Submitted:
15 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental methods
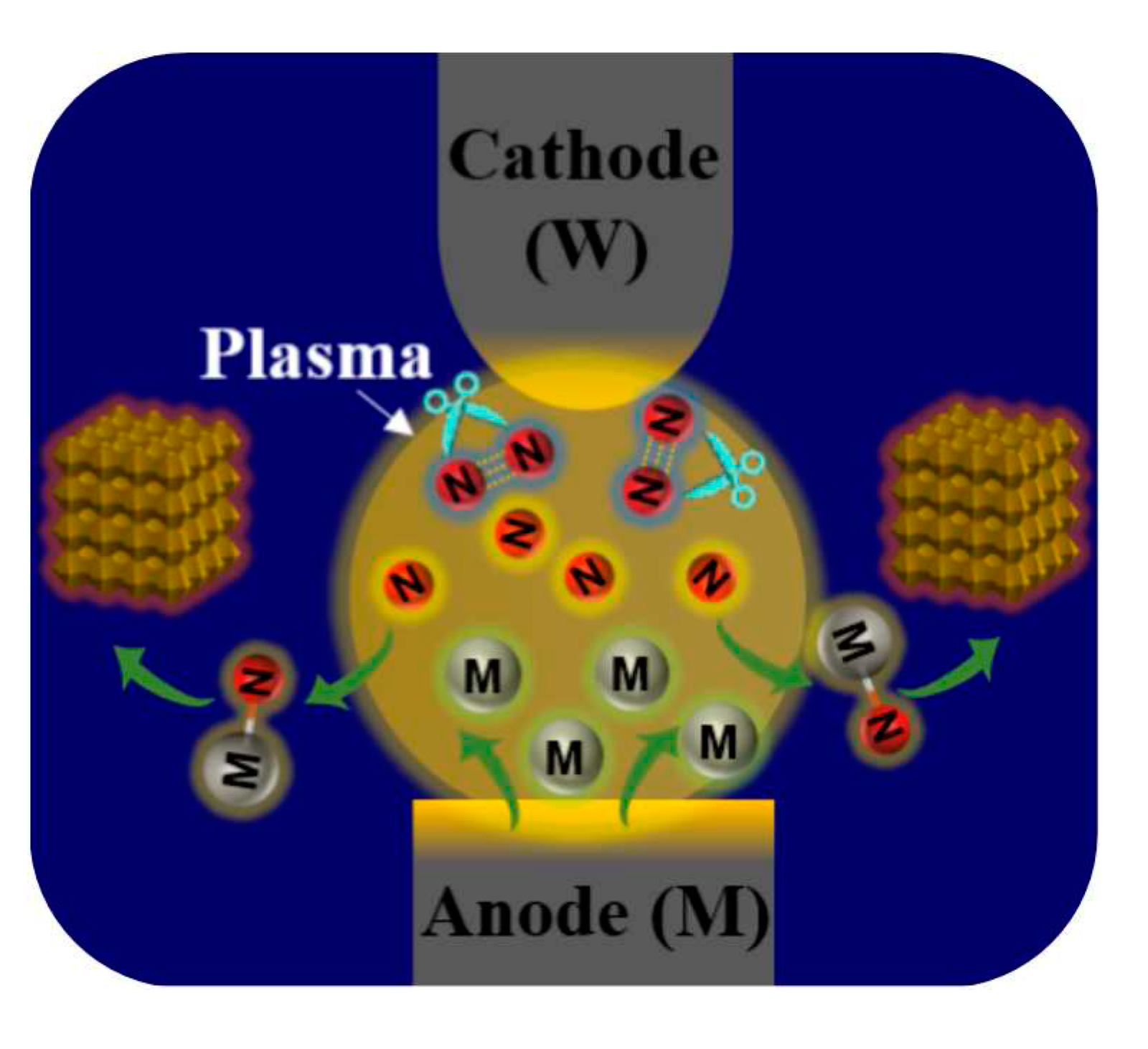
2.1. Preparation of TMNs NPs
2.2. Characterization
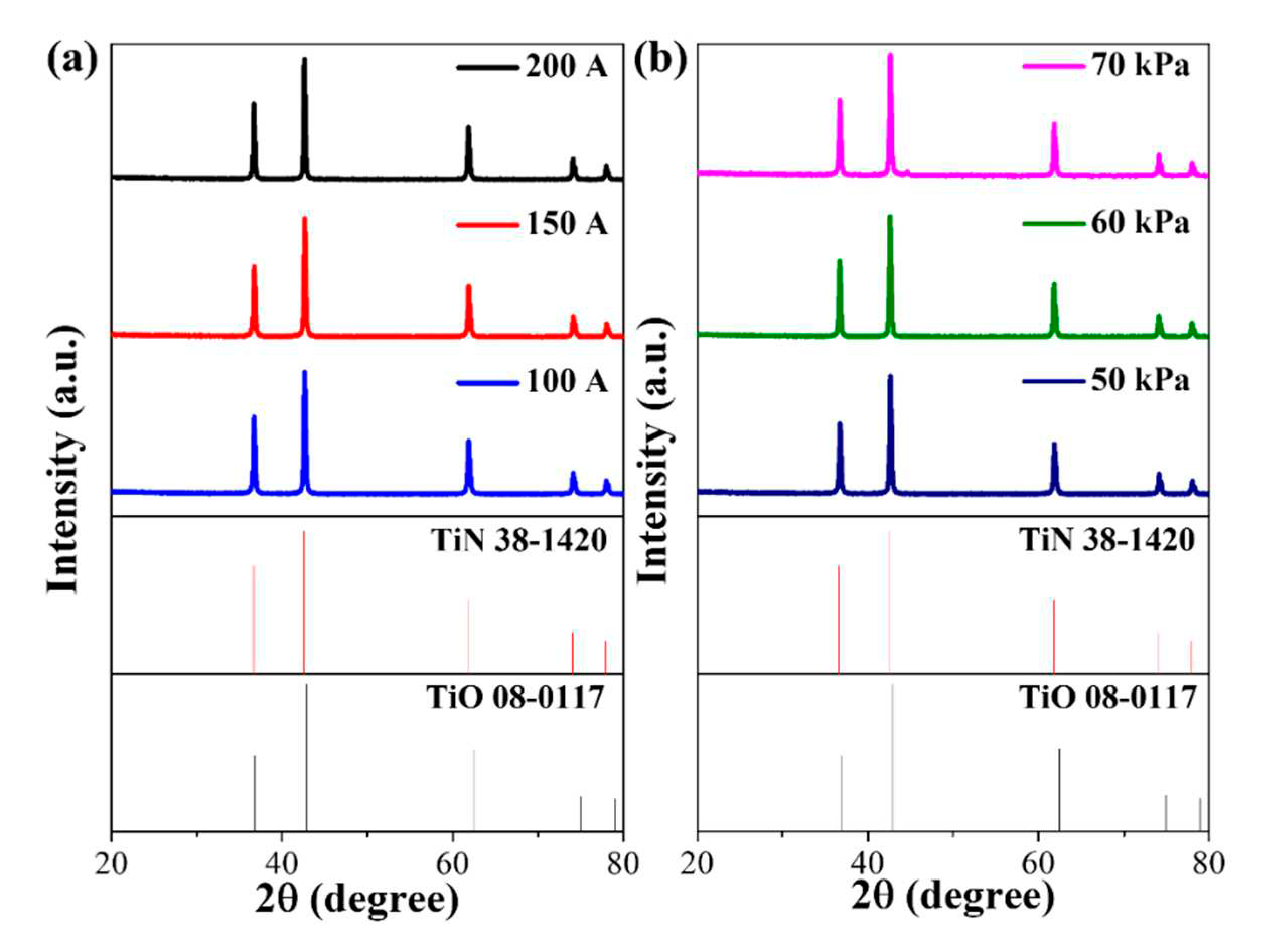
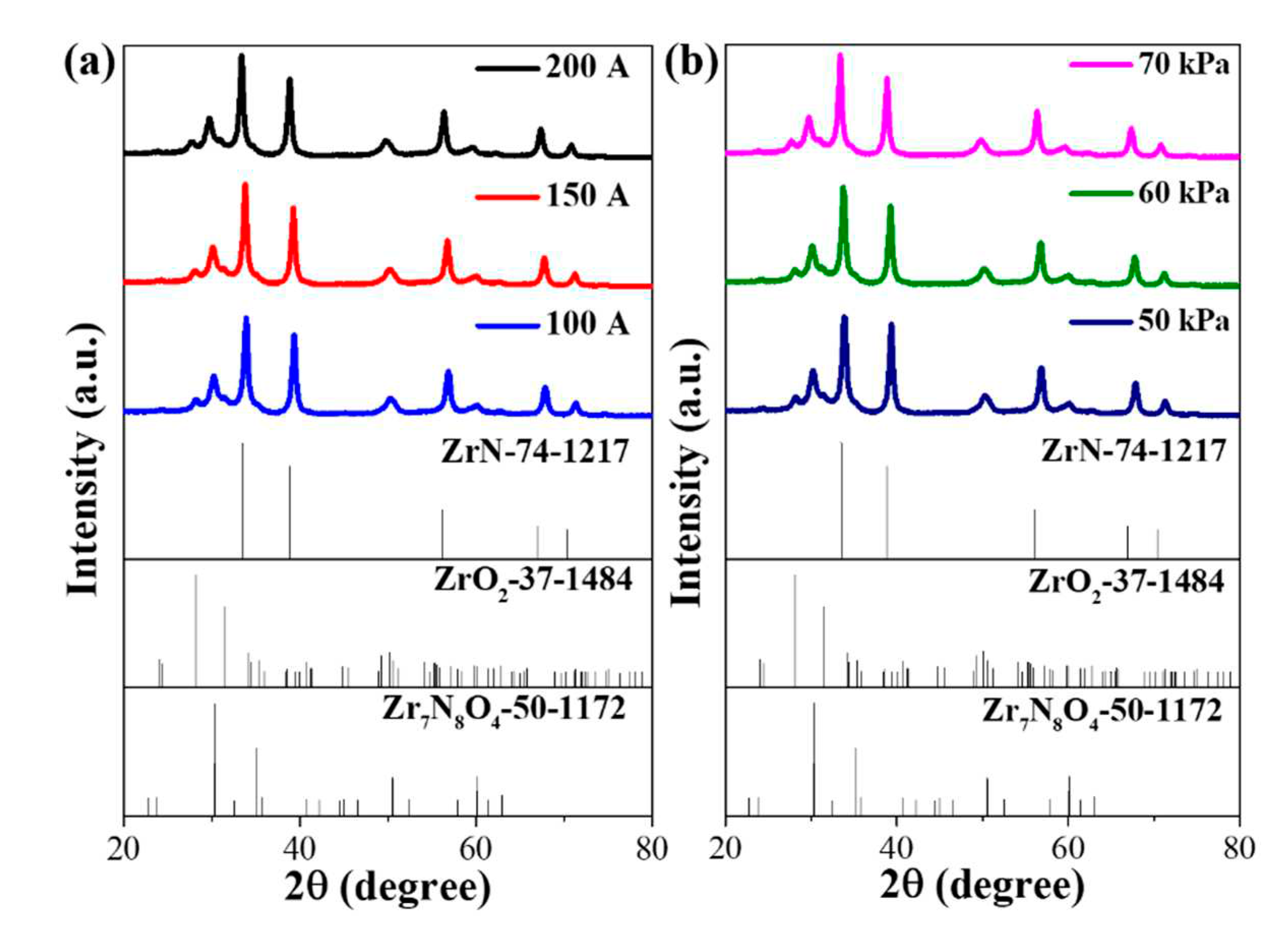
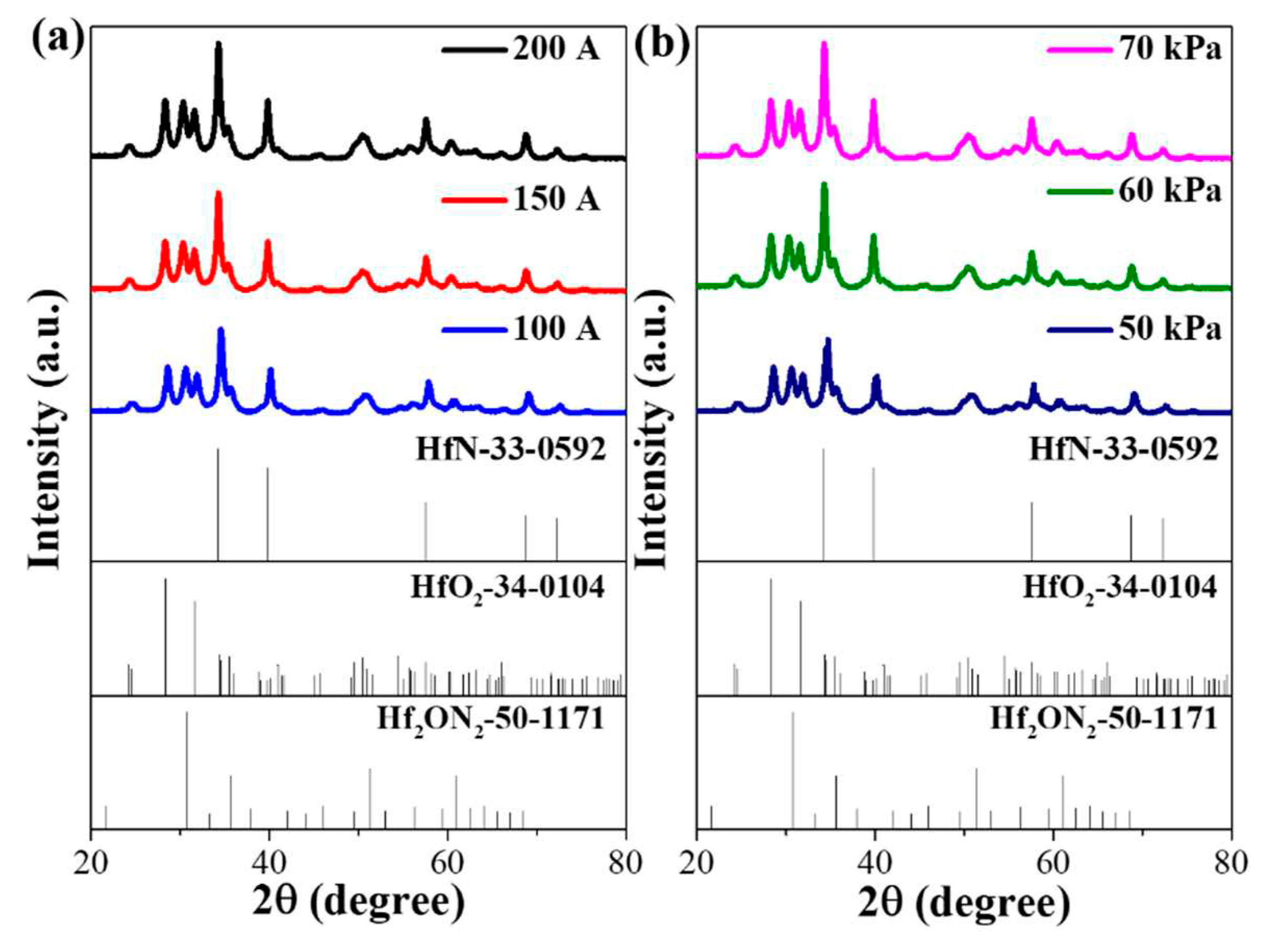
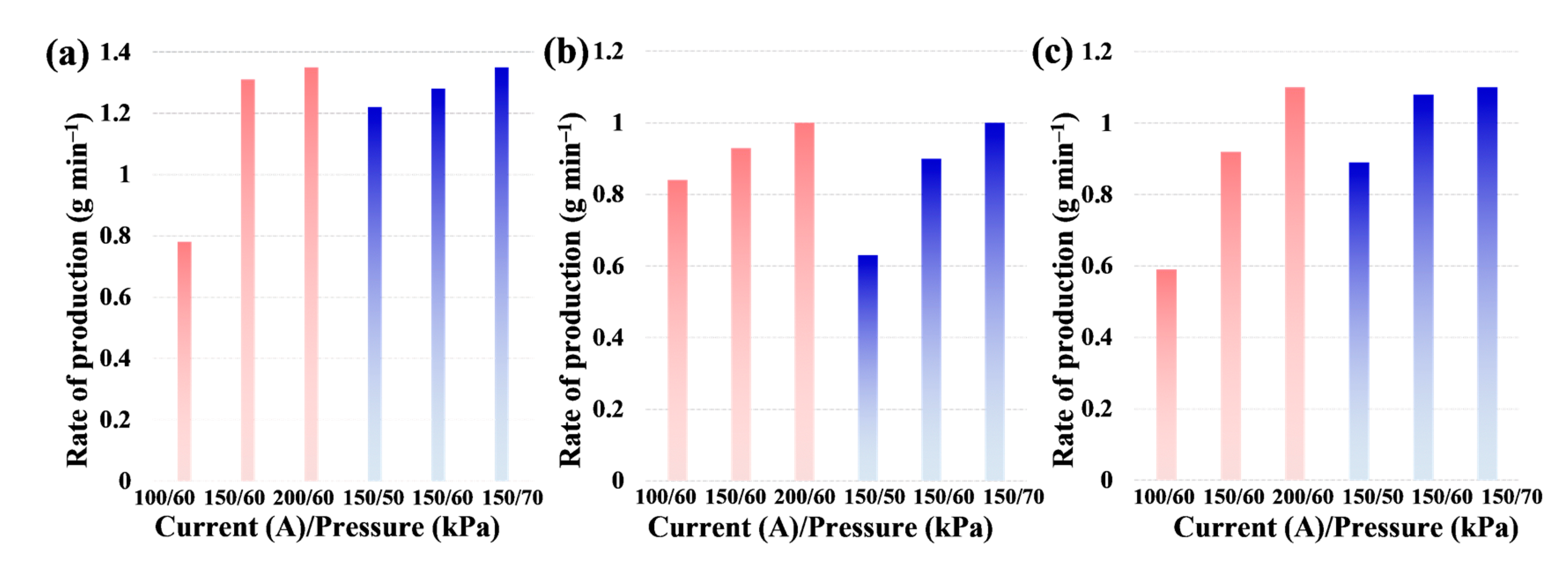
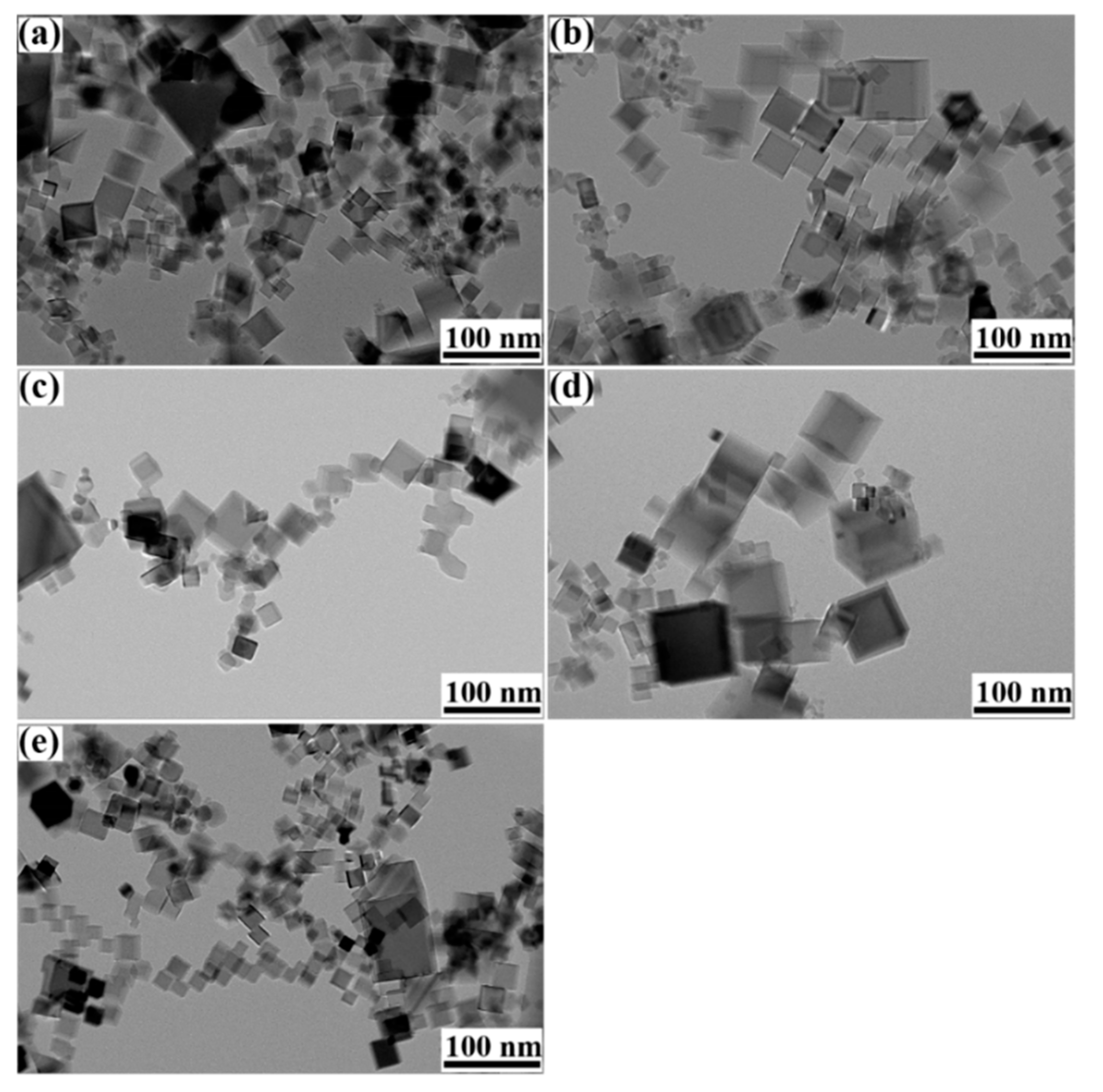
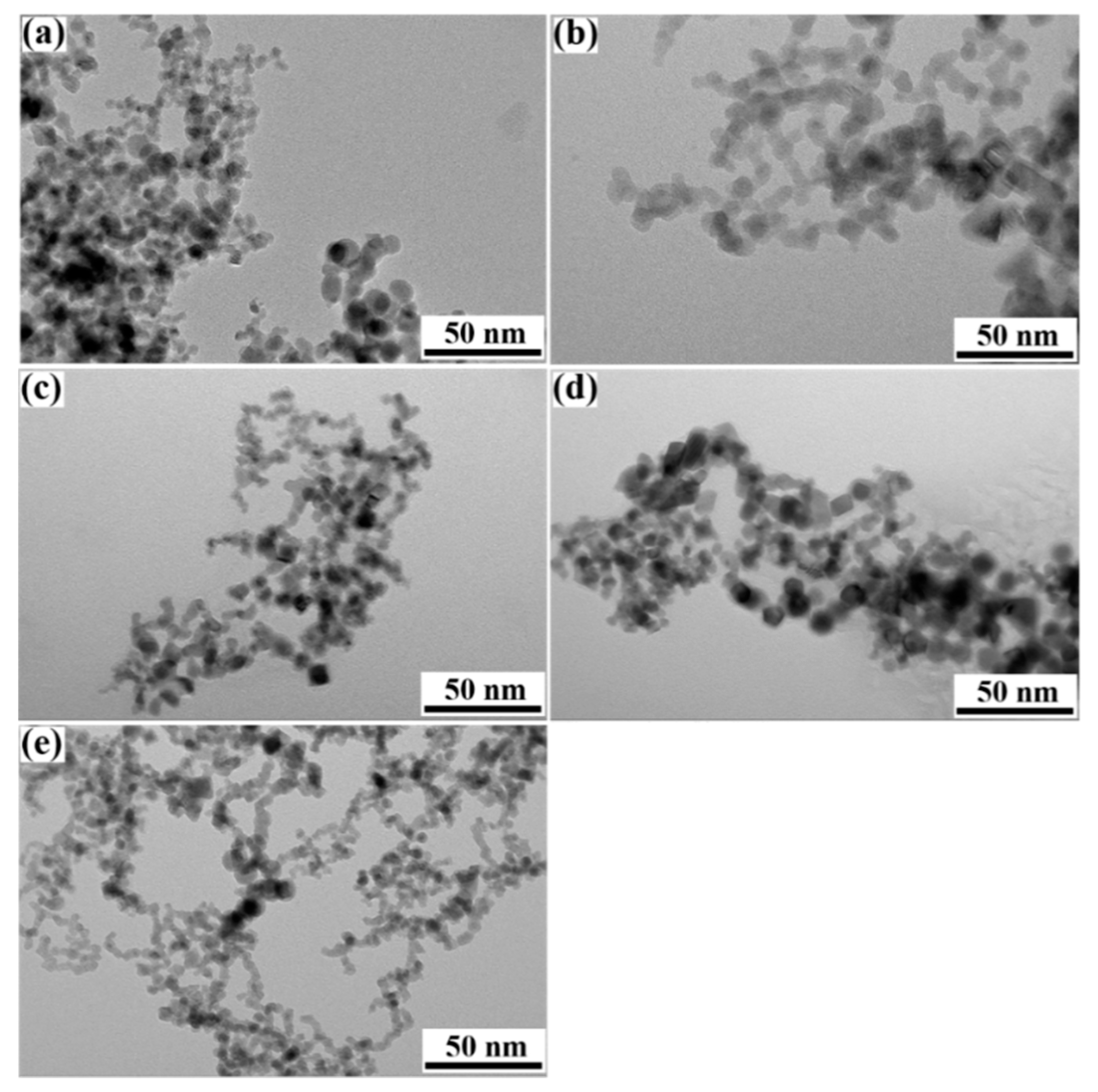
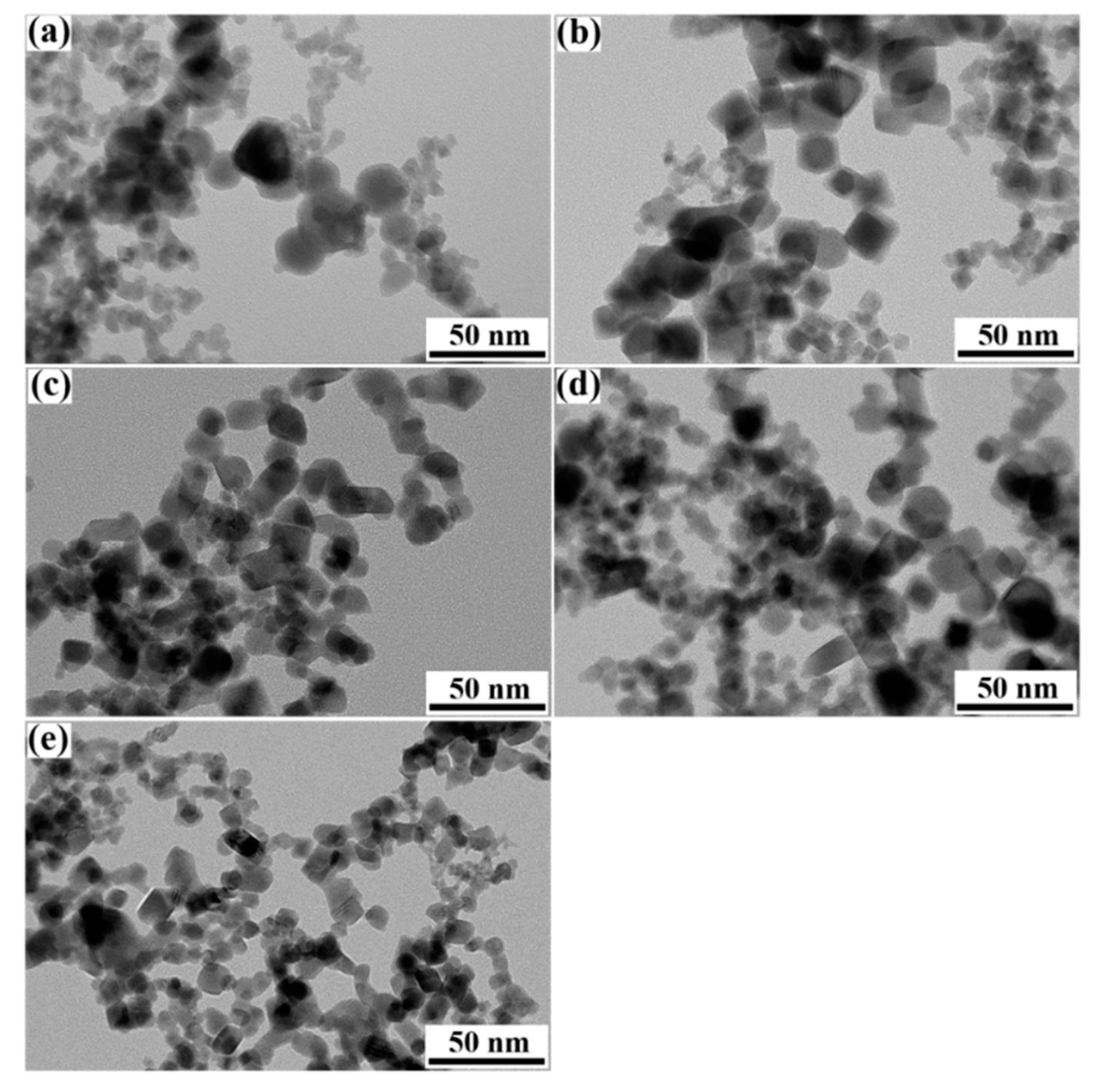
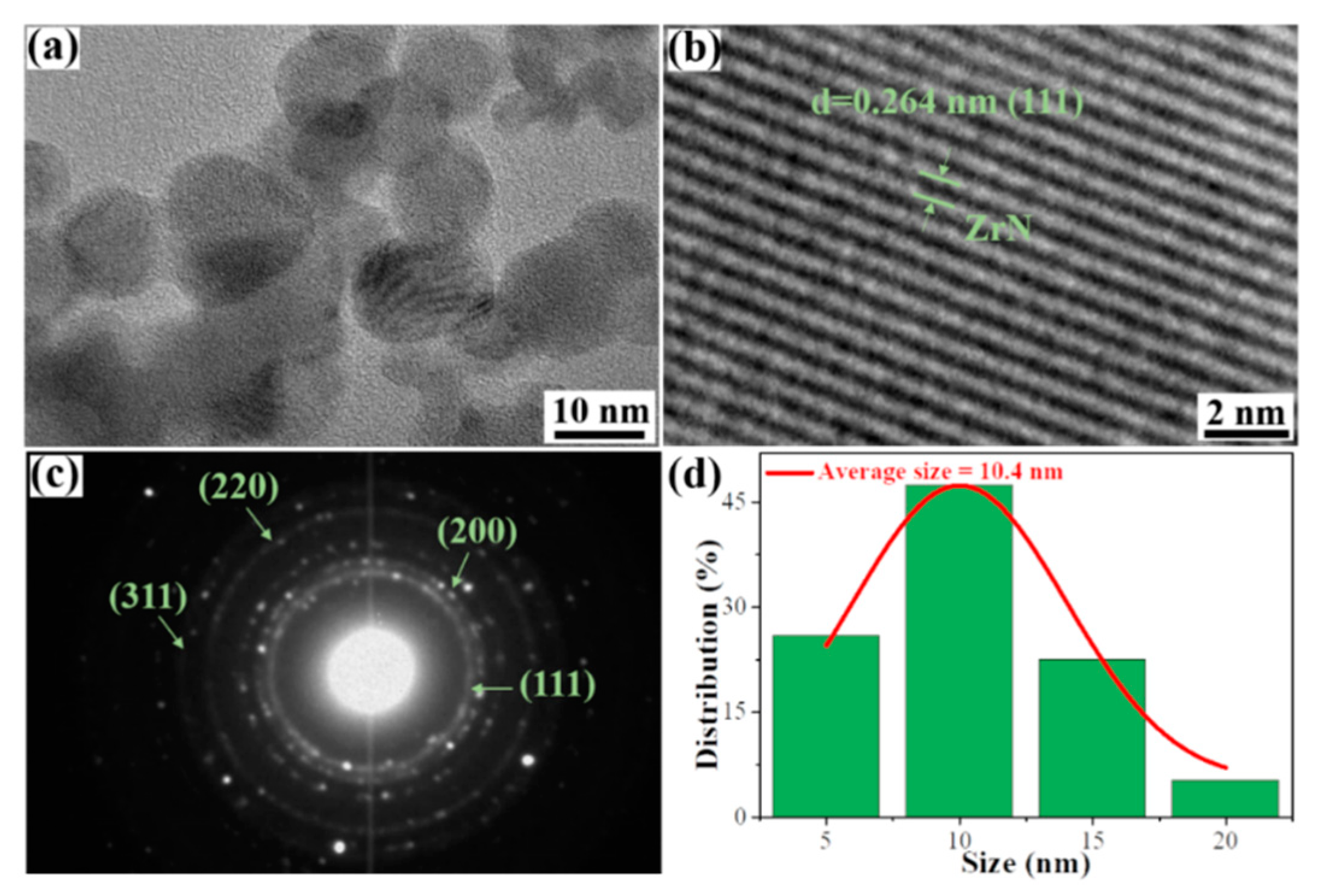
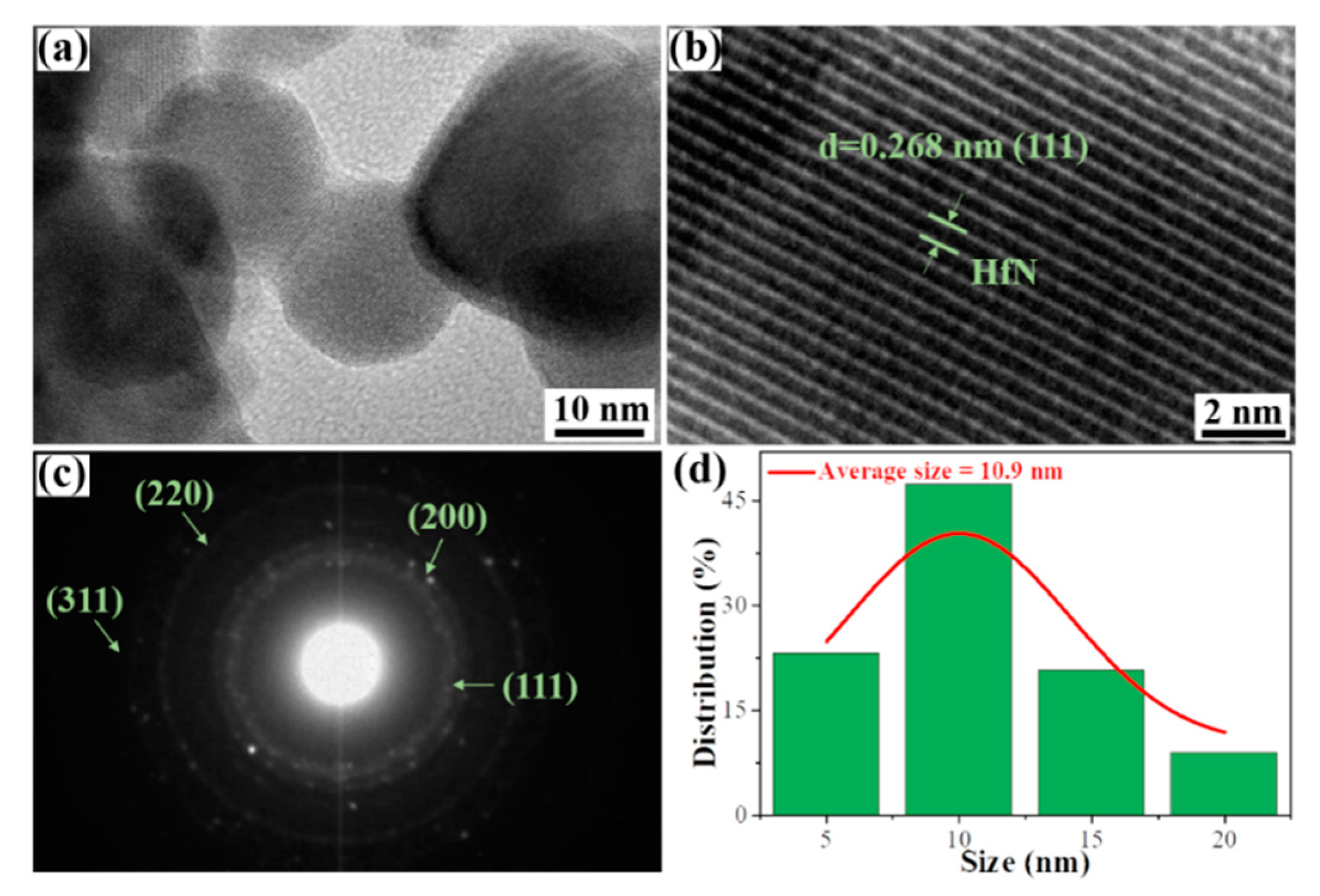
3. Results and discussion
4. Conclusions
Acknowledgments
Data availability
Conflicts of Interest
References
- Yu X, Zhou T, Ge J, Wu C. Recent advances on the modulation of electrocatalysts based on transition metal nitrides for the rechargeable Zn-air battery. ACS Mater. Lett. 2020, 2, 1423–1434. [Google Scholar] [CrossRef]
- Jamil R, Ali R, Loomba S, Xian J, Yousaf M, Khan K, Shabbir B, McConville C, Mahmood A, Mahmood N. The role of nitrogen in transition-metal nitrides in electrochemical water splitting. Chem Catal. 2021, 1, 802–854. [Google Scholar] [CrossRef]
- Choi D, Kumta P. Synthesis, structure, and electrochemical characterization of nanocrystalline tantalum and tungsten nitrides. J. Am. Ceram. Soc. 2010, 90, 3113–3120. [Google Scholar] [CrossRef]
