Submitted:
16 August 2023
Posted:
17 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell cultures
2.2. Flow cytometry analysis
2.3. RNA extraction and QRT-PCR reaction
2.4. Statistical Analysis
3. Results
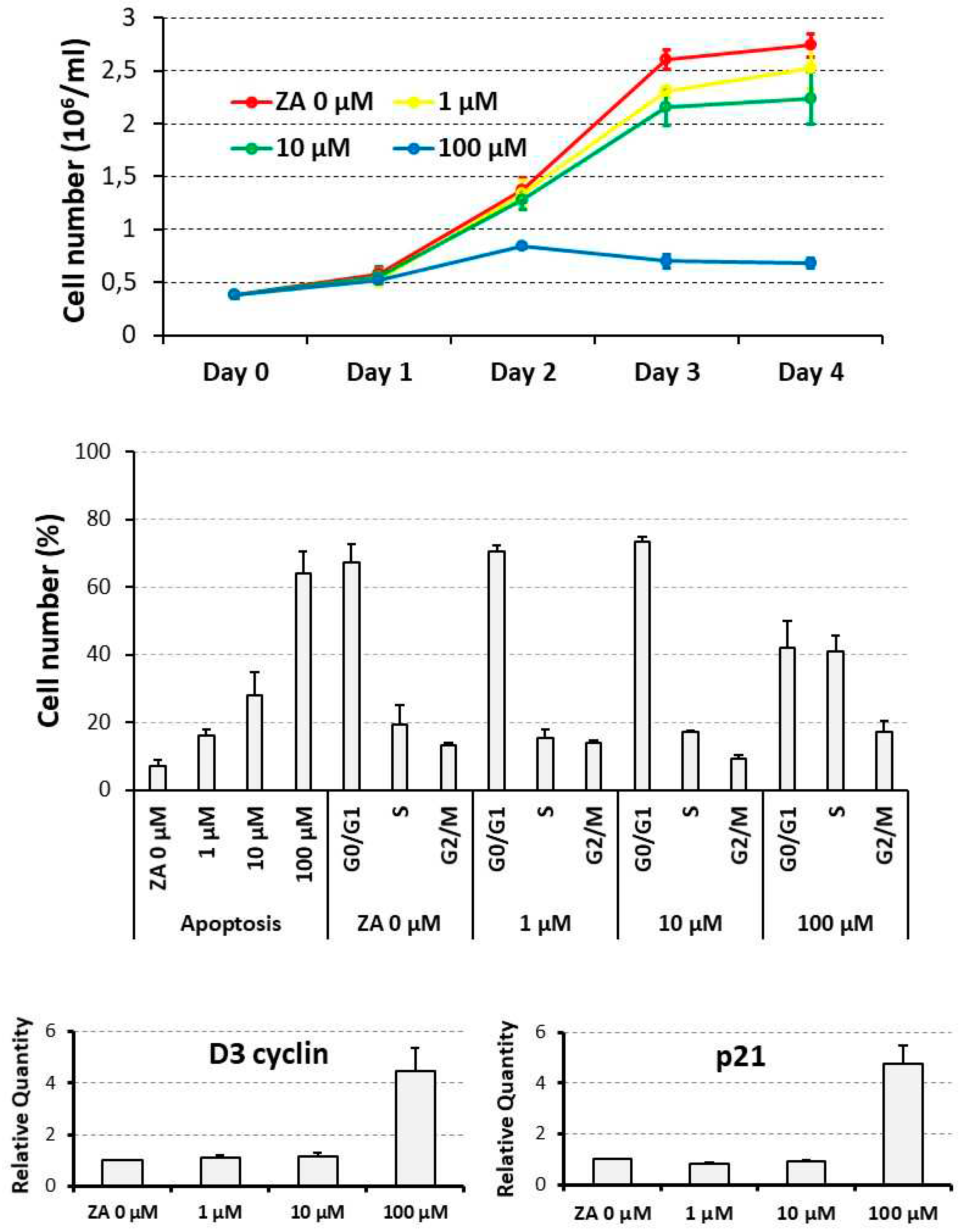
3.1. Proliferative and apoptotic effects determined on cycling U937 cells by scalar concentrations of Zoledronate
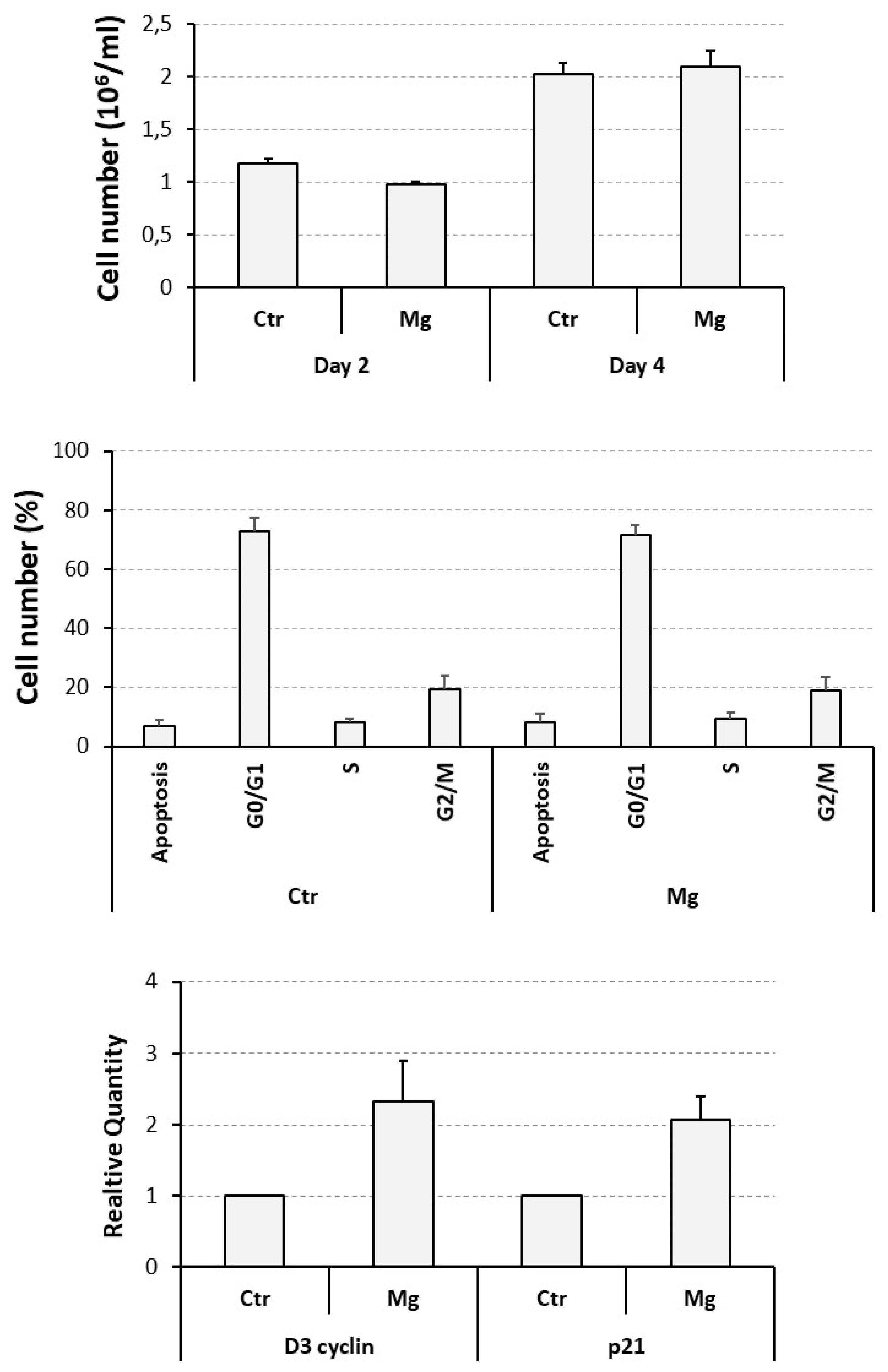
3.2. Proliferative and apoptotic effects determined on cycling U937 cells by a supra- physiological concentration of MgCl2
