Introduction
Flora should mount appropriate responses to ever-changing environmental situations via altering growth and development through specialized metabolism, adjustments in morphology, or changes in lifestyle records. Coordination of those responses is carried out through multilevel regulatory tactics; approximately a whole lot is referred to now from investigations in Arabidopsis and emerging crop version structures. Multilevel sign transduction procedures are offered here that are not regularly regarded as an included machine. This contains natural variants in regulatory processes that shape responses to significant stressors, such as drought, salt, and flooding. Transcriptional regulatory processes, alternative splicing and the fast turnover of these proteins via ubiquitination and sumoylation form complex networks that preserve cell ion homeostasis and chromatin remodelling. Plants sense signals from the surrounding environmental conditions and transmit them via a signal transduction cascade, eliciting the accrual of transcription factors requiring for initiation of gene expression, which may result in a version of demanding environmental situations (Mirouze and Paszkowski, 2011).
Few other critical mechanisms of gene expression to stresses is ‘epigenetic regulation’, which encompasses covalent modifications of DNA and histones, showing the impact on the transcriptional activity of chromatin without disconcerting DNA sequences (Iwasaki and Paszkowski, 2014). Plants incorporate sensing and signalling phenomena to activate responses against environmental stress upon exposure (Kumar and Singh, 2016).
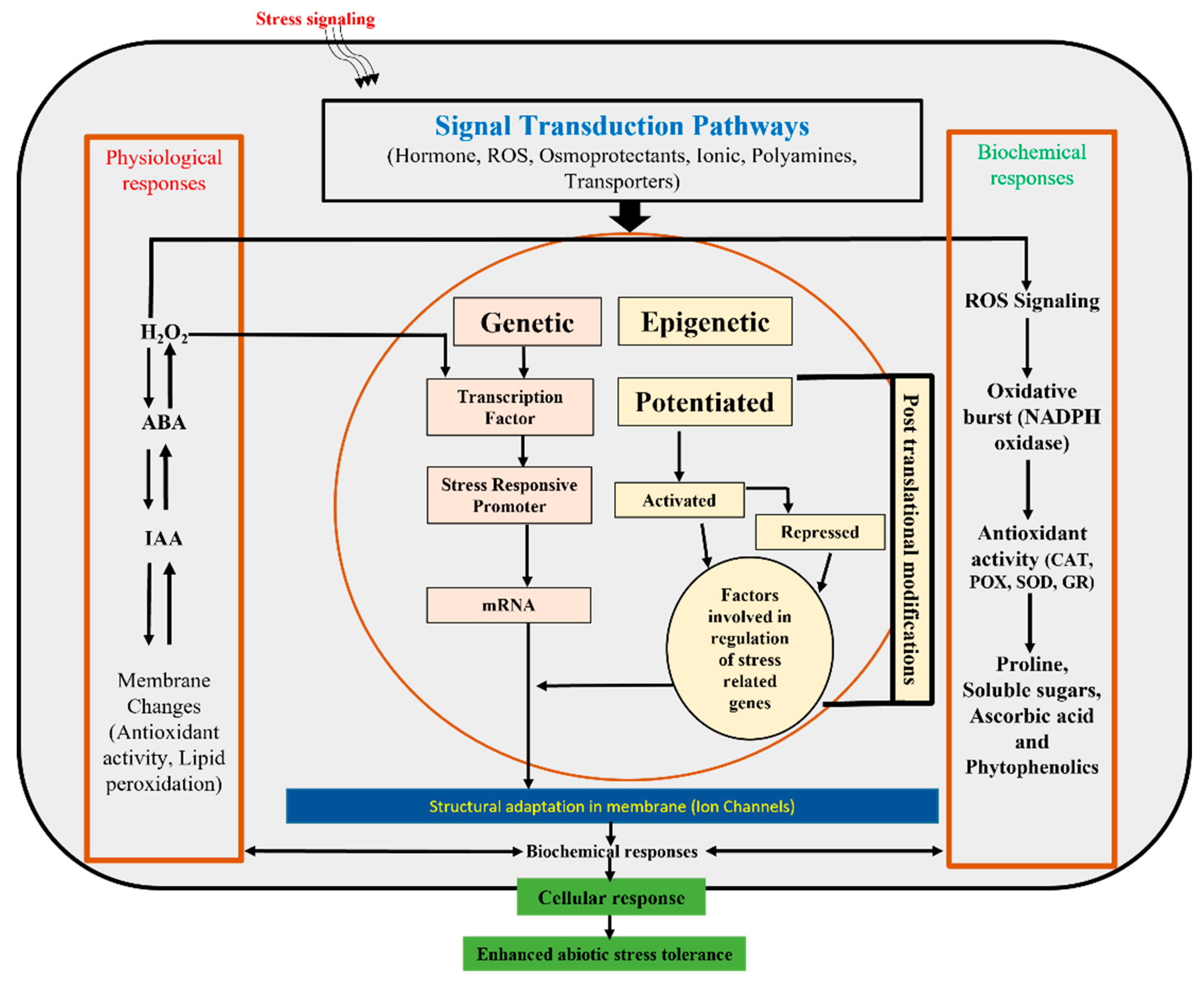
Figure 1 demonstrates the mechanism of signal transduction pathways that initiates epigenetic and genetic episodes that activate biochemical and physiological responses during abiotic stress exposure. Epigenetic phenomenon regulates these stress-associated gene regulations. DNA is not exclusively inherited genetic codes for a trait but also modulates chromatin, thereby contributing to the expression of a specific attribute. Thus environmental perturbations initiate several epigenetic and epigenomic mechanisms like DNA methylation, ubiquitination, sumoylation, post-translational modifications of histones and regulation of coding and non-coding RNA (Li et al. 2017). Chromatic shows variable epigenetic state since the transfer of specific gene traits from one generation to successive generations requires relying on the character of interest and depends on the state of epigenetics for its optimal expression. Therefore, it is mandatory to understand the mechanism of the epigenetic state for appropriate gene expression and epigenetic memory. In previously published literature, it has been shown that plants remember stress exposure and use their memory to respond promptly towards the same stress if reencountered (Kinoshita and Seki, 2014).