- Balogun M, Huang Y, Qiu W, Yang H, Ji H, Tong Y. Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today. 2017, 20, 425–451. [Google Scholar] [CrossRef]
- Deno H, Kamemoto T, Nemoto S, Koshio A, Kokai F. Formation of TiN-Ir particle films using pulsed-laser deposition and their electrolytic properties in producing hypochlorous acid. Appl. Surf. Sci. 2008, 254, 2776–2782. [Google Scholar] [CrossRef]
- Xu S, Xu J, Munroe P, Xie Z. Nanoporosity improves the damage tolerance of nanostructured tantalum nitride coatings. Scr. Mater. 2017, 133, 86–91. [Google Scholar] [CrossRef]
- Kang J, Park M, Kim J, Park S, Chung D, Yu S, Kim J, Park J, Choi J, Lee K, Jeong J, Ko M, Ahn K, Sung Y. Reactively sputtered nickel nitride as electrocatalytic counter electrode for dye-and quantum dot-sensitized solar cells. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Xiang D, Liu Y, Gao S, Tu M. Evolution of phase and microstructure during carbothermal reduction-nitridation synthesis of Ti (C, N). Mater. Charact. 2008, 59, 241–244. [Google Scholar] [CrossRef]
- Giordano C, Erpen C, Yao W, Antonietti M. Synthesis of Mo and W carbide and nitride nanoparticles via a simple “Urea Glass” route. Nano Lett. 2008, 8, 4659–4663. [Google Scholar] [CrossRef]
- Chen X, Dye J, Eick H, Elder S, Tsai K. Synthesis of transition-metal nitrides from nanoscale metal particles prepared by homogeneous reduction of metal halides with an alkalide. Chem. Mater. 1997, 9, 1172–1176. [Google Scholar] [CrossRef]
- Choi D, Kumta P. Synthesis, structure, and electrochemical characterization of nanocrystalline tantalum and tungsten nitrides. J. Am. Ceram. Soc. 2010, 90, 3113–3120. [Google Scholar] [CrossRef]
- Jiang W, Fu Q, Wei H, Yao A. TiN nanoparticles: synthesis and application as near-infrared photothermal agents for cancer therapy. J. Mater. Sci. 2019, 54, 5743–5756. [Google Scholar] [CrossRef]
- Kiesler D, Bastuck T, Theissmann R, Kruis F. Plasma synthesis of titanium nitride, carbide and carbonitride nanoparticles by means of reactive anodic arc evaporation from solid titanium. J Nanopart. Res. 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Vandenabeele C, Lucas S. Technological challenges and progress in nanomaterials plasma surface modification-a review. Mater. Sci. Eng. R Rep. 2020, 139, 100521. [Google Scholar] [CrossRef]