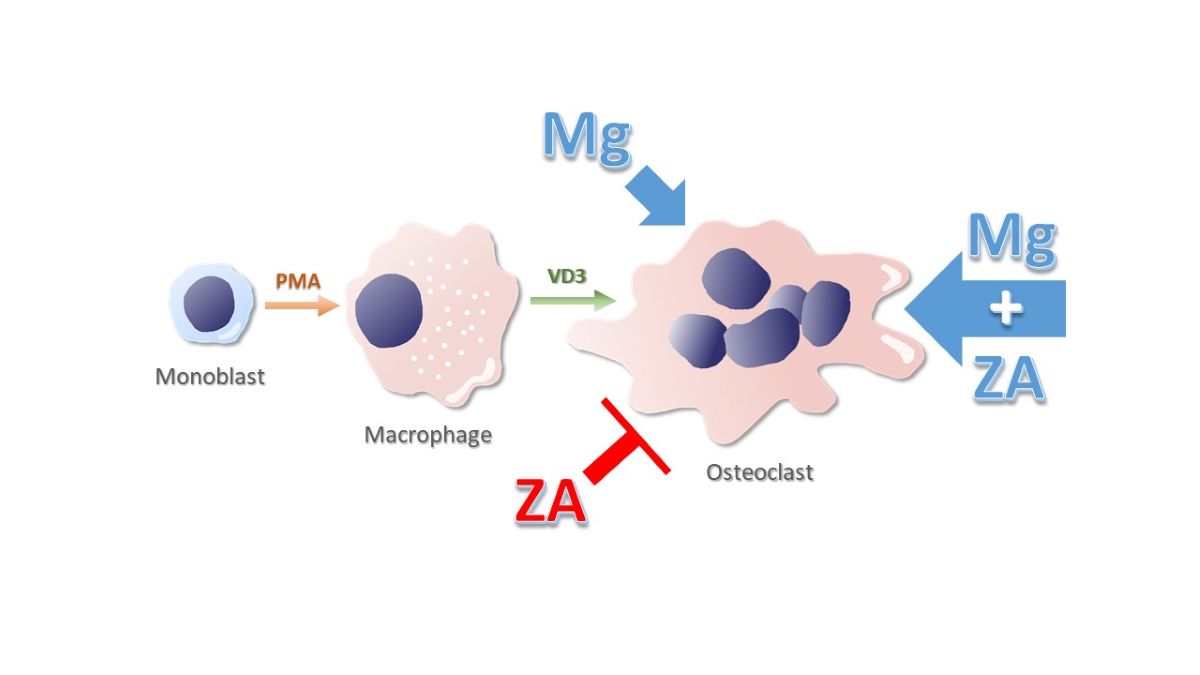
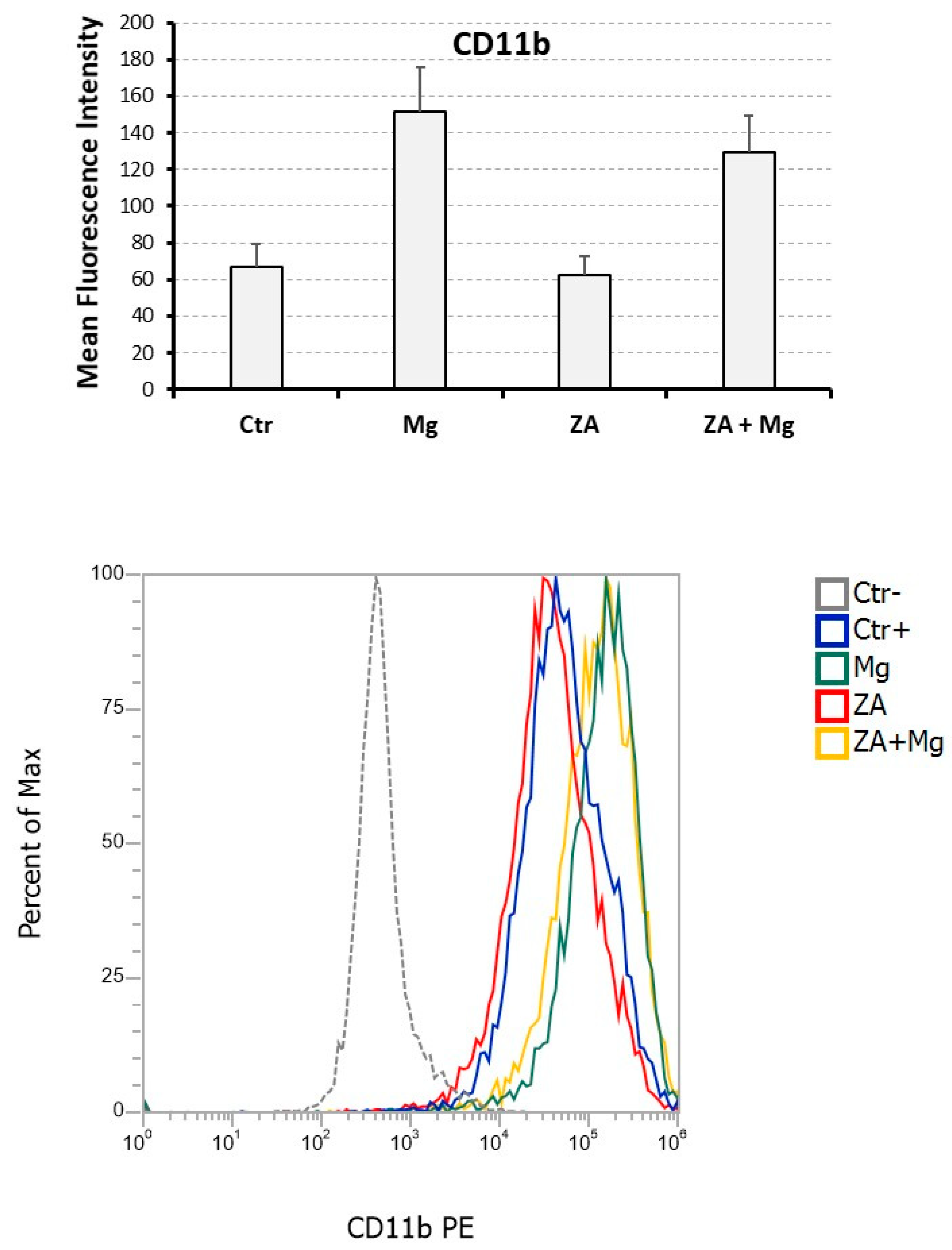
3.3. Capacity of MgCl2 and ZA to modulate the osteoclast differentiation of U937 cells induced by stimulation with Phorbol Esters and Vitamin D3
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cremers, S.; Drake M., T.; Ebetino, F.; Bilezikian J., P. ; R. Graham G. Russell. Pharmacology of Bisphosphonates. Br J Clin Pharmacol 2019, 85, 1052–62. [Google Scholar] [CrossRef]
- An, Y.; Zhao, J. Functionalized Selenium Nanotherapeutics Synergizes With Zoledronic