1. Introduction
Renewable energies are increasingly becoming an alternative solution for overcoming the problems of fossil fuel pollution and meeting the growing demand for energy from human activity. However, to overcome the problem of intermittency, which limits their use when needed, renewable energy storage systems are currently emerging as credible and effective solutions. This concern has motivated the development of various storage systems, such as batteries [
1,
2] and supercapacitors [
3,
4]. Among these energy storage technologies, batteries appear to be the most promising.
Today, of all secondary batteries, lithium-ion batteries (LIBs) are the most widely used technology and have attracted a great deal of attention from the scientific community. This is due to their excellent performance, such as compactness, high-power, high-energy density, and high cycling capacity. LIBs are the most widely used secondary batteries as energy sources in portable technologies and electric vehicles [
5,
6] and as energy storage systems in solar and wind power plants [
7]. Research into overcoming the difficulties associated with the limitations of LIBs and improving their performance is a hot topic in materials science. Although some limitations have been overcome, others are still being researched. Recent progress has focused on the preparation of innovative anode materials with optimized properties, which help to maintain the battery's capacity during its cycle. It is well known that electrode materials play a major role in determining battery efficiency. The main drawback of anode materials is that their volume expands and contracts during the battery cycle, which can lead to cracking of the anode materials. This can lead to a drastic reduction in capacity, as well as the battery exploding due to the overcharging [
8,
9].
Various strategies have been reported to overcome the reduction in anode volume expansion, including a better choice of material composition and/or architecture. In terms of material composition, TiO
2 polymorphs have been used for LIB applications and have been shown to exhibit a small volume change, less than 4%, during Li
+ lithiation/delithiation (insertion/extraction) [
10,
11]. TiO
2 polymorphs have other advantages that make them an ideal candidate for LIB applications, such as high mechanical and chemical stability, environmental friendliness, low cost, high cyclability, a relatively high theoretical capacity of 335 mA h g
-1 (anatase phase), and a flat operating potential (more than 1.7 V versus Li
+/Li) [
12,
13]. The theoretical capacity of TiO
2 is never reached due to various phenomena occurring during the LIB cycle. In fact, in most of the results reported in the literature, the capacity drops drastically after the first few cycles, and this is due both to the formation of a solid electrolyte interphase (SEI) layer on the surface of the anode materials resulting from the reaction of the electrolyte with the anode surface and to the disintegration of the anode material due to the stress induced by its volume variation during LIB cycles. To maintain a higher capacity and ensure longer reversible charge and discharge cycles, it is important to solve these problems. Identifying the morphology and architecture of materials that could accommodate the variation in anode volume and, consequently, reduce the stresses experienced by the materials during battery cycling, is of paramount importance in solving this problem. The aggregation of nanoparticles has proved to be a promising approach for obtaining materials with the desired architecture to attenuate the volume variation during LIB charge/discharge cycles [
14]. Assemblies of nanoparticles of different sizes offer numerous possibilities for tailoring the porosity of agglomerated materials, in terms of pore size and morphology [
15,
16]. In addition, nanoparticle assemblies give rise to several cases of pore connectivity [
17]. This type of material architecture is more flexible, making it possible to combine several material properties, even if they are of a conflicting nature [
15]. For LIB application, the porous materials and particularly aggregate of nanoparticles offer the possibility to accommodate the structural stress of the anode material, induced by lithium insertion, which improves the stability of LIB recycling [
16,
18]. In addition, the agglomerated porous material offers a high interface surface area in contact with the electrolyte, which greatly reduces the diffusion pathways of Li
+ ions into the material lattice [
16,
18].
In the present work, TiO2 materials with micro- and nano- structures made by TiO2 nanoparticles of different morphologies, as building unit, have been prepared using the hydrothermal method. Furthermore, on the light of the LIBs literature and the present results, the capacity decreasing with the number of discharging and charging cycles was discussed in terms of the evolution of TiO2 nanoparticles morphology, and the properties of their assembly with the LIB cycles. TiO2 materials were used as a model system to explore the underlying mechanisms behind the capacity decreasing with the first charging and discharging cycles.