- Stein M, Kruis F. Scaling-up metal nanoparticle production by transferred arc discharge. Adv Powder Technol. 2018, 29, 3138–3144. [Google Scholar] [CrossRef]
- Fu Q, Kokalj D, Stangier D, Kruis F, Tillmann W. Aerosol synthesis of titanium nitride nanoparticles by direct current arc discharge method. Adv. Powder Technol. 2020, 31, 4119–4128. [Google Scholar] [CrossRef]
- Yick S, Murdock A, Martin P, Kennedy D, Maschmeyer T, Bendavid A. Tuning the plasmonic response of TiN nanoparticles synthesised by the transferred arc plasma technique. Nanoscale. 2018, 10, 7566–7574. [Google Scholar] [CrossRef]
- Naddaf M, Abdallah B, Ahmad M, A-Kharroub M. Influence of N2 partial pressure on structural and microhardness properties of TiN/ZrN multilayers deposited by Ar/N2 vacuum arc discharge. Nucl. Instrum. Methods Phys. Res. 2016, 381, 90–95. [Google Scholar] [CrossRef]
- Zhang D, Ye K, Yao Y, Liang F, Qu T, Ma W, Yang B, Dai Y, Watanabe T. Controllable synthesis of carbon nanomaterials by direct current arc discharge from the inner wall of the chamber. Carbon. 2019, 142, 278–284. [Google Scholar] [CrossRef]
- Zhang D, Mylsamy G, Yang X, Xie Z, Su X, Liang F, Yang B, Dai Y. High purity and good dispersity AlN nanoparticles synthesized by an arc discharge with assistance of direct nitridation. Ceram. Int. 2021, 47, 16972–16979. [Google Scholar] [CrossRef]
