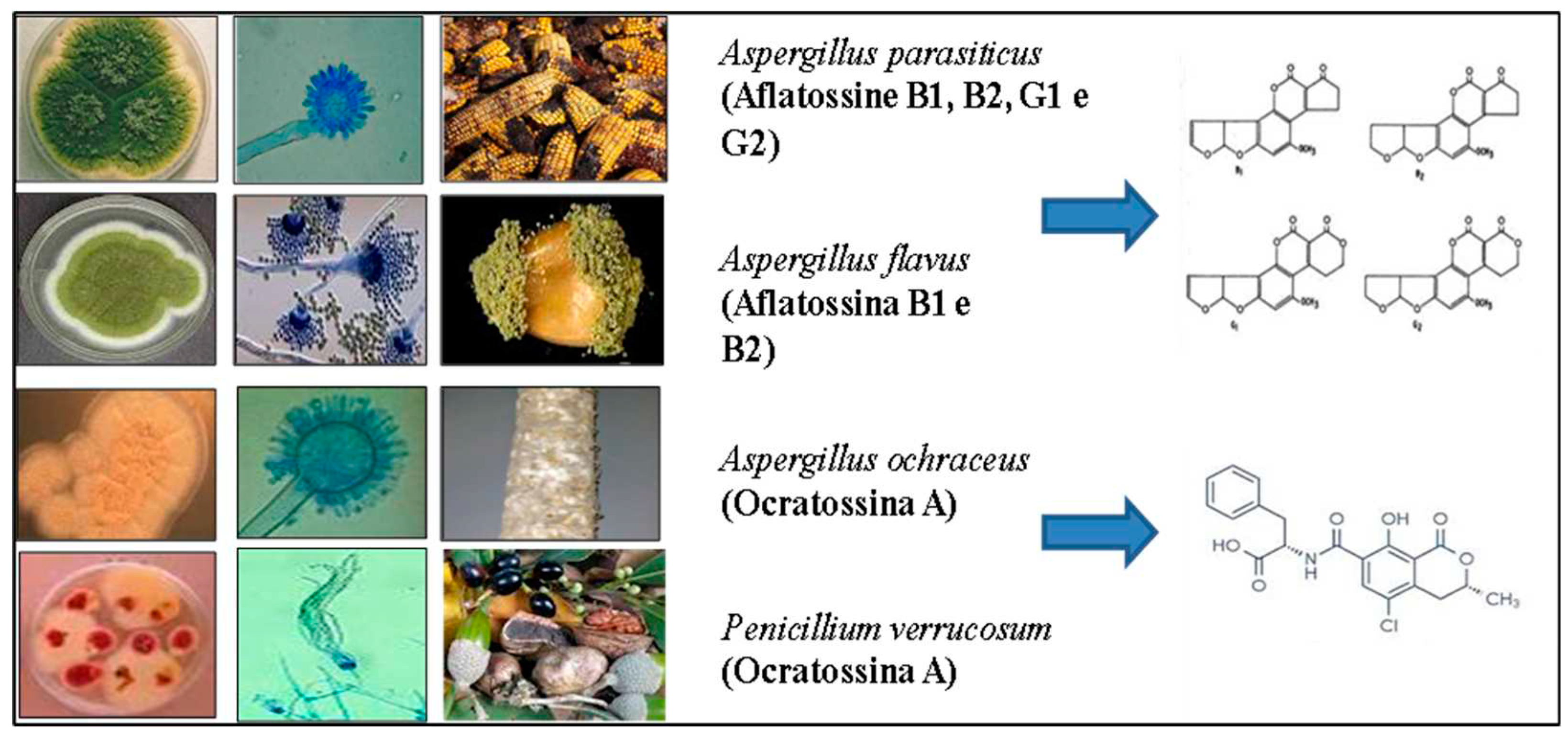
1. Introduction
2. Materials and Methods
2.1. Table Olives Samples
- -
- Nocellara del Belice (Castel Vetrano method) batch no. (335/20-402/20-409/20)
- -
- Nocellara Etnea (Natural method) batch no. (217/20-233/20-259/20)
- -
- Bella di Cerignola (Sivigliano method) batch no. (A-B-C without identification)
- -
- Itrana Bianca (Natural method) batch no. (492/19-499/19-502/19)
- -
- Nocellara del Belice (Castel Vetrano method) batch no. (335/20-402/20-409/20)
- -
- Nocellara Etnea (Natural Method) batch no. (217/20-233/20-259/20)
- -
- Bella di Cerignola (Sivigliano method) batch no. (049/20-074/20-161/20)
- -
- Itrana Bianca (Natural method) batch no. (492/19-499/19-502/19)
- -
- Nocellara del Belice (Castel Vetrano method) batch no. (408/21-422/21-474/21)
- -
- Corservolea Nera (Californian method 1st drying) batch no. (024/22-047/22-056/22)
- -
- Bella di Cerignola (Sivigliano method) batch no. (033/21-326/21-341/21)
- -
- Hojiblanca Nera (Californian pitted method) batch no. (002/22-182/22-498/21)
- -
- Nocellara del Belice (Castel Vetrano method) batch no. (408/21-422/21-474/21)
- -
- Hojiblanca Nera (Californian pitted method) batch no. (002/22-182/22-498/22)
- -
- Bella di Cerignola (Sivigliano method) batch no. (033/21-326/21-341/21)
- -
- GR 2177 (Californian pitted method) batch no. (one lot)
- -
- The Sivigliano method, which eliminates the bitterness of raw olives by using sodium solution. This treatment takes between eight and fifteen hours from start to finish depending on the temperature, the size of the olives, and their degree of ripeness. This process is repeatedly followed by washing to remove the soda.
