Submitted:
14 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient’s Identification and Inclusion Criteria
2.2. Patients’ follow-up
2.3. Clinical Assessment of the Patients
2.4. Primary and Secondary Objectives and Definitions
2.5. Interventional Procedure
2.6. Covariates included in the study
2.7. Statistical Analysis
3. Results
3.1. Pre-operative Characteristics
3.2. Intra-operative and post-operative characteristics
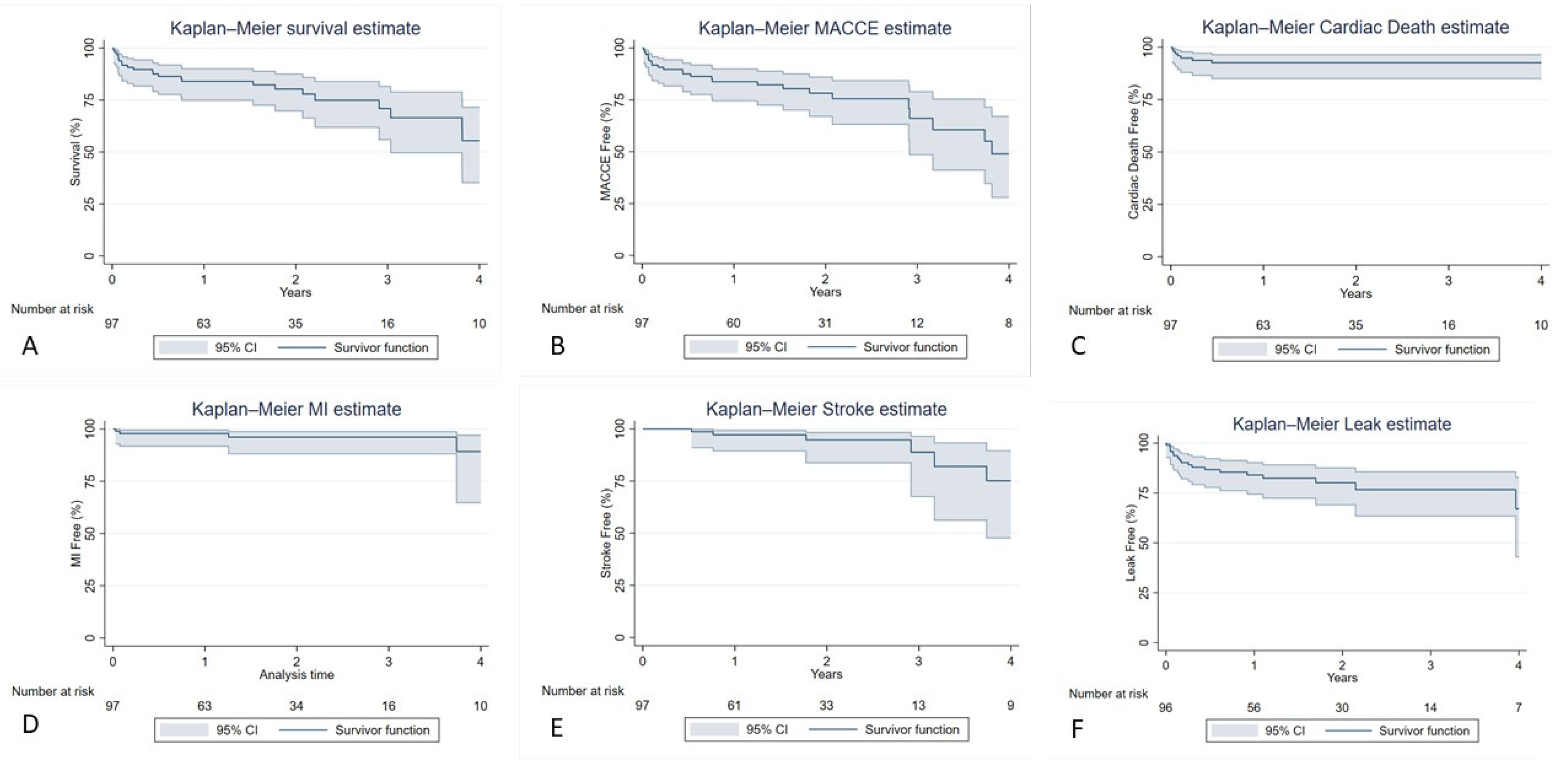
3.3. Patients Follow-up
3.4. Emergent TEVAR procedure
3.5. Urgent TEVAR procedure
3.6. Aortic Dissection Presentation
3.7. Aortic Aneurysm Presentation
3.8. Cox-regression analysis for analyses of periprocedural risk predictors that impact long-term prognosis
4. Discussion
4.1. All-cause mortality
4.2. Neurological Outcomes
4.3. Predictors Impacting Long-term Prognosis
4.4. Incidence of Endoleaks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weissler, E.H.; Osazuwa-Peters, O.L.; Greiner, M.A.; Hardy, N.C.; Kougias, P.; O’brien, S.M.; Mark, D.B.; Jones, W.S.; Secemsky, E.A.; Vekstein, A.M.; et al. Initial Thoracic Endovascular Aortic Repair vs Medical Therapy for Acute Uncomplicated Type B Aortic Dissection. JAMA Cardiol. 2023, 8, 44–53. [Google Scholar] [CrossRef]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Ayala, J.E.; Cheema, Z.F.; Davies, M.G.; et al. Hybrid thoracic endovascular aortic repair via right anterior minithoracotomy. J Thorac Cardiovasc Surg. 2011, 142, 314–8. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.; Ramlawi, B. The current status of endovascular repair of thoracic aortic aneurysms (TEVAR). Methodist Debakey Cardiovasc J. 2011, 7, 15–9. [Google Scholar] [CrossRef] [PubMed]
- Ramlawi, B.; Reardon, M.J. Aortic arch debranching: advanced and hybrid techniques. Methodist Debakey Cardiovasc J. 2011, 7, 43–7. [Google Scholar] [CrossRef]
- Leurs, L.J.; Bell, R.; Degrieck, Y.; Thomas, S.; Hobo, R.; Lundbom, J. Endovascular treatment of thoracic aortic diseases: Combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J. Vasc. Surg. 2004, 40, 670–679. [Google Scholar] [CrossRef]