- Duan C, Tanaka M, Kishida M, Watanabe T. Treatment of pyridine in industrial liquid waste by atmospheric DC water plasma. J. Hazard. Mater. 2022, 430, 128381. [Google Scholar] [CrossRef] [PubMed]
- Zhang D, Xie Z, Zhang K, Wang H, Qu T, Ma W, Yang B, Dai Y, Liang F, Lei Y, Watanabe T. Controlled regulation of the transformation of carbon nanomaterials under H2 mixture atmosphere by arc plasma. Chem. Eng. Sci. 2021, 241, 116695. [Google Scholar] [CrossRef]
- Cheng Y, Choi S, Watanabe T. Effect of nucleation temperature and heat transfer on synthesis of Ti and Fe boride nanoparticles in RF thermal plasmas. Powder Technol. 2013, 246, 210–217. [Google Scholar] [CrossRef]
- Park Y, Kodama S, Sekiguchi H. Preparation of metal nitride particles using arc discharge in liquid nitrogen. nanomaterials. 2021, 11, 2214. [Google Scholar] [CrossRef]
- Lu Y, Zhang J, Zhao C, Nie M, Wang C, Wang T. Zirconium nitride as a highly efficient nitrogen source to synthesize the metal nitride cluster fullerenes. Sci. China: Chem. 2021, 64, 29–33. [Google Scholar] [CrossRef]