Acid to Suppress Prostate Cancer Cell Growth Through Induction of Mitochondria-Mediated Apoptosis and Cell Cycle S Phase Arrest. Front. Oncol. 6857. [Google Scholar] [CrossRef]
- Wang, B.; Zhan, Y.; Yan, L.; Hao, D. How Zoledronic Acid Improves Osteoporosis by Acting on Osteoclasts. Front. Pharmacol. 9619. [Google Scholar] [CrossRef]
- Wang, L.; Fang, D.; Xu, J.; Luo, R. Various Pathways of Zoledronic Acid against Osteoclasts and Bone Cancer Metastasis: A Brief Review. BMC Cancer, 1059. [Google Scholar] [CrossRef]
- Gong, L.; Altman, R. B.; Klein, T. E. Bisphosphonates Pathway. Pharmacogenet 2011, 21, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D. B. Mechanism of Action, Pharmacokinetic and Pharmacodynamic Profile, and Clinical Applications of Nitrogen-Containing Bisphosphonates. J. Dent. Res. 2007, 86, 1022–33. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Woodlock, T.; Kriegelstein, S.; Steiner, T.; Messlinger, K.; Troeltzsch, M. Physiology and Pharmacology of Nonbisphosphonate Drugs Implicated in Osteonecrosis of the Jaw. J Can Dent Assoc.