- -
- The Castelvetrano method, also known as the ‘soda method’, which proceeds through the following stages: sorting of the olives, grading, sodium-saline solution, softening and fermentation, which are essential for final consumption.
- -
- The Natural method proceeds through the following stages: sorting of the olives, grading, possibly crushing and stoning, brine, fermentation, grading, packaging, and possibly pasteurisation, which are essential for final consumption.
- -
- While for black olives, the method used was:
- -
- The Californian method proceeds through the following stages: sorting of the olives, grading, brine, treatment with soda, air oxidation, washing, treatment with ferrous salts, brine, eventual pasteurisation, grading, packaging, and sterilisation, which are essential for final consumption.
2.2. Obtaining Olive Paste
2.3. Solvents Used and Olive Pasta Drying
2.4. Study of the Preparatory Phase
2.5. Adjustments to the Preparatory Phase
2.6. ELISA Method
2.7. LC-MS/MS Method
2.8. Statistical Analysis
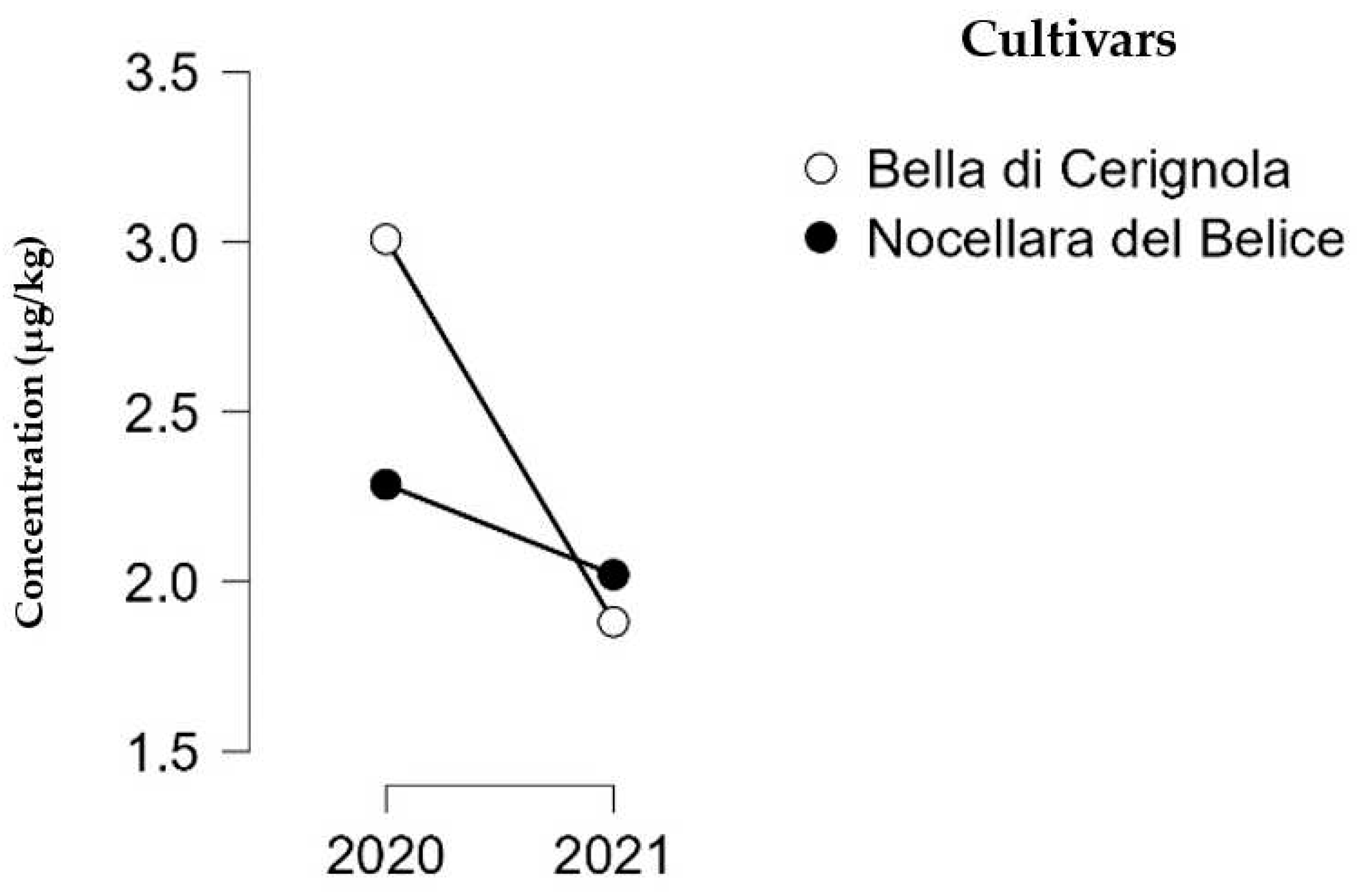
3. Results
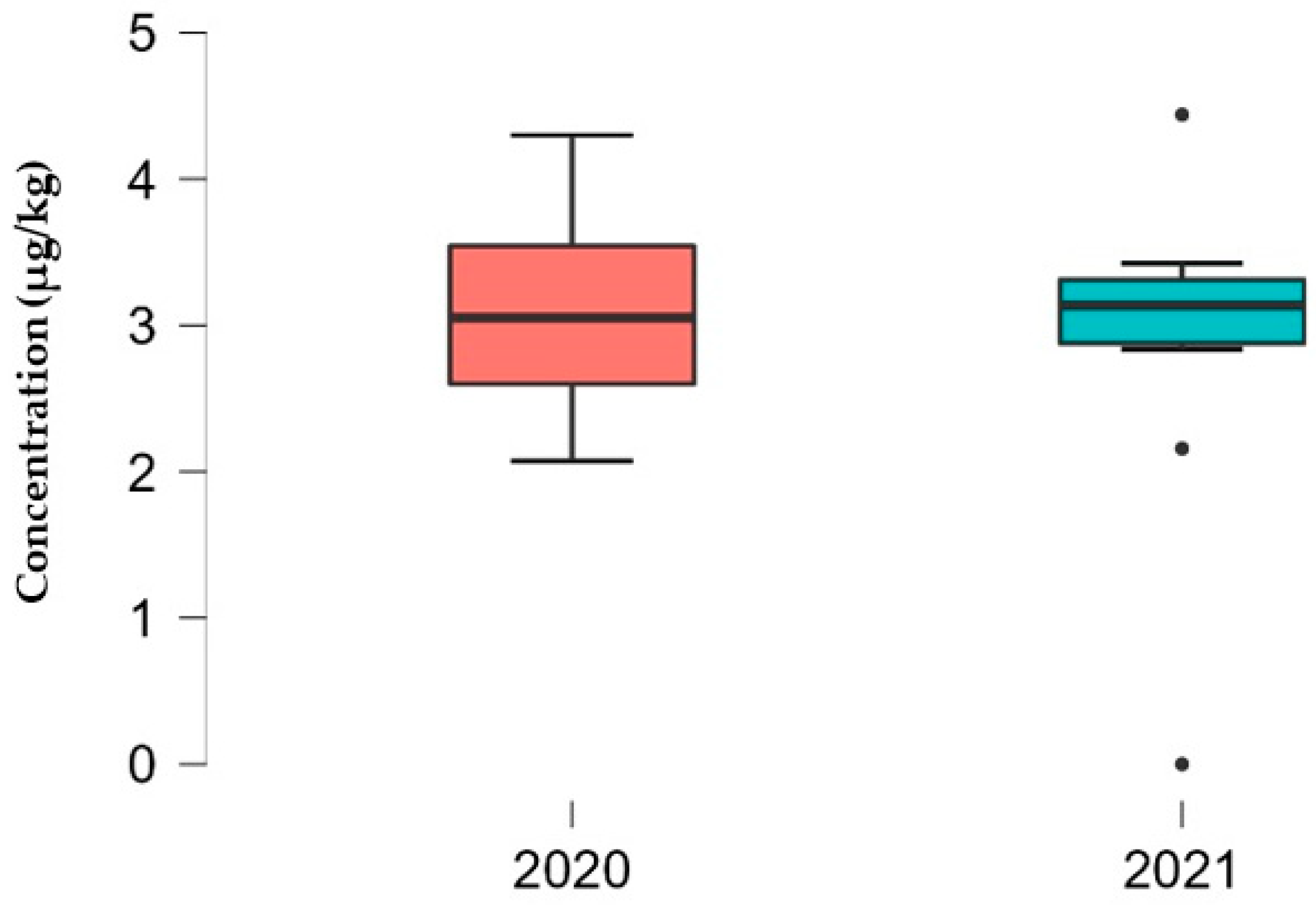
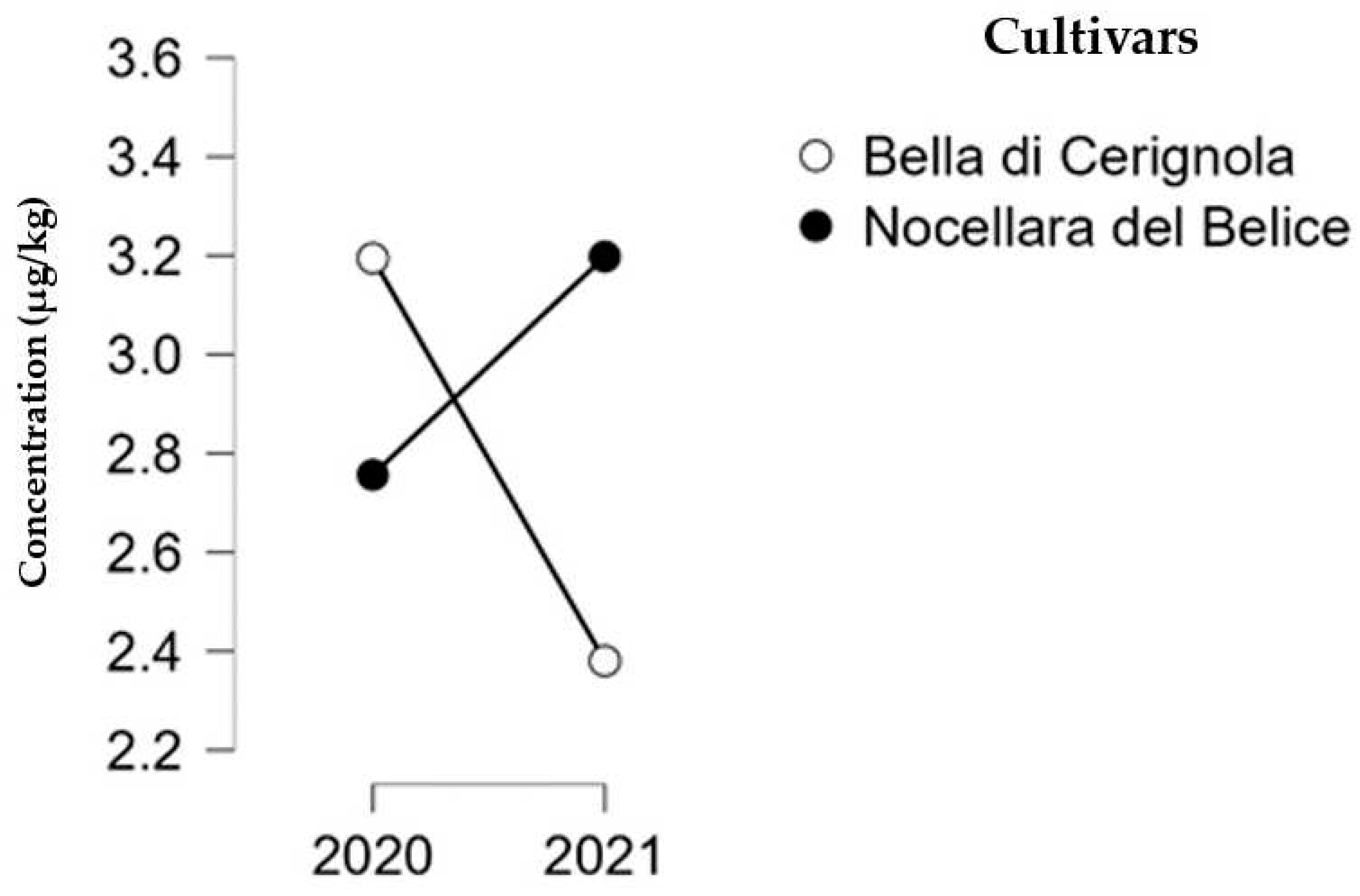
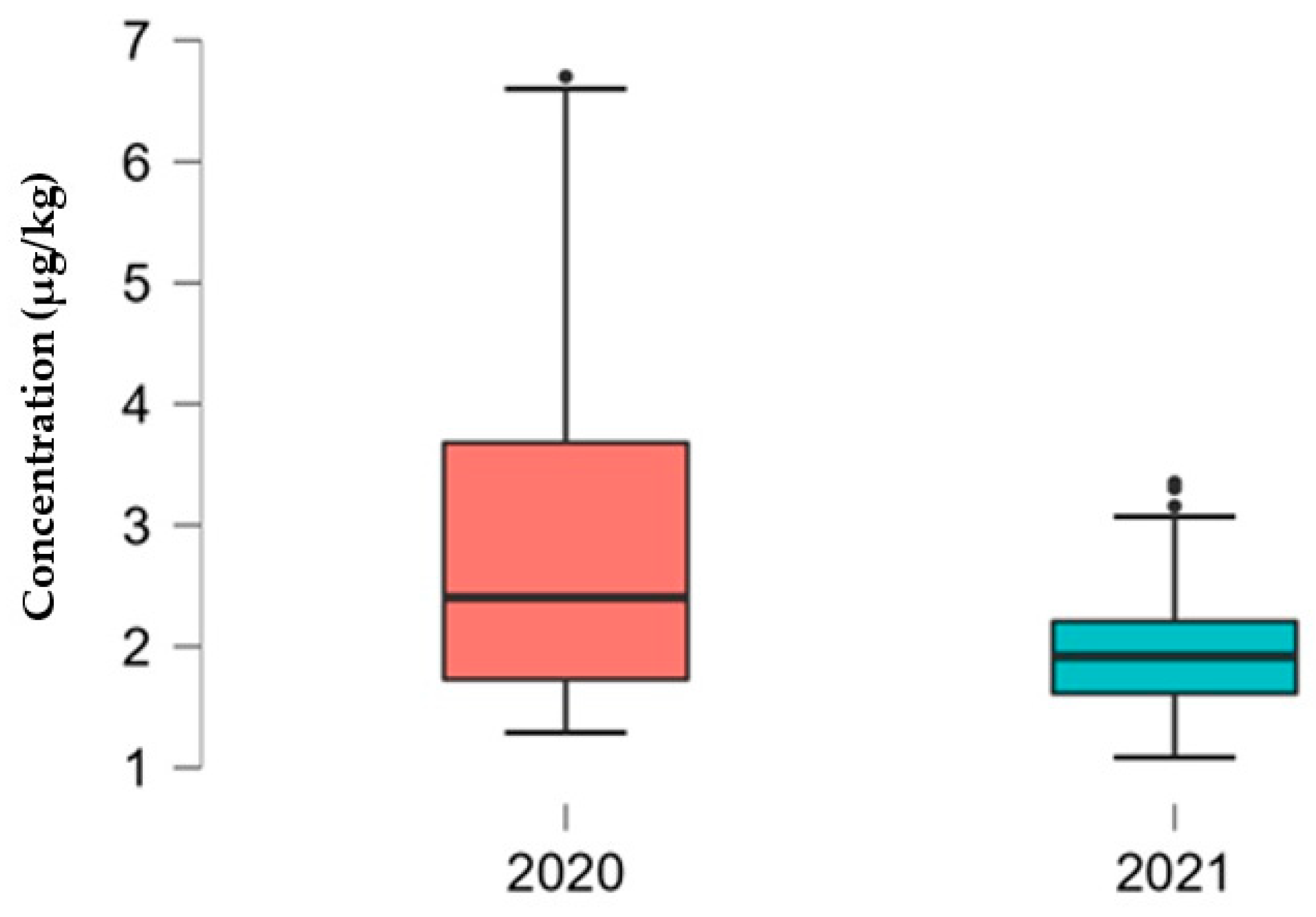
3.1. Determination of Mycotoxin Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rämö, S.; Kahala, M.; Joutsjoki, V. Aflatoxin B1 Binding by Lactic Acid Bacteria in Protein-Rich Plant Material Fermentation. Appl. Sci. 2022, 12, 12769. [Google Scholar] [CrossRef]