Plant Memory Mechanism
Plant memory is primarily attributed by tuned molecular responses against any adverse stress. Currently, several molecular mechanisms have been proposed for responding to plant memory. One device responsible for plant memory is sustained alterations levels of metabolites and transcription factors involved in signaling (Conrath, 2011). Another potential avenue could be alterations to chromatin granule states, like simple protein tail modifications, deoxyribonucleic acid methylation, or inhibited ribonucleic acid enzyme II (Pol II), that may play a huge role within the coordinated alterations in the organic phenomenon of memory responses (Avramova, 2015). The underlying mechanisms behind this phenomenon need a lot of analysis. Plant memories can necessitate the persistence of proteins or transcription factors required for signaling post stress exposure. A classic example involved the unrelenting expression of micro-RNAs to control transcription factor Squamosa Promoter Binding Protein-Like (SPL) that is important for heat shock memory (Stief et al. 2014). Beta-amino-butyric acid (BABA) induced priming of salicylate dependent mechanism requires activation of secondary metabolites and several signaling molecules. Mutation in the cyclin-dependent kinases impairs this priming mechanism (Ton et al. 2005). In another mechanism of priming of defense, associated genes involve the phosphorylation of Mitogen-Activated Protein Kinases (MPKs) like MPK3 and MPK6 along with their mRNAs post-treatment with salicylic acid (Beckers et al. 2009). A heat shock protein, like HsB1 is also associated with systemic acquired resistance (Pick et al. 2012). These are some typical examples of bequest maternal phenomenon.
Epigenetic Memories in Plants
The most common definition of epigenetics refers to information existence beyond contained in DNA template, thereby enabling the functional genome of cells. The word epi means beyond or upon, while the word genetic refers to a DNA sequence. The definition of epigenetics involves total chromatin and DNA alterations, along with their transcriptional controls (Greally, 2018). In plants, DNA methylation and histones modifications are already well known inherited mechanism of cells occurs via cell division (mitosis). Plants may circumvent such modifications processes particularly by vegetative propagation and genome demethylation in germ cells as inherited in animal cells (Feng et al. 2010). Therefore, it is extremely plausible that mitosis and meiotic transmission of stress-induced epigenetic modifications might occur among higher plants. Many environmental stresses modified chromatin content and associated its epigenetic marks (Eichten et al. 2014). Previously, it has been studied that DNA methylation can be induced by stresses like drought, flood, nutrient paucity, temperature, ultraviolet radiation, salinity, pathogen infections, chemicals and mechanical shock (Boyko et al. 2010). Histones modification pattern due to environmental perturbations is associated with selected loci within the gene (Dijk et al. 2010). In a recent study conducted on Oryza sativa and Arabidopsis, it was found that phosphate stress initiates the chromatin variation by targeting transposable elements present near the vicinity of the gene (Secco et al. 2015). Numerous reports published on epigenetic memory demonstrated that plants with hampered epigenetic machinery of RNA mediated DNA methylation showed reduced transgenerational transmission of traits (Boyko et al. 2010; Luna et al. 2012).
Epigenetic Mechanism Involvement in Abiotic Stress and Memory
Responses to stress initiate genome-wide changes in chromatin composition and gene transcription or maybe even related to modifications to the genomic sequence. Exposure to various abiotic stresses induces alterations in chromatin organization, deoxyribonucleic acid methylation, nucleosome habitation and ingredient, and the presence of simple histones protein variants with histone PTMs and chromatin orientation. Disentanglement of the direct effects of stress on chromatin and nuclear organization from its effects on transcription factors has been extremely difficult. In plant species, heterochromatin decondensation and release of transcriptional gene silencing (TGS) from transposons elements were reported due to abiotic stresses. It has been reported that heat and salinity stress initiates decondensation of 45S rDNA clusters mechanism (Santos et al. 2011) and heat stress in rye plants (Tomás et al. 2013). In maize, the cold was found responsible for the loss of tandem repeat transcriptional silencing of heterochromatic knobs (Hu et al. 2012).