- Moriyama, Y.; Nomura, M. Clodronate: A Vesicular ATP Release Blocker. Trends in Pharmacological Sciences 2018, 39, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J. S.; Bassett, J. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, A. A.; Morrison, A.; Hanley, D. A.; Felsenberg, D.; McCauley, L. K.; O’Ryan, F.; Reid, I. R. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015; 30. [Google Scholar] [CrossRef]
- Hoefert, S.; Schmitz, I.; Weichert, F.; Gaspar, M.; Eufinger, H. Macrophages and Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ): Evidence of Local Immunosuppression of Macrophages in Contrast to Other Infectious Jaw Diseases. Clin Oral Investig 2015, 19, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Marx, R. E. Pamidronate (Aredia) and Zoledronate (Zometa) Induced Avascular Necrosis of the Jaws: A Growing Epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–17. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Kouri, M.; Papadopoulou, E.; Vardas, E.; Galiti, D. ; Epstein,J. B.; Elad, S. et al. Osteonecrosis of the Jaw Related to Non-Antiresorptive Medications: A Systematic Review. Support Care Cancer. [CrossRef]
- Ruggiero, S. L.; Dodson, T. B.; Aghaloo, T.; Carlson, E. R.; Ward, B. B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg 2022, 80, 920–43. [Google Scholar] [CrossRef]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac Surg Clin North Am 2015, 27, 489–96. [Google Scholar] [CrossRef]
- Chang, J.; Hakam, A. E.; McCauley L., K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–95. [Google Scholar] [CrossRef]
- Otto, S.; Tröltzsch, M.; Jambrovic, V.; Panya, S.; Probst, F.; Ristow, O.; Ehrenfeld, M.; Pautke, C. Tooth Extraction in Patients Receiving Oral or Intravenous Bisphosphonate Administration: A Trigger for BRONJ Development? J Craniomaxillofac Surg 2015, 43, 847–54. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased Incidence of Osteonecrosis of the Jaw after Tooth Extraction in Patients Treated with Bisphosphonates: A Cohort Study. Int J Oral Maxillofac Surg 2012, 41, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical Considerations for Medication-Related Osteonecrosis of the Jaw: A Comprehensive Literature Review. Int. J. Implant Dent. [CrossRef]