- Bang J, Suslick K. Dual templating synthesis of mesoporous titanium nitride microspheres. Adv. Mater. 2009, 21, 3186–3190. [Google Scholar] [CrossRef]
- Yugeswaran S, Ananthapadmanabhan P, Kumaresan L, Kuberan A, Sivakumar S, Shanmugavelayutham G, Ramachandran K. Synthesis of zirconium nitride from zircon sand by transferred arc plasma assisted carbothermal reduction and nitridation process. Ceram. Int. 2018, 44, 14789–14796. [Google Scholar] [CrossRef]
- Lam D, Suematsu H, Ogawa T. Characterization of ZrN, ZrO2 and β′-Zr7O11N2 nanoparticles synthesized by pulsed wire discharge. J. Am. Ceram. Soc. 2017, 100, 4884–4892. [Google Scholar] [CrossRef]
- O'Neill D, Frehan S, Zhu K, Zoethout E, Mul G, Garnett E, Huijser A, Askes S. Ultrafast photoinduced heat generation by plasmonic HfN nanoparticles. Adv. Opt. Mater. 2021; 2100510. [CrossRef]
- Vandenabeele, Lucas S. Technological challenges and progress in nanomaterials plasma surface modification-A review. Mater. Sci. Eng. R Rep. 2019, 139, 100521. [Google Scholar] [CrossRef]
- Liu M, Dong Y, Wu Y, Feng H, Li J. Titanium nitride nanocrystals on nitrogen-doped graphene as an efficient electrocatalyst for oxygen reduction reaction. Chem. Eur. J. 2013, 19, 14781–14786. [Google Scholar] [CrossRef]