Abiotic stresses initiate the synthesis of long non-coding RNAs and several small RNAs of varied classes involved in gene expression at different stress levels (Borges and Martienssen 2015). MicroRNAs (miRNAs) amend in stress conditions via post-transcriptional gene silencing (PTGS) (Borges and Martienssen 2015). It was also reported that miRNAs were involved in the memory of abiotic stresses. One study found upregulation of miR156 due to heat stress, which further downregulated the Squamosa Promoter Binding Protein-Like (SPL) transcription factor (Stief et al. 2014; Cui et al. 2014).
A maintained level of miR156 is required for the memory of recurring heat stress, and miR156, an evolutionarily conserved miRNA in plants, may mediate crosstalk between responses to environmental conditions and plant development (Stief et al. 2014; Cui et al. 2014). Abiotic stresses modulate the activity of histone acetyltransferases (HATs) and deacetylases (HDACs) (Luo et al. 2017). Acetylation is a highly dynamic post-transcriptional modification mechanism, and in the chromatin of stress-associated genes, it was recovered back to the naïve state (Friedrich et al. 2018). It was reported that histone methylation is regulated by H3K4me3, H3K9me3, H3K36me3 (initiate activation) and H3K27me3 (deactivation). The stress response mechanism involves methylation and acetylation and is also valid for sumoylated proteins, e.g., heat stress activates the histone 2B (H2B) sumoylation. Sumoylation is a post-translational modification mechanism demonstrated by adding small ubiquitin-like modifiers (SUMOs). Chromatin-associated factors like SWI/SNF, HMTs, and GCN5 were also sumoylated post-stress exposure (Miller et al. 2010).
It is interesting to know that PTMs are involved in somatic memory rather than intergenerational abiotic stress. Stress-induced epigenetic memory in chromatin is a subject of detailed research (Friedrich et al. 2018). It was reported that epigenetic memory created due to PTMs of histones is of short duration and ends within ten days, followed by initial stress exposure (Lämke et al. 2016). Another study found that the PTM phenomenon might be mitotically transmitted in a state of recurring stress (Lämke and Bäurle 2017). Another study on the molecular vernalization of Arabidopsis found that histone PTM of chromatin content can be transmitted successfully somatically and inter-generationally (Lämke and Bäurle 2017). The authors also reported that this transmission was long-lasting somatic memory due to exposure to abiotic stress. Bodily stress memory, or type 1 memory, is contributed by the unrelenting expression of stress-responsive genes (Bäurle, 2017).
Moreover, abiotic stress transcriptional memory, also termed type 2 memory, comes into play upon repeated stress exposure due to initial transcription activation of stress-associated genes (Bäurle, 2017). It was reported that type 2 memory initiates the upregulation of H3K4me3, which is responsible for heat-stress-related memory, along with the acetylation of H3K9 and H3K4me2 in plants (Liu et al. 2018b). Assessment of the transgenerational epi-marks to heritable phenotypes is a significant challenge in the epigenetic memory system. It was evident that plants acquire inheritable epiallelic variations that can be further exploited for crop improvement. Recently CRISPR-Cas9, a genome-editing tool, was used to manipulate locus-specific DNA methylation/acetylation of chromatic content (Liu et al. 2016).
Exoribonuclease 4 and mRNA Association with Plant Stress Memory
It has been reported that Arabidopsis, in connection with the cytoplasmic RNA degrading enzyme known as exoribonuclease four, has a pioneering role in stress tolerance and recovery. It has been found that heat stress initiates the decay of several transcripts that are temperature labile (Merret et al. 2013). It has been found that LA-related protein 1A and XRN4 are associated with heat stress and smooth the process of heat-induced mRNA degradation process targeting 4500 mRNAs. The LARP1 variant mediates the association between XRN4 and polysomes; therefore xrn4 mutants showed a disposition towards continuous mild heat stress. A recent study demonstrated that the xrn4 mutant is a heat-tolerable variant up to 44oC for 5 hours compared to the previous one that showed susceptibility up to 37oC for seven days (Nguyen et al. 2015). The heat-tolerant counterpart depended on heat shock proteins and transcription factors A2 (HSFA2). It is interesting to know that the xrn4 mutant retains a stress memory for a prolonged time, while XRN4 plays a vital role in the recovery and resetting of the transcriptome.
The Intelligence of Plants from the Environmental Learning
Abundant articles were published that showed the intelligent behaviour of plants towards environmental exposure. These articles stated that plants efficiently store information about their environment from generation to generation and evaluate their ambience (Latzel et al. 2010, 2014). Moreover, plants are intelligent enough to predict future conditions based on past environmental interactions. This plant’s chloroplast is responsible for quantum computing to predict future changes related to light based on previous light quality and exposure so that plants may adjust for the mechanism of photosynthesis accordingly (Latzel et al. 2016). It has been found that plants, as per their memory, can modify their growth according to the resources, nutrient availability and predict future competition (Latzel et al. 2016).