- Locatelli, S.; Scarpino, V.; Lanzanova, C.; Romano, E.; Reyneri, A. Multi-Mycotoxin Long-Term Monitoring Survey on North-Italian Maize over an 11-Year Period (2011–2021): The Co-Occurrence of Regulated, Masked and Emerging Mycotoxins and Fungal Metabolites. Toxins 2022, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, S.L.; Susca, A.; Frisvad, J.C.; Tufariello, M.; Chytiri, A.; Perrone, G.; Mita, G.; Logrieco, A.F.; Bleve, G. 2017; 8. [CrossRef]
- Medina-Pradas, E.; Arroyo-López, F.N. Presence of toxic microbial metabolites in table olives. Front. Microbiol. 2015, 6, 873. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, L.; Scarpellini, M.; Vecchio, A.; Planeta, D. Microbiological and safety evaluation of green table olives marketed in Italy. Ann. Microbiol 2011, 61, 843–851. [Google Scholar] [CrossRef]
- Ghitakou, S.; Koutras, K.; Kanellou, E.; Markaki, P. Study of aflatoxin B1 and ochratoxin A production by natural microflora and Aspergillus parasiticus in black and green olives of Greek origin. Food Microbiology 2006, 23, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Eltem, R. Growth and aflatoxin B1 production on olives and olive paste by moulds isolated from ‘Turkish-style’ natural black olives in brine. International Journal of Food Microbiology 1996, 32, 217–223. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs, Volumes 1–119, last update:. 28 June.
- Galvano, F.; Ritieni, A.; Piva, G.; Pietri, A. Mycotoxins in the human food chain. In: Diaz D, editor. Mycotoxins Blue Book. Nottingham: Nottingham University Press.