- Kaneko K, Kitawaki K, Sadayama S, Razavi H, Hernandez-Garrido J, Midgley P, Okuyama H, Uda M, Sakka Y. Fabrication and characterization of TiN nanocomposite powders fabricated by DC arc-plasma method. J. Alloys Compd. 2010, 492, 685–690. [Google Scholar] [CrossRef]
- Tavares J, Coulombe S, Meunier J. Synthesis of cubic-structured monocrystalline titanium nitride nanoparticles by means of a dual plasma process. J. Phys. D: Appl. Phys. 2009, 42, 102001. [Google Scholar] [CrossRef]
- Yuan Y, Wang J, Adimi S, Shen Hangjia, Thomas Tiju, Ma Ruguang, Attfield J, Yang M. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 2020, 19, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Li J Y, Sun Y, Tan Y, Xu F, Shi X, Ren N. Zirconium nitride (ZrN) fibers prepared by carbothermal reduction and nitridation of electrospun PVP/zirconium oxychloride composite fibers. Chem. Eng. J. 2008, 144, 149–152. [Google Scholar] [CrossRef]
- Chen Y, Deng C, Yu C, Ding J, Zhu H. Molten-salt nitridation synthesis of cubic ZrN nanopowders at low temperature via magnesium thermal reduction. Ceram. Int. 2018, 44, 8710–8715. [Google Scholar] [CrossRef]
- Reddy R, Kamaraj M, Mudali U, Chakravarthy S, Sarathi R. Generation and characterization of zirconium nitride nanoparticles by wire explosion process. Ceram. Int. 2012, 38, 5507–5512. [Google Scholar] [CrossRef]