- Bonacchi, M.; Cabrucci, F.; Bacchi, B.; Haranal, M.; Gelsomino, S.; Ramlawi, B.; Dokollari, A. Editorial: Novel insights into aortic arch repair. Front. Cardiovasc. Med. 2022, 9, 1087952. [Google Scholar] [CrossRef]
- Dokollari, A.; Bisleri, G. Watchful waiting during visceral malperfusion in DeBakey type I aortic dissection: A possible paradigm shift? JTCVS Tech. 2020, 4, 81–82. [Google Scholar] [CrossRef]
- Coselli, J.S.; Rosu, C.; Amarasekara, H.S.; Green, S.Y.; Zhang, Q.; Price, M.D.; LeMaire, S.A. Reoperative surgery on the thoracoabdominal aorta. J. Thorac. Cardiovasc. Surg. 2018, 155, 474–485. [Google Scholar] [CrossRef]
- Augoustides, J.; Mz, T.; Mj, E.; Ee, R.; Eh, B.; F, X.; M, I. ; Johnston; Eg, S. ; Fg, B.; et al. Faculty Opinions recommendation of Outcomes of open versus endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms. 2021. [Google Scholar] [CrossRef]
- Braconi, L.; Cabrucci, F.; Bacchi, B.; Bonacchi, M. A threatening meteor for cardiac surgeons: anomalous left main coronary origin in type A aortic dissection. Eur. J. Cardio-Thoracic Surg. 2022, 62. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, J.S.; Cambria, R.P.; Dake, M.D.; Moore, R.D.; Svensson, L.G.; Snyder, S. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J. Vasc. Surg. 2008, 47, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; Daniels, M.J.; Beaver, T.M.; Klodell, C.T.; Raghinaru, D.E.; Hess, P.J. Late Outcomes of a Single-Center Experience of 400 Consecutive Thoracic Endovascular Aortic Repairs. Circulation 2011, 123, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Olsen, D.M.; Shtutman, A.; Lucas, L.A.; Wheatley, G.; Alpern, J.; Ramaiah, V.; Diethrich, E.B. Application of endograft to treat thoracic aortic pathologies: A single center experience. J. Vasc. Surg. 2007, 46, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Riambau, V.; Zipfel, B.; Coppi, G.; Czerny, M.; Tealdi, D.G.; Ferro, C.; Chiesa, R.; Sassi, C.; Rousseau, H.; Berti, S. Final operative and midterm results of the European experience in the RELAY Endovascular Registry for Thoracic Disease (RESTORE) study. J. Vasc. Surg. 2011, 53, 565–573. [Google Scholar] [CrossRef]