Moreover, stress memory fabricated within plant machinery may help overcome the future stress environment. Learning is a memory-based mechanism; some memories, like stability and adaptability, run parallel with the developmental tool. Most plants’ memories rely on molecular mechanisms known as epigenetics. Epigenetic memory created due to environmental stress may either be limited to one generation or transferred to successive generations (Latzel et al. 2016). Epigenetic modifications help the plants recognize and remember their former stress, enabling them to learn and acquire essential traits for their survival and better adaptability (Latzel et al. 2016).
Epigenetic Variation Inheritance
The change of epigenetic stamps, for example, ‘DNA methylation’, could result in an epigenetic reaction, and the epigenetic imprints can be passed on to the descendants through the procedure of mitosis and sometimes also meiosis (Hauser et al. 2011). The same gene is methylated differently than it is denoted as ‘epiallele.’ The epiallele variations are depicted mainly based on either numbers or allocations of methylated nucleotides at the exact gene sequence. Different epialleles are inherited in successive generations and can bring about various phenotypes. Studies on transposable element regulation show the effect of epialleles on the inheritance pattern (Martienssen et al.1990), deciphered the contribution of transposable elements (TE) regulation during the period of maize plant development and its effect on the inheritance pattern of epialleles.Furthermore, Linaria vulgaris is an instance of a naturally occurring mutant that suggests the ‘transgenerational epigenetic inheritance’ (Cubas et al. 1999). Various investigators also portrayed the DNA methylation level of CYCLOIDEA as being responsible for causing changes in phenotypes of flower symmetry, and these modifications have been maintained for many years. It also becomes imperative to emphasize epigenetic marks that some ‘epigenetic marks’ can bring about variation inheritable phenotypes while others show an inability to do so (Baulcombe and Dean, 2014). However, the research did not completely explain the mechanisms involved but gathered information from research that exhibited DNA methylation (as an epigenetic mark).Many cells, particularly germline cells of plants, carry epigenetic marks, and the descent of such cells from somatic cells may bestow the inheritability of epigenetic marks. Intensive research studies on epigenetic inheritance are mainly centred on deciphering the pattern of DNA methylation (Kalisz and Purugganan, 2004). It is mandatory to know the mechanistic view behind a transgenerational epigenetic inheritance to decipher the propagation of epigenetic imprints during gametophyte development. In Arabidopsis, the loss of epigenetic marks in the somatic cells of pollen triggers the activation of transposons. Still, this RNA molecule can be an antecedent for the siRNA formation.This immediate product, ‘siRNA’, can result in the silencing of this transposon in germ cells and give an ascent to the ensuing generation (Slotkin and Martienssen, 2007). Many types of research reveal the consequences of inheritable epigenetic imprints in inheritable phenotypic variation, demonstrating an impact on wellness/fitness, and can be liable to natural selection (Baulcombe and Dean, 2014). In contrast to warm-blooded creatures such as mammals, DNA methylation at ‘CG’ and ‘CHG’ were kept in three haploid cell types from mounting pollen (Calarco et al. 2012). Even though the collapse of methylation at ‘CHH’ in retrotransposons of sperm cells and microspores, the activity of siRNAs containing nucleotides (mainly 24 nucleotides) can reinstate the process of methylation through de novo DNA methyltransferase activity. Moreover, such results exhibited the significance of sRNA (small RNA) molecules during the methylation process. Furthermore, sRNA-mediated DNA methylation also contributes to the TEs and repeats, whose decline in the process of DNA methylation results in increased TEs movement, which may further show its impact on genetic variation (Matzke and Mosher, 2014). The replication or copying of DNA strands possessing methylated DNA sequences bestows the ‘hemimethylated’, a process where the methylation of a solitary strand of the DNA duplex occurs. Plants contain MET1 (METHYLTRANSFERASE1), which participates in the replication of ‘CG’ methylation, and in this manner, the ‘hemimethylated DNA’perform as a duplicate of the recently synthesized strand. Fascinatingly, an examination of a mutant for MET1 uncovered that the maintenance of methylation in tissues, specifically somatic tissues, vanished at the stage of gametogenesis (Saze et al. 2003). During vegetative growth, plants can sense the various environmental conditions resulting in epigenetic changes in a cell lineage, which can generate a germline (Mirouze and Paszkowski, 2011). Research on the model plants, such as ‘Arabidopsis’, delineated the reliance upon ‘stress-induced transgenerational responses’ on DNA methylation modification (Boyko et al. 2010; Lang-Mladek et al. 2010). In light of such perception, the concluding remarks show the possibility of inheritability of epialleles caused phenotypic effects crosswise over generations, which are affected by environmental situations and local plants. Hence, inheritable epialleles will impact plant evolution/development, particularly phenotypic attribute distributions and fitness. Additionally, numerous plants are propagated through clonal reproduction, where meiotic epigenetic readapt doesn’t occur. Among clonal generations, epigenetic information is much more productive than sexual reproduction (Latzel et al. 2016). Few investigations indicating epigenetic inheritance other than model plants (non-model plants) have been published. The following topic describes studies exhibiting the function of ‘epigenetic regulation in non-model plants adaptation,’ some of this investigation accentuated the jobs of assumed epigenetic inheritance.