- Bircan, C. Determination of aflatoxin contamination in olives by immunoaffinity column using High-Performance Liquid Chromatography. Journal of Food Quality 2005, 29, 126–138. [Google Scholar]
- El Adlouni, C.; Tozlovanu, M.; Naman, F.; Faid, M.; Annle Pfohl-Leszkowlcz, A. Preliminary data on the presence of mycotoxins (ochratoxin A, citrinin and aflatoxin B1) in blacktable olives “Greek style” of Moroccan origin. Mol. Nutr. Food Res. 2006, 50, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, K.; Khedri, S.; Alizadeh, L.; Javanmardi, F.; Oliveira, C.A.F.; Khaneghah, A.M. The mycotoxins in edible oils: An overview of prevalence, concentration, toxicity, detection and decontamination techniques. Trends in Food Science & Technology 2021, 115, 500–511. [Google Scholar] [CrossRef]
- Lin, H.; Ni, L.; Chen, H.; Xu, W. A simple and versatile strategy for sensitive SIDA-UHPLC-MS/MS analysis of Alternaria toxins in olive oil. Analytica Chimica Acta 2022, 232, 340451. [Google Scholar] [CrossRef] [PubMed]
- Timpanaro, N.; Rutigliano, C.A.C.; Benincasa, C.; Foti, P.; Mangiameli, S.; Nicoletti, R.; Muzzalupo, I.; Romeo, F.V. Comparing Spanish-Style and Natural Fermentation Methods to Valorise Carolea, Nocellara Messinese and Leccino as Table Olives. Horticulturae 2023, 9, 496. [Google Scholar] [CrossRef]
- Mougiou, N.; Tsoureki, A.; Didos, S.; Bouzouka, I.; Michailidou, S.; Argiriou, A. Microbial and Biochemical Profile of Different Types of Greek Table Olives. Foods 2023, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Prete, R.; Gracia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table