- Fillinger, M.F.; Greenberg, R.K.; McKinsey, J.F.; Chaikof, E.L. Reporting standards for thoracic endovascular aortic repair (TEVAR). J. Vasc. Surg. 2010, 52, 1022–1033. [Google Scholar] [CrossRef]
- Alric, P.; Canaud, L.; Branchereau, P.; Marty-Anne, C. Traitement endovasculaire des anevrysmes de l’aorte thoracique descendante. EMC Techniques chirurgicales – Chirurgie vasculaire. 2012, 7, 1–20. [Google Scholar] [CrossRef]
- Dokollari, A.; Cameli, M.; Wang, L.; Bisleri, G. Aortic intimo-intimal intussusception in Stanford type A acute aortic dissection. Eur. Hear. J. 2020, 42, 3410–3410. [Google Scholar] [CrossRef]
- Bonacchi, M.; Dokollari, A.; Parise, O.; Sani, G.; Prifti, E.; Bisleri, G.; Gelsomino, S. Ministernotomy compared with right anterior minithoracotomy for aortic valve surgery. J. Thorac. Cardiovasc. Surg. 2021, 165, 1022–1032. [Google Scholar] [CrossRef]
- Dokollari, A.; Cameli, M.; Mandoli, G.E.; Kalra, D.-K.S.; Poston, R.; Coku, L.; Pernoci, M.; Miri, M.; Bonacchi, M.; Gelsomino, S. Early and Midterm Clinical Outcomes of Transcatheter Valve-in-Valve Implantation Versus Redo Surgical Aortic Valve Replacement for Aortic Bioprosthetic Valve Degeneration: Two Faces of the Same Medal. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 3223–3231. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Stana, J.; Konstantinou, N.; Chen, M.; Zhou, Q.; Kölbel, T. Identifying risk factors for early neurological outcomes following thoracic endovascular aortic repair using the SUMMIT database. Eur. J. Cardio-Thoracic Surg. 2021, 62. [Google Scholar] [CrossRef]