- Asagiri, M.; Takayanagi, H. The Molecular Understanding of Osteoclast Differentiation. Bone 2007, 40, 251–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-L.; Huang, L.-Y.; Cheng, Y.-T.; Li, F.; Zhou, Q.; Wu, C.; Shi, Q.-H.; Guan, Z.-Z.; Liao, J.; Hong, W. Zoledronic Acid Inhibits Osteoclast Differentiation and Function through the Regulation of NF-ΚB and JNK Signalling Pathways. Int. J.Mol. Med. 2019, 44, 582–92. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yu, W.; Zhao, H.; Su, J.; Sheng, Q. Skeletal Site-Specific Effects of Zoledronate on in Vivo Bone Remodeling and in Vitro BMSCs Osteogenic Activity. Sci. Rep. 3612. [Google Scholar] [CrossRef]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment Strategies and Outcomes of Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) with Characterization of Patients: A Systematic Review. Int J Oral Maxillofac Surg 2015, 44, 568–85. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Morrison, A.; Cheung, A.; Hashem, W.; Compston, J. Osteonecrosis of the Jaw (ONJ): Diagnosis and Management in 2015. Osteoporos Int 2016, 27, 853–59. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017; 32. [Google Scholar] [CrossRef]
- Rupel, K.; Ottaviani, G. ; Gobbo, M; Contardo, L.; Tirelli, G.; Vescovi, P.; Di Lenarda, R.; Matteo Biasotto. A Systematic Review of Therapeutical Approaches in Bisphosphonates-Related Osteonecrosis of the Jaw (BRONJ). Oral Oncol. 1049. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S. ; Grande. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 4925. [Google Scholar] [CrossRef]
- Boyle, W. J.; Scott Simonet, W.; Lacey, D. L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–42. [Google Scholar] [CrossRef] [PubMed]