Mechanism of Priming and Memory in Plants
As the plants experience stress, they become resistant to future exposure through memory acquisition. Several instances of memory in higher plants have been revealed across numerous species and presented in detail in past reviews (Herman and Sultan, 2011; Crisp et al., 2016). Stress memory in response to stimuli example includes abscisic acid (ABA), β-aminobutyric acid (BABA), drought, salinity, methyl jasmonate, oxidative stress, excess light, and cold (Crisp et al. 2016). Evidence suggests that priming can persist among generations, and this process is determined as adaptive transgenerational plasticity (Crisp et al. 2016). Similarly, selecting events referring to transgenerational memory includes short-wavelength radiation (ultraviolet C), herbivory damage and treatment with jasmonic acid, flagellin (an elicitor of plant defence), day length, BABA priming, drought, temperature and heat (Crisp et al. 2016). These consequences highlight the potential for memories to be passed to progeny through transgenerational inheritance to augment offspring success.
Transgenerational Mechanism and Epigenetic Memory
Phenotypic agility in plants allows them to adapt for a better future and adjust their morphology according to the existing environment (Rendinaet al. 2018). A study showed that phenotypes of plants reflect their grandparent’s level of stress exposure due to transgenerational effects (TGE)(Lampei et al. 2017). TGE had been exploited most in sexual generations but rarely in clonal generations. However, clonal mode of reproduction is the most common method found in plant species. Every time each newly formed ramet might be adjusted in its functions and independent of the mother plant in terms of environmental information (Rendina et al. 2018). Clonal plant behaviour depends on the actual ecological exposure and is inherited from parent plants due to TGE (Rendina et al. 2018). Although, TGE is often designed to be beleaguered by a pre-programming mediated through epigenetic mechanisms (Rendina et al. 2018). It has believed that clonal plants maintain environmental-induced epigenetic alterations better than sexually reproduced generations because clonal plants originate through asexual mode, thereby bypassing the meiosis process. One of the classical studies showed that environmental stress impairs the DNA methylation mechanism in the parental generation that can be passed to successive apomictic generations with a high fidelity rate in Taraxacumofficinale (Rendina et al. 2018). Another study on poplar plants found that epigenetic variation contributes to drought stress response in clonal offspring (Raj et al. 2011).
Epimarks Transgenerational Inheritance
Epigenetic mechanisms have been illustrated as an important mediator of plant responses to different environmental stresses. However, their contribution to long-term adaptation and stress memory is still debatable. The changes in genome-wide epigenetics are linked with alterations in gene expression at the time of stress exposures and development processes. However, if the stress is withdrawn, the epigenetic changes and the gene expression level may revert to the pre-stress condition. Moreover, some of these epigenetic modifications are maintained, which could be conceded forward over the generation as stress memory (Kumar, 2018). Indeed, in response to the environment, epigenetic variations can be accumulated in the first generation, and transgenerational epigenetic memory ensures flexibility and adaptability in the plant. In Taraxacumofficinale, the genome-wide DNA methylation pattern was found to be altered once the parental plants were enforced with environmental stress. Hence, the progenies exhibited changes in the leaf morphology, shoot/root biomass ratio and stress tolerance capability compared to the control plant (Verhoeven and Gurp, 2017). For example, tissue culture practice upon stress imposition regenerated rice plants that showed genome-wide DNA methylation pattern alterations. The changes were determined predominately as the loss rather than the gain in DNA methylation, and the changes continued in the regenerated plants and their progenies (Pellegrini et al. 2013). These are determined as ‘indicators’ rather than the proof of transgenerational inheritance of epigenetic alterations which affect adaptive phenotypes. An example related to the defence priming shows excellent proof of the transgenerational epigenetic effect. Descendants of bacterial-infected Arabidopsis weree determined to possess additionalresistancey towards secondary infection of oomycete compared to that of the descendants of unprimed or control plants (Luna et al. 2012). The inherited priming was due to the epigenetic mechanisms, as was confirmed by chromatin analysis of the defence genes. The up-regulated expression of defence genes was related to histone acetylation, a known transcriptional activation mark in the promoter region. On the contrary, down-regulated expression of the genes was found to be linked with the elevated level of a repressive Primark ‘H3K27me3′. However, the plants showing a defect in DNA methylation at CHG sites mimicked the transgenerational priming effects (Luna and Ton, 2012). Thus, assuming that transgenerational priming might be mediated by DNA demethylation at the CHG sites would be suitable. Hence, this involves a series of epigenetic changes wherein the biotic stress causes loss of repressive Primark, which sequentially triggers activating Primark. Investigation of 30 generations of Arabidopsis exhibited spontaneous loss or gain in epigenetic marks (Becker et al. 2011). Though the reasons for some loci being more prone to spontaneous epigenetic alterations are not apparent, the presence of overlapping and diverging transcripts might be responsible for such loss or gain in epigenetic marks. Such configuration might affect the chromatin structure might be affected by such configuration because of which the epigenetic marks are gained or lost more easily than may take place in any other region of the genome. In allotetraploids of Arabidopsis, the loss of repressive histone marks from the circadian clock regulators (CCA1 and LHY) resulted in the up-regulation of 130 genes (Niet al. 2009). Alterations in the methylation level at many associated loci in the hybrids of cultivated- and wild-tomato and the biogenesis of siRNAs indicated that wide-hybridization caused a genome-shock in the hybrid, leading to induced epigenetic changes. Therefore, future research priorities on heterosis must involve understanding the role of different epigenetic components in providing hybrid vigour. Zheng et al. (2017) found improved rice plant drought adaptability because of exposure to multi-generational stress. Moreover, they also identified the manifestation of non-random drought-induced epimutations and a high proportion of the stress-induced epimutations ‘DNA methylation’ that could be retained in the following generations. Determination of the drought-associated genes has shown the level of DNA methylation of the genes that were affected by exposure to multi-generational drought stress. These outcomes further suggest the imperative role of epigenetic mechanisms in acquiring plant adaptation in response to environmental stresses. Hence, the heritable epigenetic variations can be considered significant resources in the improvement of the plant that may help improve crop plants’ adaptation towards adverse environmental conditions. Mechanistic insights of transgenerational stress memory are not understood appropriately and require further investigation. The present understanding suggests the proficient involvement of DNA methylation, histone modifications and siRNA pathways in adapting to environmental stress and stress memory in numerous plants.