- Zha, Z.; Pan, Y.; Zheng, Z.; Wei, X. Prognosis and Risk Factors of Stroke After Thoracic Endovascular Aortic Repair for Stanford Type B Aortic Dissection. Front. Cardiovasc. Med. 2022, 8, 787038. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Corriere, M.A.; Veeraswamy, R.K.; Kasirajan, K.; Milner, R.; Dodson, T.F.; Salam, A.A.; Chaikof, E.L. Risk factors for late mortality after endovascular repair of the thoracic aorta. J. Vasc. Surg. 2010, 52, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.P.; Greenberg, R.K.; Lu, Q.; Cury, M.; Hernandez, A.V.; Mohabbat, W.; Moon, M.C.; Morales, C.A.; Bathurst, S.; Schoenhagen, P. Endoleaks Following Endovascular Repair of Thoracic Aortic Aneurysm: Etiology and Outcomes. J. Endovasc. Ther. 2008, 15, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.S.; Geisbüsch, P.; Kotelis, D.; Müller-Eschner, M.; Hyhlik-Dürr, A.; Böckler, D. Clinical significance of type II endoleaks after thoracic endovascular aortic repair. J. Vasc. Surg. 2013, 58, 643–650. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Ohki, T.; Maeda, K.; Baba, T. Analysis of Risk Factors for Early Type I Endoleaks After Thoracic Endovascular Aneurysm Repair. J. Endovasc. Ther. 2016, 24, 89–96. [Google Scholar] [CrossRef]
- Bonacchi, M.; Prifti, E.; Giunti, G.; Frati, G.; Sani, G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann. Thorac. Surg. 2002, 73, 460–465. [Google Scholar] [CrossRef]
- Prifti E, Bonacchi M, Frati G, et al. Early and long-term outcome in patients undergoing aortic root replacement with composite graft according to the Bentall’s technique. Eur J Cardiothorac Surg. 2002, 21, 15–21. [Google Scholar] [CrossRef]
- De Jong, M.M.; Lorusso, R.; Al Awami, F.; Matteuci, F.; Parise, O.; Lozekoot, P.; Bonacchi, M.; Maessen, J.G.; Johnson, D.M.; Gelsomino, S. Vascular complications following intra-aortic balloon pump implantation: an updated review. Perfusion 2017, 33, 96–104. [Google Scholar] [CrossRef]
- Seike, Y.; Matsuda, H.; Shimizu, H.; Ishimaru, S.; Hoshina, K.; Michihata, N.; Yasunaga, H.; Komori, K. ; on behalf of the Japanese Committee for Stentgraft Management (JACSM)* Nationwide Analysis of Persistent Type II Endoleak and Late Outcomes of Endovascular Abdominal Aortic Aneurysm Repair in Japan: A Propensity-Matched Analysis. Circulation 2022, 145, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