- Zerr A, Miehe G, Riedel R. Synthesis of cubic zirconium and hafnium nitride having Th3P4 structure. Nat. Mater. 2003, 2, 185–189. [Google Scholar] [CrossRef]
- Defilippi C, Shinde D, Dang Z, Manna L, Hardacre C, Greer A, D'Agostino C, Giordano C. HfN nanoparticles: An unexplored catalyst for the electrocatalytic oxygen evolution reaction. Angew. Chem. 2019, 58, 15464–15470. [Google Scholar] [CrossRef]
- Hu C, Zhang X, Gu Z, Huang H, Zhang S, Fan X, Zhang W, Wei Q, Zheng W. Negative effect of vacancies on cubic symmetry, hardness and conductivity in hafnium nitride films. Scr. Mater. 2015, 108, 141–146. [Google Scholar] [CrossRef]
- Gu Z, Hu C, Huang H, Zhang S, Fan X, Wang X, Zheng W. Identification and thermodynamic mechanism of the phase transition in hafnium nitride films. Acta Mater. 2015, 90, 59–68. [Google Scholar] [CrossRef]

| Samples(1kg) | Cost accounting (¥) | ||||
|---|---|---|---|---|---|
| Raw Materials | Gas | Electricity | Water | Total | |
| TiN | 65 | 25 | 12 | 6 | 108 |
| ZrN | 321 | 30 | 16 | 8 | 375 |
| HfN | 21540 | 30 | 16 | 8 | 31594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