Prospects
The role of plant intelligence is the prime topic for research in the scientific world for challenges to the problem of abiotic stresses. However, multiple environmental challenges must be addressed, decoding further progression in plant memory for stress response. Future research is required to use plant intelligence for developing libraries of plants already bearing ‘stress responsive memory.’ Furthermore, an improvement in plant breeding based on understanding stress responding memory of plants can be a significant and striking achievement for developing crops with the ability to tolerate stress conditions. The current research is also directing the future for using plant smartness in revolutionizing agricultural biotechnology’ for solving the purpose of improved food crops grown under different environmental stresses (abiotic and biotic).
Conclusion
Understanding ethe co-physiology of plants is the current need to alleviate the consequences of abiotic stresses and to decipher the mechanisms behind imparting stress-conscious memory to the subsequent generation of plants. Advancement in epigenetic studies has provided sufficient evidence for engendering abiotic sstress-responsivememory. Abiotic stresses of various kinds showed their efficiency tin reducing plant growth, eliciting plant intelligence, and developing further memory to sense environmental pressures. Due to abiotic stress perturbation; several epigenetic mechanisms are modified, and patterns from DNA or chromatin modification to post-translation are seen. The modified epigenetic traits giprovidentelligence to successive plant gegenerationshat are necessary for maintaining plants’ healthy ecophysiological behaviour in challenging environmental conditions. Multiple epigenetic strategies conclude the splendid role of the brilliant performance of plants in abiotic stresses in near future challenges.
Acknowledgemnts
Dr. Prashant Kumar Singh thanks the Science and Engineering Research Board (SERB), New Delhi, India, for financial support through a startup research grant (SRG/2021/000511).
References
- Mirouze, M.; Paszkowski, J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef]
- Iwasaki, M.; Paszkowski, J. Epigenetic memory in plants. EMBO J. 2014, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A. Epigenetic regulation of abiotic stress tolerance in plants. Adv Plants Agric Res 2016, 5, 00179. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Qian, W. Active DNA demethylation: Mechanism and role in plant development. Plant Cell Rep 2017, 37, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Eichten, S.R.; Schmitz, R.J.; Springer, N.M. Epigenetics: Beyond chromatin modifications and complex genetic regulation. Plant Physiol. 2014, 165, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.; Blevins, T.; Yao, Y.; Golubov, A.; Bilichak, A.; Ilnytskyy, Y.; Hollander, J.; Meins, F., Jr.; Kovalchuk, I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of dicer-like proteins. PLOS One 2010, 5, e9514. [Google Scholar] [CrossRef]
- van Dijk, K.; Ding, Y.; Malkaram, S.; Riethoven, J.-J.M.; Liu, R.; Yang, J.; Laczko, P.; Chen, H.; Xia, Y.; Ladunga, I.; Avramova, Z.; Fromm, M. , Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 238. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Shou, H.; Schultz, M.D.; Chiarenza, S.; Nussaume, L.; Ecker, J.R.; Whelan, J.; Lister, R. , Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. eLife 2015, 4, e09343. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef]
- Santos, A.P.; Ferreira, L.; Maroco, J.; Oliveira, M.M. Abiotic stress and induced DNA hypomethylation cause interphase chromatin structural changes in rice rDNA loci. Cytogenet Genome Res. 2011, 132, 297–303. [Google Scholar] [CrossRef]
- Tomás, D.; Brazão, J.; Viegas, W.; Silva, M. Differential effects of high-temperature stress on nuclear topology and transcription of repetitive noncoding and coding rye sequences. Cytogenet. Genome Res 2013, 139, 119–127. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.U.; He, S.; et al. Cold stress selectively unsilences tandem repeats in heterochromatin associated with accumulation of H3K9ac. Plant Cell Environ 2012, 35, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015, 83, 149–159. [Google Scholar] [CrossRef]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.-R.; Bäurle, I. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Métraux, J.-P.; Mauch-Mani, B. Dissecting the b-aminobutyric acid–induced priming phenomenon in Arabidopsis. Plant Cell 2005, 17, 987–999. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Jaskiewicz, M.; Liu, Y.; Underwood, W.R.; He, S.Y.; Zhang, S.; Conrath, U. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsisthaliana. Plant Cell 2009, 21, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Pick, T.; Jaskiewicz, M.; Peterhäensel, C.; Conrath, U. Heat shock factor HsfB1 primes gene transcription and systemic acquired resistance in Arabidopsis. Plant Physiol. 2012, 159, 52–55. [Google Scholar] [CrossRef]