| Pre-operative Patients Characteristics | Patients n = 97 |
|---|---|
| Male n (%) | 48 (49.5%) |
| Female n (%) | 49 (50.5%) |
| White n (%) | 57 (59.4%) |
| Non-White n (%) | 39 (40.6%) |
| Age Years (Mean/SD) | 70.4 (13.5) |
| BMI kg/m² (Mean/SD) | 28.6 (5.5) |
| Obese ≥25 kg/m² n (%) | 30 (30.9%) |
| EF (Mean/SD) | 61.1 (9.9) |
| EF<50% n (%) | 8 (8.2%) |
| Creatinine level (Median/IQR) | 1.1 (0.9-1.3) |
| Creatinine Clearance (Mean/SD) | 71.6 (34.2) |
| Creatinine Clearance <60 (Yes) n (%) | 36 (37.1%) |
| INR (Median/IQR) | 1.1 (1-1.2) |
| Hemoglobin level (Median/IQR) | 11.7 (10.1-12.9) |
| Dialysis n (%) | 4 (4.2%) |
| Diabetes n (%) | 17 (17.1%) |
| Smoking n (%) | 65 (67.7%) |
| COPD n (%) | 26 (27.1%) |
| Hypertension n (%) | 84 (87.5%) |
| Dyslipidemia n (%) | 24 (24.7%) |
| Pneumonia n (%) | 4 (4.3%) |
| Cerebrovascular Disease n (%) | 22 (23.2%) |
| PVD n (%) | 39 (40.6%) |
| Endocarditis n (%) | 1 (1.0%) |
| Immunocompromise n (%) | 5 (5.2%) |
| Home O2 therapy n (%) | 4 (4.2%) |
| Cancer (within 5 years) n (%) | 8 (8.6%) |
| Aortic Aneurysm n (%) | 61 (62.9%) |
| Aortic Dissection n (%) | 27 (27.8%) |
| Aortic Other n (%) | 7 (7.22%) |
| Previous Mediastinal Radiation n (%) | 2 (2.1%) |
| Prior PCI n (%) | 11 (11.3%) |
| Syncope n (%) | 3 (3.1%) |
| Previous Cardiac Surgery n (%) | 49 (51.6%) |
| Prior CABG n (%) | 7 (7.2%) |
| Prior Valve Surgery n (%) | 18 (18.6%) |
| Prior Aortic Valve replacement n (%) | 8 (8.5%) |
| Prior Aortic Valve repair n (%) | 12 (12.8%) |
| Prior AICD Implantation n (%) | 3 (3.2%) |
| Preoperative Arrhythmia n (%) | 18 (18.6%) |
| Atrial Fibrillation n (%) | 12 (12.8%) |
| Paced Rhythm n (%) | 4 (4.3%) |
| MI n (%) | 11 (11.6%) |
| TIA n (%) | 9 (9.3%) |
| Cardiogenic Shock n (%) | 2 (2.1%) |
| Heart Failure n (%) | 21 (21.9%) |
| Coronary Artery Disease n (%) | 22 (22.7%) |
| Internal Mammary Artery use n (%) | 4 (4.3%) |
| Aortic Valve Disease n (%) | 25 (25.8%) |
| Aortic Valve Regurgitation n (%) | 35 (36.1%) |
| Aortic Valve Stenosis n (%) | 6 (6.2%) |