- Parenti, S.; Sandoni, L.; Montanari, M.; Zanocco-Marani, T.; Anesi, A.; Iotti, S.; Manfredini, R.; Frassineti, C.; Davalli, P.; Grande, A. Magnesium Favors the Capacity of Vitamin D3 to Induce the Monocyte Differentiation of U937 Cells. Magnes. Res. 2021, 34, 114–29. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. [CrossRef]
- Amoui, M.; Suhr, S.-M.; Baylink, D. J.; Lau, K.-H. W. An Osteoclastic Protein-Tyrosine Phosphatase May Play a Role in Differentiation and Activity of Human Monocytic U-937 Cell-Derived, Osteoclast-like Cells. Am. J. Physiol., Cell Physiol. [CrossRef]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D Endocrine System and Osteoclasts. BoneKEy Rep. [CrossRef]
- Li, P.; Zhao, Z.; Wang, L.; Jin,X. ; Shen, Y.; Nan, C.; Liu, H. Minimally Effective Concentration of Zoledronic Acid to Suppress Osteoclasts in Vitro. Exp. Ther. Med. 2018, 15, 5330–36. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A. F.; Willumeit-Römer, R.; Luthringer, B. J. C. Effects of Extracellular Magnesium Extract on the Proliferation and Differentiation of Human Osteoblasts and Osteoclasts in Coculture. Acta Biomater 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; et al. Autoamplification of NFATc1 Expression Determines Its Essential Role in Bone Homeostasis. Exp. Biol. Med. (Maywood) 2005, 202, 1261–69. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T. Regulators of Osteoclast Differentiation and Cell-Cell Fusion. Keio J Med 2011, 60, 101–5. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Villarroel-Espindola, F.; Schalper, K. A.; Pusztai, L.; Rimm, D. L. CD68, CD163, and Matrix Metalloproteinase 9 (MMP-9) Co-Localization in Breast Tumor Microenvironment Predicts Survival Differently in ER-Positive and -Negative Cancers. Breast Cancer, 20. [CrossRef]