- Greally, J.M. A user's guide to the ambiguous word'epigenetics. Nature Reviews Molecular Cell Biology 2018, 19, 207–208. [Google Scholar] [CrossRef]
- Feng, S.; Jacobsen, S.E.; Reik, W. Epigenetic reprogramming in plant and animal development. Science 2010, 330, 622–627. [Google Scholar] [CrossRef]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-G.; Shan, J.-X.; Shi, M.; et al. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J 2014, 80, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Stief, A.; Altmann, S.; Hoffmann, K.; et al. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, K.; Xu, Y.; et al. Plant responses to abiotic stress regulated by histone deacetylases. Front Plant Sci 2017, 8, 2147. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Faivre, L.; Bäurle, I.; Schubert, D. Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ. 2018. [CrossRef]
- Sequeira-Mendes, J.; Araguez, I.; Peiro, R.; et al. The functional topography of the Arabidopsis genome is organized in a reduced number of linear motifs of chromatin states. Plant Cell 2014, 26, 2351–2366. [Google Scholar] [CrossRef]
- Miller, M.J.; Barrett-Wilt, G.A.; Hua, Z.; Vierstra, R.D. Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc Natl Acad Sci 2010, 107, 16512–16517. [Google Scholar] [CrossRef]
- Lämke, J.; Brzezinka, K.; Altmann, S.; et al. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J 2016, 35, 162–175. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol 2017, 18, 124. [Google Scholar] [CrossRef]
- Bäurle, I. Can’t remember to forget you: chromatin-based priming of somatic stress responses. Semin Cell Dev Biol 2017, 83, 133–139. [Google Scholar] [CrossRef]
- Liu, H.; Lämke, J.; Lin, S.; et al. Distinct heat shock factors and chromatin modifications mediate the organ-autonomous transcriptional memory of heat stress. Plant J 2018, 95, 401–413. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; et al. Editing DNA methylation in the mammalian genome. Cell 2016, 167, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Alonso, C.; Becker, C.; Bossdorf, O.; Bucher, E.; Colome-Tatche, M.; Durka, W.; Engelhardt, J.; Gaspar, B.; Gogol-Doring, A.; et al. Ecological plant epigenetics: evidence from model and non-model species, and the way forward. Ecology Letters 2017, 20, 1576–1590. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Huang, S.C.; Jupe, F.; Sasaki, E.; Schmitz, R.J.; Urich, M.A.; Castanon, R.; Nery, J.R.; Barragan, C.; He, Y.; et al. Epigenomic diversity in a global collection of Arabidopsisthaliana accessions. Cell 2016, 166, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Farlik, M.; Halbritter, F.; Muller, F.; Choudry, F.; Ebert, P.; Klughammer, J.; Farrow, S.; Santoro, A.; Ciaurro, V.; Mathur, A.; et al. DNA methylation dynamics of human hematopoietic stem cell differentiation. Cell Stem Cell 2016, 19, 808–822. [Google Scholar] [CrossRef]
- Juhling, F.; Kretzmer, H.; Bernhart, S.H.; Otto, C.; Stadler, P.F.; Hoffmann, S. metilene: fast and sensitive calling of differentially methylated regions from bisulfite sequencing data. Genome Research 2016, 26, 256–262. [Google Scholar] [CrossRef]
- Hagmann, J.; Becker, C.; Muller, J.; Stegle, O.; Meyer, R.C.; Wang, G.; Schneeberger, K.; Fitz, J.; Altmann, T.; Bergelson, J.; et al. Century-scale methylome stability in a recently diverged Arabidopsisthaliana lineage. PLoS Genetics 2015, 11, e1004920. [Google Scholar] [CrossRef] [PubMed]
- Omony, J.; Nussbaumer, T.; Gutzat, R. DNA methylation analysis in plants: review of computational tools and future perspectives. Briefings in Bioinformatics 2019, bbz039. [Google Scholar] [CrossRef]
- Merret, R.; Descombin, J.; Juan, Y.-T.; Favory, J.-J.; Carpentier, M.-C.; Chaparro, C.; Charng, Y.-Y.; Deragon, J.-M.; Bousquet-Antonelli, C. XRN4 and LARP1 Are Required for a Heat-Triggered mRNA Decay Pathway Involved in Plant Acclimation and Survival during Thermal Stress. Cell Rep. 2013, 5, 1279–1293. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Matsui, A.; Tanaka, M.; Mizunashi, K.; Nakaminami, K.; Hayashi, M.; Iida, K.; Toyoda, T.; Nguyen, D.V.; Seki, M. Loss of Arabidopsis 5′–3′ exoribonuclease AtXRN4 function enhances heat stress tolerance of plants subjected to severe heat stress. Plant Cell Physiol. 2015, 56, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Latzel, V.; Janeček, Š.; Doležal, J.; Klimešová, J; Bossdorf, O. Adaptive transgenerational plasticity in the perennial Plantagolanceolata. Oikos 2014, 123, 41–46. [Google Scholar] [CrossRef]