Olives More than a Fermented Food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Pérez-Nevado, F.; Montero-Fernández, I.; Lozano, J.; Meléndez, F.; Martín-Vertedor, D. Application of Electronic Nose to Discriminate Species of Mold Strains in Synthetic Brines. Frontiers in Microbiology May 2022, 13, 897178. [Google Scholar] [CrossRef] [PubMed]
- Planeta, D.; Mineo, V.; Finoli, C.; Vecchio, A.; Franzetti, L. Table olives contamination by mycotoxins. Olive bio teq, 5 November 2006. [Google Scholar]
- Bangar, S.P.; Sharma, N.; Bhardwaj, A.; Phimolsiripol, Y. Lactic acid bacteria: A bio-green preservative against mycotoxins for food safety and shelf-life extension. Quality Assurance and Safety of Crops & Foods 2022, 14, 13–31. [Google Scholar]
- Simões, L.; Fernandes, N.; Teixeira, J.; Abrunhosa, L.; Dias, D.R. Brazilian Table Olives: A Source of Lactic Acid Bacteria with Antimycotoxigenic and Antifungal Activity. Toxins 2023, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- European Commission (2006). Commission regulation (EC) No 1881/2006 of setting maximum levels for certain contaminants in foodstuffs. In: Official Journal of the European Union, L 364/5. 19 December.
- European Commission (2007) Commission regulation of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize maize, p.r.o.d.u.c.t.s. European Commission (2007) Commission regulation of amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize maize, p.r.o.d.u.c.t.s.; 1126/2007/EC In: Official Journal of the European Union, L 255/14. 28 September.