- Kim, K.; Kim J., A.; Lee, J. J.; Jin, H. J.; Kook, H.; Kim, K. K.; Lee, S. Y.; Kim, N. MafB Negatively Regulates RANKL-Mediated Osteoclast Differentiation. Blood 2007, 109, 3253–59. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Kou, X.; Luo, Q.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; et al. Enhanced M1/M2 Macrophage Ratio Promotes Orthodontic Root Resorption. J. Dent. Res. 2015, 94, 129–39. [Google Scholar] [CrossRef] [PubMed]
- 40. Fukui, Shoichi, Naoki Iwamoto, Ayuko Takatani, Takashi Igawa, Toshimasa Shimizu, Masataka Umeda, Ayako Nishino, et al.. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Frontiers in Immunology, 1958. [CrossRef]
- Yang, G.; Chen, X.; Yan, Z.; Zhu, Q.; Yang, C. CD11b Promotes the Differentiation of Osteoclasts Induced by RANKL through the Spleen Tyrosine Kinase Signalling Pathway. J. Cell. Mol. Med. 2017, 21, 3445–52. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, X.; Xia, Y.; Yu, Z.; Cai, N.; Malwal, S. R.; Han, X.; Oldfield, E.; Zhang, Y. Farnesyl Pyrophosphate Synthase as a Target for Drug Development: Discovery of Natural-Product-Derived Inhibitors and Their Activity in Pancreatic Cancer Cells. J. Med. Chem. 2019, 62, 10867–96. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G. A. Bone Mass Homeostasis and Bisphosphonate Action. Bone 1997, 20, 1–4. [Google Scholar] [CrossRef]
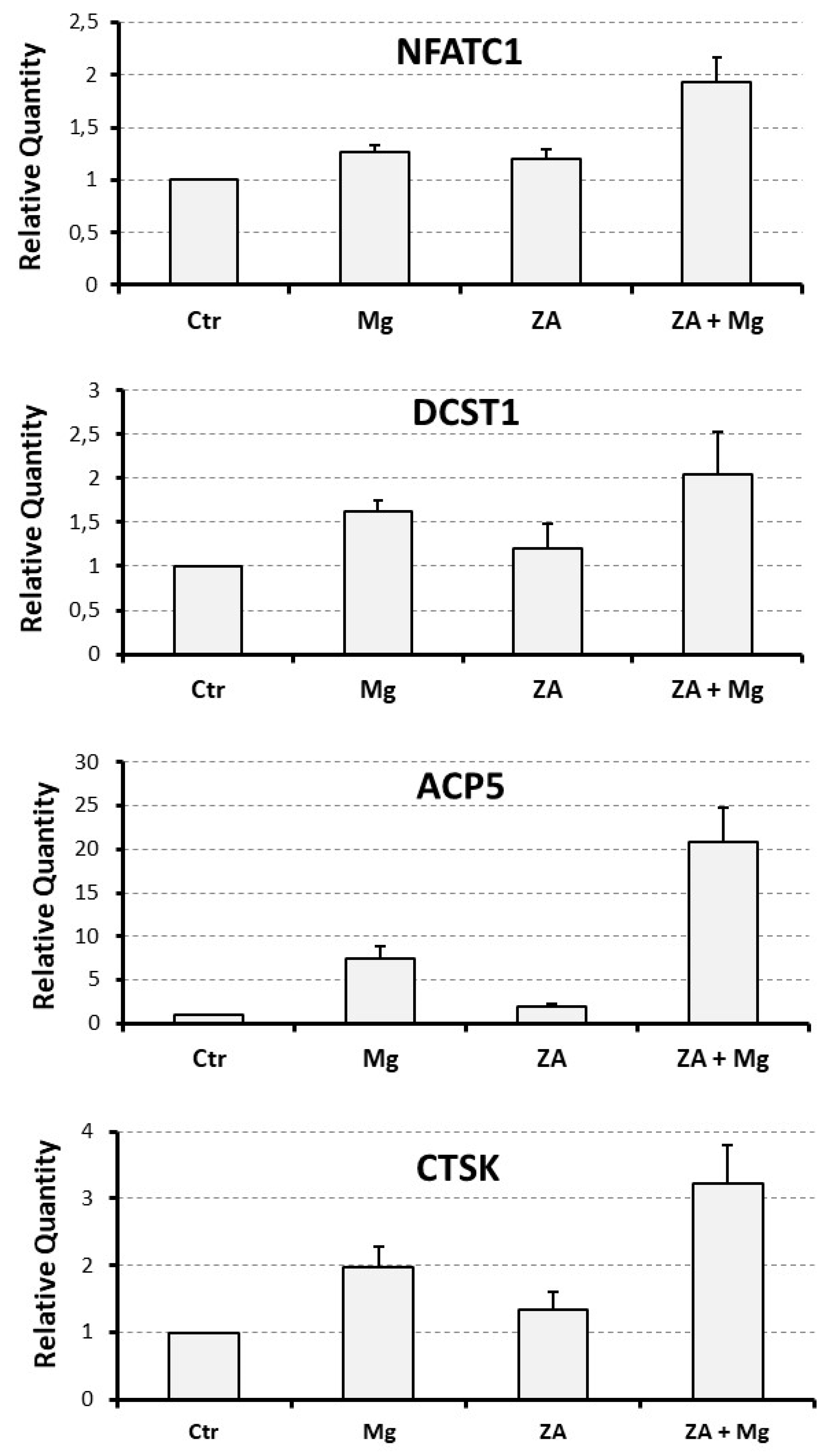
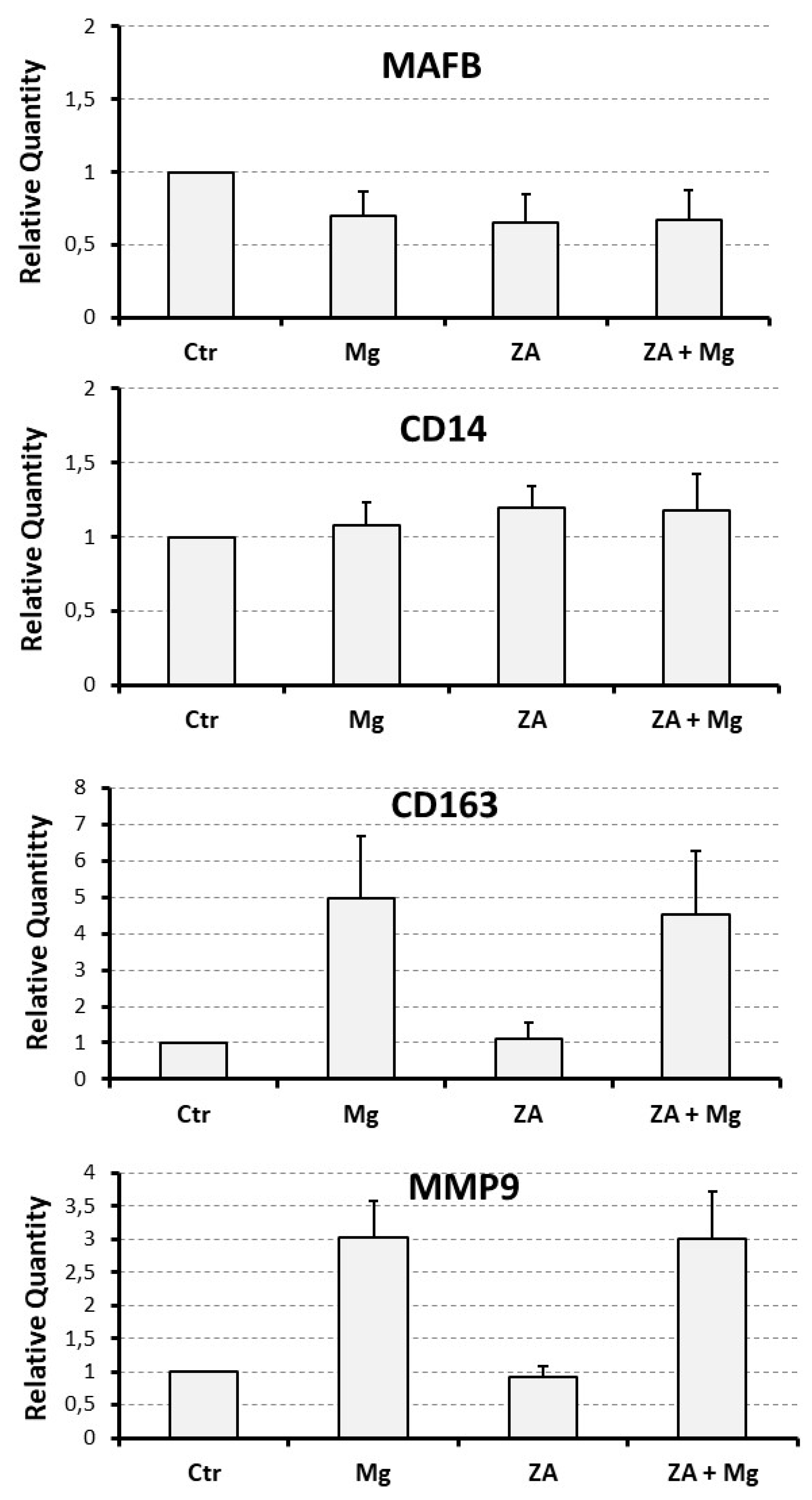
| Analyzed Marker |
Ctr | Mg | ZA | ZA + Mg | Anova, p value |
|---|---|---|---|---|---|
| NFATC1 | 1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.2 | 0.005 |
| DCST1 | 1 | 1.6 ± 0,1 | 1.2 ± 0.3 | 2.1 ± 0.5 | 0.3 |
| ACP5 | 1 | 7.5 ± 1.4 | 2.0 ± 0.3 | 20.9 ± 4.0 | 0.002 |
| CTSK | 1 | 2.0 ± 0.3 | 1.3 ± 0.3 | 3.2 ± 0.6 | 0.01 |
| MMP9 | 1 | 3.0 ± 0.6 | 0.9 ± 0.2 | 3.0 ± 0.7 | 0.01 |
| MAFB | 1 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.4 |
| CD14 | 1 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.3 | 0.8 |
| CD163 | 1 | 5.0 ± 1.7 | 1.1 ± 0.4 | 4.5 ± 1.7 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