| Mitral Valve Stenosis n (%) | 3 (3.1%) |
| Mitral Valve Regurgitation n (%) | 66 (69.5%) |
| Tricuspid Valve Regurgitation n (%) | 76 (80.0%) |
| Aortic Aneurysm Etiology n (%) | 60 (61.8%) |
| Aortic Dissection Etiology n (%) | 28 (28.8%) |
| Intramural Hematoma Etiology n (%) | 4 (4.1%) |
| Other Etiology n (%) | 5 (5.1%) |
| Variables | Patients n = 97 |
|---|---|
| Intra-operative | |
| Time in OR (Hours) Mean/SD | 4.3 (2.8) |
| Blood Products Used n (%) | 16 (16.5%) |
| RBC Units n (%) | 16 (16.5%) |
| Cryoprecipitate Units n (%) | 6 (6.2%) |
| Platelet Units n (%) | 8 (8.3%) |
| FFP Units n (%) | 6 (6.2%) |
| Cross Clamp Time (Median/IQR) | 87 (76-145) |
| CBP utilization n (%) | 8 (8.2%) |
| CBP time (Median/IQR) | 172 (119-178) |
| Extubated in OR n (%) | 70 (72.2%) |
| Elective n (%) | 54 (55.7%) |
| Emergent n (%) | 6 (6.2%) |
| Urgent n (%) | 37 (38.1%) |
| Percutaneous n (%) | 76 (78.3%) |
| Partial Sternotomy n (%) | 5 (5.1%) |
| Combined Procedure | 10 (10.3%) |
| Other | 6 (6.2%) |
| Post-operative | |
| Total ICU (Hours) (Median/IQR) | 56.2 (28.3-114.5) |
| Total LOS (Days) (Median/IQR) | 6 (4-10) |
| Blood Products Used n (%) | 31 (32.0%) |
| RBC Units n (%) | 28 (29.2%) |
| Cryoprecipitate Units n (%) | 2 (2.1%) |
| Platelet Units n (%) | 7 (7.2%) |
| FFP Units n (%) | 5 (5.2%) |
| Creatine level (Median/IQR) | 1.1 (0.9-1.7) |
| Creatinine Clearance (mean/SD) | 63.1 (32.8) |
| Creatinine Clearance < 60 ml/min n (%) | 46 (47.4%) |
| Reoperation for Bleeding n (%) | 3 (3.1%) |
| Prolonged Ventilation >24 hours n (%) | 18 (18.6%) |
| Superficial Infection n (%) | 2 (2.1%) |
| Deep Sternal Infection n (%) | 3 (3.1%) |
| Cerebrovascular Accident n (%) | 3 (3.1%) |
| TIA n (%) | 0 (0.0%) |
| Cardiac Arrest n (%) | 4 (4.12%) |
| Renal Failure n (%) | 9 (9.3%) |
| Dialysis n (%) | 6 (6.2%) |
| New Atrial Fibrillation n (%) | 12 (12.4%) |
| ICU Readmission n (%) | 4 (4.1%) |
| Operative Mortality n (%) | 6 (6.2%) |
| In-hospital Mortality n (%) | 7 (7.2%) |
| Readmission n (%) | 59 (60.8%) |