- Ferracane, R. , Tafuri, A., Logieco A., Galvano F., Balzano D., Ritieni A., 2007. Simultaneous Determination of Aflatoxin B1 and Ochratoxin A and Natural Occurrence in Mediterranean Virgin Olive Oil, Food Additives and Contaminants 24(2):173-80.
- 24. European Commission. Commission Regulation (EC) No. 401/2006 of 23 February 2006 Laying Down the Methods of Sam-pling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Official J. Eur. Union, 23 February.
- 25. R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- De Mendiburu, F. , 2021. Agricolae: Statistical Procedures for Agricultural Research. R package version 1.3-5, https://CRAN.R-project.org/package=agricolae.
- Fox, J. , and Weisberg, S. (2020). car: Companion to Applied Regression. [R package]. Retrieved from https://cran.r-project.org/package=car.
- Lenth, R. (2020). Emmeans: Estimated Marginal Means, aka Least-Squares Means. [R package]. Retrieved from https://cran.r-project.org/package=emmeans.
| Cultivar | ELISA method | LC-MS/MS analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I shipment | II shipment | III shipment | IV shipment | ||||||
| Veratox® | Agraquant® | Veratox® | Agraquant® | Veratox® | Agraquant® | Veratox® | Agraquant® | ||
| Nocellara del Belice | <LOQ | 3.41 | 1.82±0.08 | 1.67±0.08 | <LOQ | <LOQ | 2.04±0.32 | 1.54±0.55 | <LOQ |
| Castelvetrano method | 2.15±0.35 | <LOQ | <LOQ | <LOQ | <LOQ | 1.29±0.35 | 2.42±0.72 | 1.79±0.46 | <LOQ |
| 2.43±0.10 | <LOQ | <LOQ | <LOQ | <LOQ | 2.06±0.38 | 3.00±0.37 | <LOQ | <LOQ | |