- Latzel, V. ; Klimešová, J Transgenerational plasticity in clonal plants. Evol. Ecol. 2010, 24, 1537–1543. [Google Scholar] [CrossRef]
- Latzel, V.; Rendina González, A.P.; Rosenthal, J. Epigenetic Memory as a Basis for Intelligent Behavior in Clonal Plants. Front. Plant Sci. 2016, 7, 1354. [Google Scholar] [CrossRef]
- Rendina González, A.P.; Preite, V.; Verhoeven, K.J.F.; Latzel, V. Transgenerational Effects and Epigenetic Memory in the Clonal Plant Trifoliumrepens. Front. Plant Sci. 2018, 9, 1677. [Google Scholar] [CrossRef] [PubMed]
- Lampei, C.; Metz, J.; Tielborger, K. Clinal population divergence in an adaptive parental environmental effect that adjusts seed banking. New Phytol. 2017, 214, 1230–1244. [Google Scholar] [CrossRef]
- Raj, S.; Bräutigam, K.; Hamanishi, E.T.; Wilkins, O.; Schroeder, W.; Mansfield, S.D.; et al. Clone history shapes populus drought responses. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 12521–12526. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.-T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational epigenetic inheritance in plants. Biochim. Biophys. Acta.
- Martienssen, R.; Barkan, A.; Taylor, W.C.; Freeling, M. Somatically heritable switches in the DNA modification of Mu transposable elements monitored with a suppressible mutant in maize. Genes Dev. 1990, 4, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Vincent, C.; Coen, E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature.
- Baulcombe, D.C.; Dean, C. Epigenetic regulation in plant responses to the environment. Cold Spring Harb. Perspect. Biol. 2014, 6, a019471. [Google Scholar] [CrossRef]
- Kalisz, S.; Purugganan, M.D. Epialleles via DNA methylation: consequences for plant evolution. Trends Ecol. Evol. 2004, 19, 309–314. [Google Scholar] [CrossRef]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef]
- Calarco, J.P.; Borges, F.; Donoghue MT, A.; Van Ex, F.; Jullien, P.E.; Lopes, T.; et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell.
- Matzke, M.A.; Mosher, R.A. A. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Saze, H.; Scheid, O.M.; Paszkowski, J. Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat. Genet. 2003, 34, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Mirouze, M.; Paszkowski, J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.; Blevins, T.; Yao, Y.; Golubov, A.; Bilichak, A.; Ilnytskyy, Y.; et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of dicer-like proteins. PLoS One 2010, 5, e9514. [Google Scholar] [CrossRef]
- Lang-Mladek, C.; Popova, O.; Kiok, K.; Berlinger, M.; Rakic, B.; Aufsatz, W.; et al. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Mol. Plant 2010, 3, 594–602. [Google Scholar] [CrossRef]
- Latzel, V.; Rendina González, A.P.; Rosenthal, J. Epigenetic memory as a basis for intelligent behavior in clonal plants. Front. Plant Sci. 2016, 7, 1354. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E. Adaptive transgenerational plasticity in plants: Case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef]
- Crisp Peter, A.; Ganguly Diep Eichten Steven, R.; Borevitz Justin, O.; Pogson Barry, J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef]
- Kumar, S. Epigenetic memory of stress responses in plants. J. Phytochem. Biochem. 2018, 2, e102. [Google Scholar]
- Verhoeven, K.J.F.; VanGurp, T.P. Transgenerational effects of stress exposure on offspring phenotypes inapomictic dandelion. PLoS ONE 2012, 7, e38605. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Wang, G.; Meyers, B.C.; Fellowship, D.Y. Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2013, 2, e00354. [Google Scholar]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Ton, J. The epigenetic machinery controlling transgenerational systemic acquired resistance. Plant Signal. Behav. 2012, 7, 615–618. [Google Scholar] [CrossRef]
- Becker, C.; Hagmann, J.; Müller, J.; Koenig, D.; Stegle, O.; Borgwardt, K.; Weigel, D. Spontaneous epigenetic variation in the Arabidopsisthalianamethylome. Nature 2011, 480, 245–249. [Google Scholar] [CrossRef]
- Ni, Z.; Kim, E.D.; Ha, M.; Lackey, E.; Liu, J.; Zhang, Y.; Sun, Q.; Chen, Z.J. Altered circadian rhythms regulategrowth vigour in hybrids and allopolyploids. Nature 2009, 457, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Li, T.; Luo, L. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).