| Cardiac Readmission n (%) | 26 (26.8%) |
| Follow-up Outcomes | Patients n = 97 |
|---|---|
| Survival (all cause) | |
| Yes n (%) | 74 (76.3%) |
| No n (%) | 23 (23.7%) |
| MACCE n (%) | 25 (25.8%) |
| Stroke n (%) | 6 (6.2%) |
| MI n (%) | 4 (4.1%) |
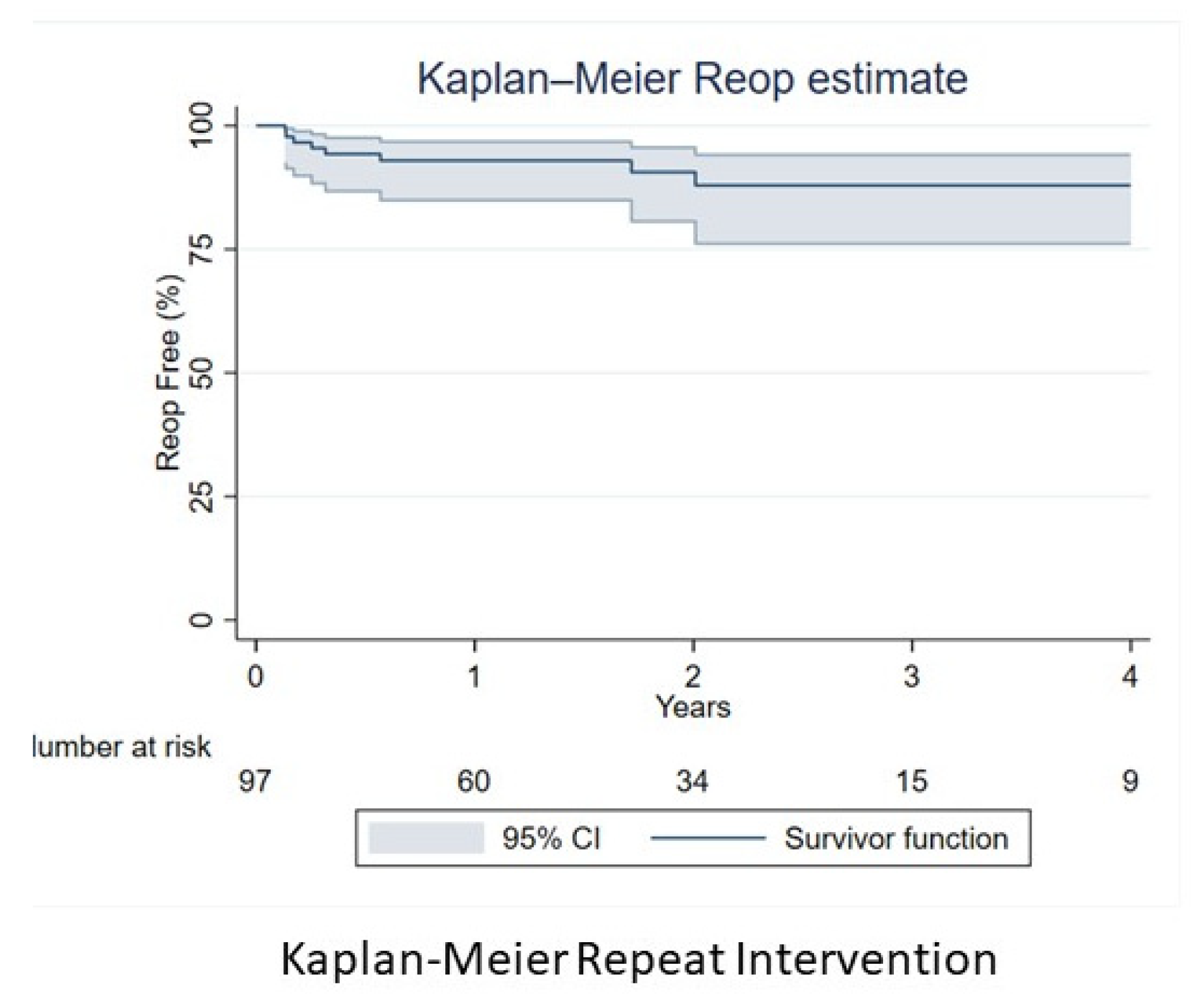
| Reoperation n (%) | 10 (10.3%) |
| Cardiac Death n (%) | 7 (7.2%) |
| Presence of endoleak n (%) | 21 (21.6%) |
| Variables | Patients n = 97 |
|---|---|
| All-Cause Mortality | |
| 1 year | 15 (15.5%) |
| 2 years | 17 (17.5%) |
| 5 years | 23 (23.7%) |
| MACCE | |
| 1 year | 15 (15.5%) |
| 2 years | 18 (18.6%) |
| 5 years | 24 (24.7%) |
| Stroke | |
| 1 year | 2 (2.1%) |
| 2 years | 3 (3.1%) |
| 5 years | 6 (6.2%) |
| MI | |
| 1 year | 2 (2.1%) |
| 2 years | 3 (3.1%) |
| 5 years | 4 (4.1%) |
| Reoperation | |
| 1 year | 6 (6.2%) |
| 2 years | 7 (7.2%) |
| 5 years | 8 (8.2%) |
| Cardiac Death | |
| 1 year | 7 (7.2%) |
| 2 years | 7 (7.2%) |
| 5 years | 7 (7.2%) |
| All-type Endoleak | |
| 1 year | 15 (15.5%) |
| 2 years | 17 (17.5%) |
| 5 years | 19 (19.6%) |
| All-Cause Mortality | HR (95% CI) | p-value |
|---|---|---|
| Aortic valve regurgitation | 4.01 (1.56, 10.3) | 0.004 |
| COPD | 9.42 (3.47, 25.6) | <0.001 |
| Aortic valve stenosis | 4.32 (1.27, 14.72) | 0.019 |
| CBVD | 2.88 (1.09, 7.57) | 0.032 |
| Cardiac Death | HR (95% CI) | p-value |
| Aortic valve stenosis | 7.95 (1.15, 54.89) | 0.035 |
| COPD | 7.29 (1.37, 38.78) | 0.02 |
| MACCE | HR (95% CI) | p-value |
| Aortic valve stenosis | 6.20 (1.98, 19.49) | 0.002 |
| COPD | 3.27 (1.41,7.57) | 0.006 |
| CBVD | 2.73 (1.13, 6.62) | 0.026 |
| STROKE | HR (95% CI) | p-value |
| EF | 0.95 (0.91, 0.99) | 0.043 |
| MI | HR (95% CI) | p-value |
| Syncope | 38.57 (4.22, 352.11) | 0.001 |
| Cardiogenic Shock | 22.42 (1.78, 282.64) | 0.016 |
| Reoperation | HR (95% CI) | p-value |
| Prior Mediastinal Radiation | 8.88 (1.76, 44.7) | 0.008 |
| Endoleak Leak | HR (95% CI) | p-value |
| Aortic valve disease | 3.29 (1.39, 7.79) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