| Nocellara Etnea | 3.16±0.90 | 5.53±0.98 | 2.41±0.15 | <LOQ | - | - | - | - | <LOQ |
| Natural method | 2.18±0.99 | 5.59±0.52 | 1.73±0.16 | <LOQ | - | - | - | - | <LOQ |
| 2.96±0.13 | 4.88±0.82 | 2.40±0.92 | <LOQ | - | - | - | - | <LOQ | |
| Bella di Cerignola | 1.85±0.59 | 6.21±0.77 | <LOQ | 3.98±0.18 | <LOQ | <LOQ | 2.07±0.30 | <LOQ | <LOQ |
| Sivigliano method | 2.05±0.79 | 5.16±0.59 | 2.05±0.79 | <LOQ | <LOQ | <LOQ | 1.82±0.26 | 1.64±0.47 | <LOQ |
| 1.67±0.19 | 3.95±1.00 | 1.67±0.19 | 2.80±0.23 | 1.91±0.07 | <LOQ | 2.11±0.45 | 1.90±0.33 | <LOQ | |
| Itrana Bianca | <LOQ | 3.00±0.19 | <LOQ | 3.00±0.19 | - | - | - | - | <LOQ |
| Natural method | <LOQ | 2.96±0.41 | <LOQ | 2.96±0.41 | - | - | - | - | <LOQ |
| <LOQ | <LOQ | <LOQ | <LOQ | - | - | - | - | <LOQ | |
| Conservolea Nera | - | - | - | - | 1.62±0.24 | <LOQ | - | - | <LOQ |
| Californiano I° drying method | - | - | - | - | <LOQ | 1.66±0.28 | - | - | <LOQ |
| - | - | - | - | 2.53±0.52 | <LOQ | - | - | <LOQ | |
| Hojiblanca Nera | - | - | - | - | 1.99±0.53 | <LOQ | 1.90±0.48 | <LOQ | <LOQ |
| Pitted | - | - | - | - | 4.57±0.12 | <LOQ | 2.85±0.44 | <LOQ | <LOQ |
| - | - | - | - | 3.86±0.30 | <LOQ | 1.70±0.28 | <LOQ | <LOQ | |
| GR 2177 | - | - | - | - | - | - | <LOQ | <LOQ | <LOQ |
| Californiano pitted method | - | - | - | - | - | - | <LOQ | <LOQ | <LOQ |
| - | - | - | - | - | - | <LOQ | <LOQ | <LOQ | |
| Cultivar | ELISA method | LC-MS/MS analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I shipment | II shipment | III shipment | IV shipment | ||||||
| Veratox® | Agraquant® | Veratox® | Agraquant® | Veratox® | Agraquant® | Veratox® | Agraquant® | ||
| Nocellara del Belice | <LOQ | 2.78±0.23 | <LOQ | <LOQ | <LOQ | 2.88±0.63 | <LOQ | <LOQ | <LOQ |
| Castelvetrano method | <LOQ | 2.74±0.31 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| Nocellara Etnea | <LOQ | 2.49±0.28 | <LOQ | 2.49±0.28 | - | - | - | - | <LOQ |
| Natural method | 3.74±0.05 | <LOQ | <LOQ | <LOQ | - | - | - | - | <LOQ |
| <LOQ | 2.29±0.08 | <LOQ | <LOQ | - | - | - | - | <LOQ | |
| Bella di Cerignola | <LOQ | <LOQ | <LOQ | 2.93±0.80 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Sivigliano method | <LOQ | <LOQ | <LOQ | 3.42±0.32 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| <LOQ | <LOQ | <LOQ | 3.00±0.56 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| Itrana Bianca | <LOQ | 3.86±0.41 | <LOQ | <LOQ | - | - | - | - | <LOQ |
| Natural method | <LOQ | <LOQ | <LOQ | <LOQ | - | - | - | - | <LOQ |
| <LOQ | <LOQ | <LOQ | <LOQ | - | - | - | - | <LOQ | |
| Conservolea Nera | - | - | - | - | <LOQ | <LOQ | - | - | <LOQ |
| Californiano I° drying method | - | - | - | - | <LOQ | <LOQ | - | - | <LOQ |
| - | - | - | - | <LOQ | <LOQ | - | - | <LOQ | |
| Hojiblanca Nera | - | - | - | - | <LOQ | 4.00±0.39 | <LOQ | <LOQ | <LOQ |
| Pitted | - | - | - | - | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| - | - | - | - | <LOQ | 3.44±0.69 | <LOQ | <LOQ | <LOQ | |
| GR 2177 | - | - | - | - | - | - | <LOQ | <LOQ | <LOQ |
| Californiano pitted method | - | - | - | - | - | - | <LOQ | <LOQ | <LOQ |
| - | - | - | - | - | - | <LOQ | <LOQ | <LOQ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