3. Results and discussion
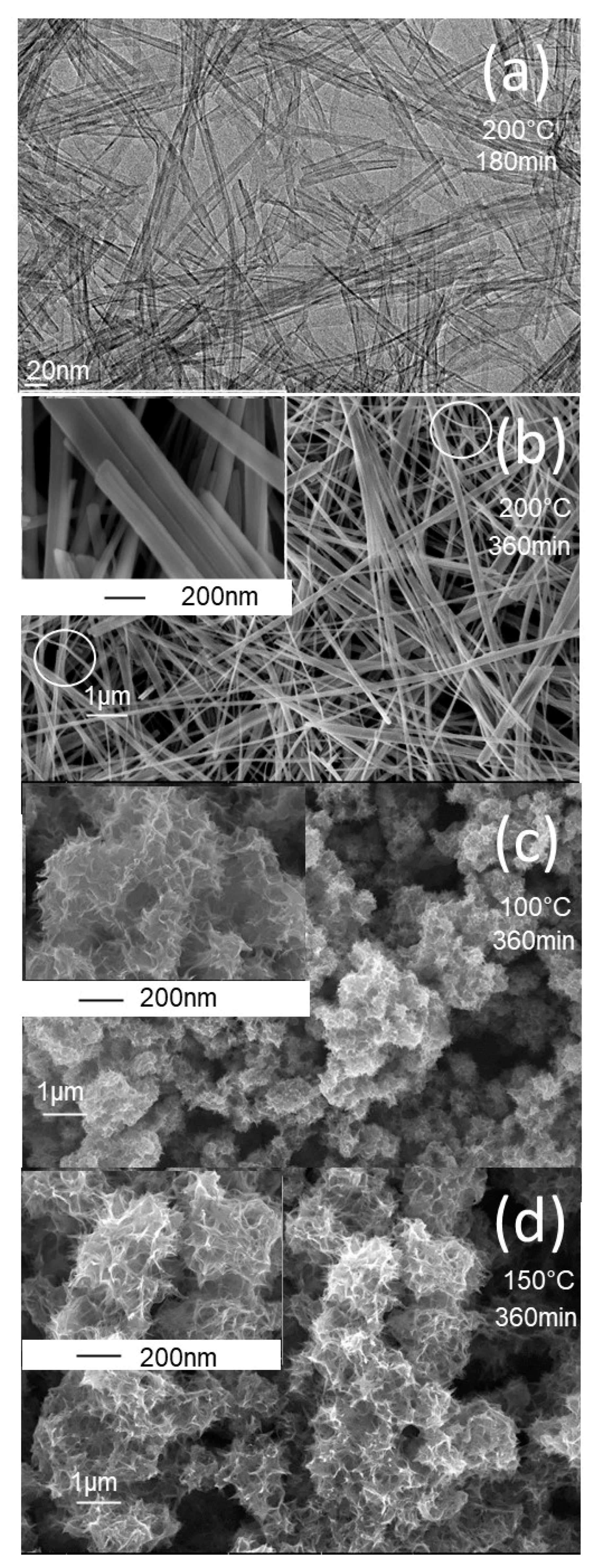
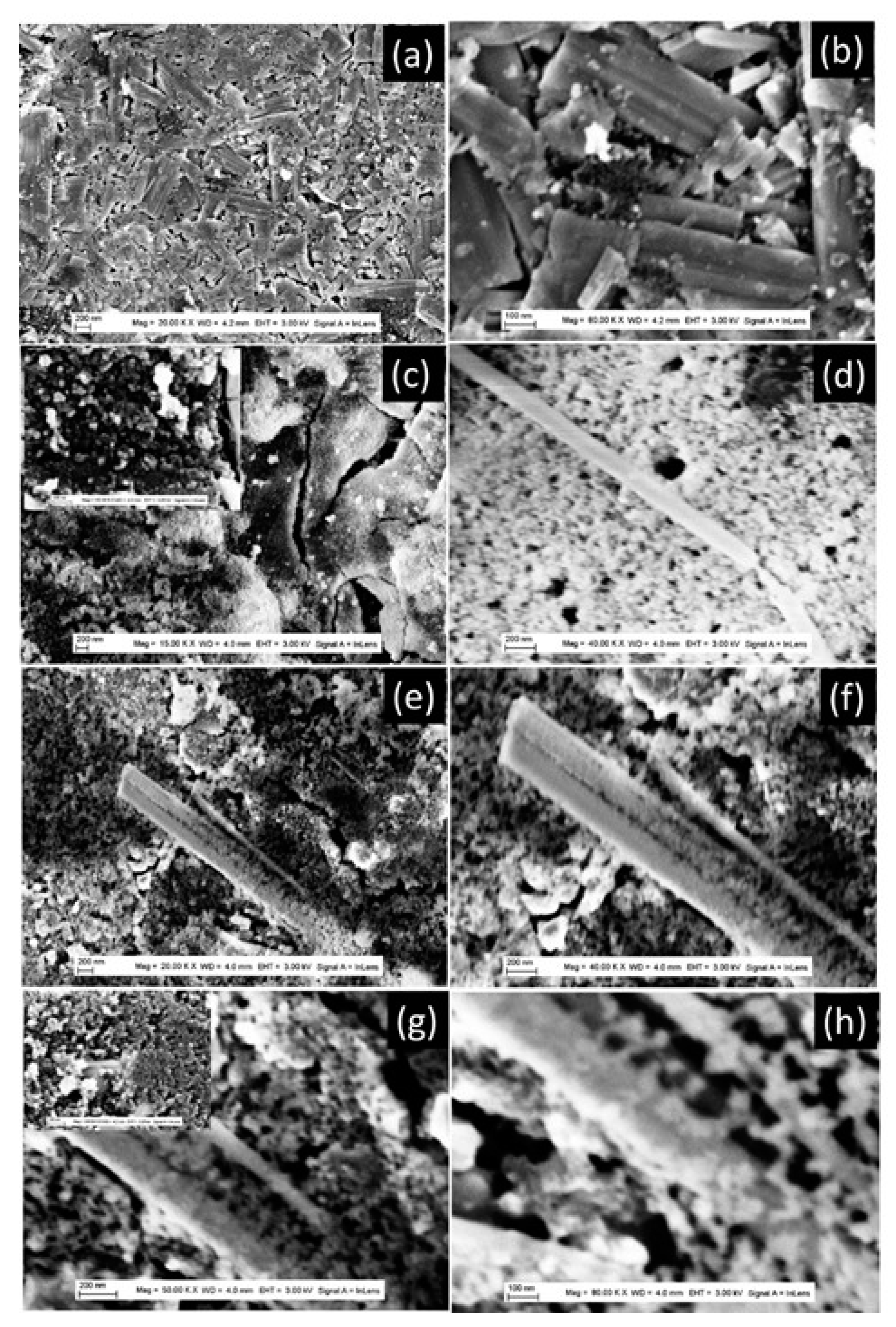
The synthesis protocols described in the experimental section yielded white powders that were characterized using a variety of techniques. The morphology of the prepared powders was characterized by FEGSEM, and the images obtained are shown in
Figure 1. At a higher synthesis temperature of 200°C (
Figure 1a,b), the TiO
2 powder exhibits a nanotube morphology at a short synthesis time of 180 min, and a nanobelt morphology at a longer synthesis time of 360 min. The insert in
Figure 1b shows a bundle of several nanobelts stacked along their longitudinal axis. It can also be seen that the nanobelts prepared have a homogeneous thickness of around 10 nm, a diameter ranging from 50 to 100 nm, and a length of more than 10 micrometers. At higher magnifications, the nanobelts have a smooth surface with no contamination. In addition, the regions shown in
Figure 1b display curved nanobelts, illustrating their high elasticity. At the synthesis temperature of 100°C, the prepared powder has a sea-urchin-like morphology, with stretched sheets connected to form a randomly connected network (
Figure 1c). At the synthesis temperature of 150°C, the morphology is like that obtained at a temperature of 150°C, but with more coiled sheets (
Figure 1d).
The EDS spectrometry was used to analyze the chemical composition of prepared TiO
2 powders, just after synthesis (
Figure 2a) and after the washing and annealing steps (
Figure 2b). The EDS spectra show the peak corresponding to Na just after synthesis
Figure 2a, whereas after washing and annealing steps, the Na peak is completely absent (
Figure 2b), which indicates the complete exchange of Na
+ ions by H
+ during the washing step.
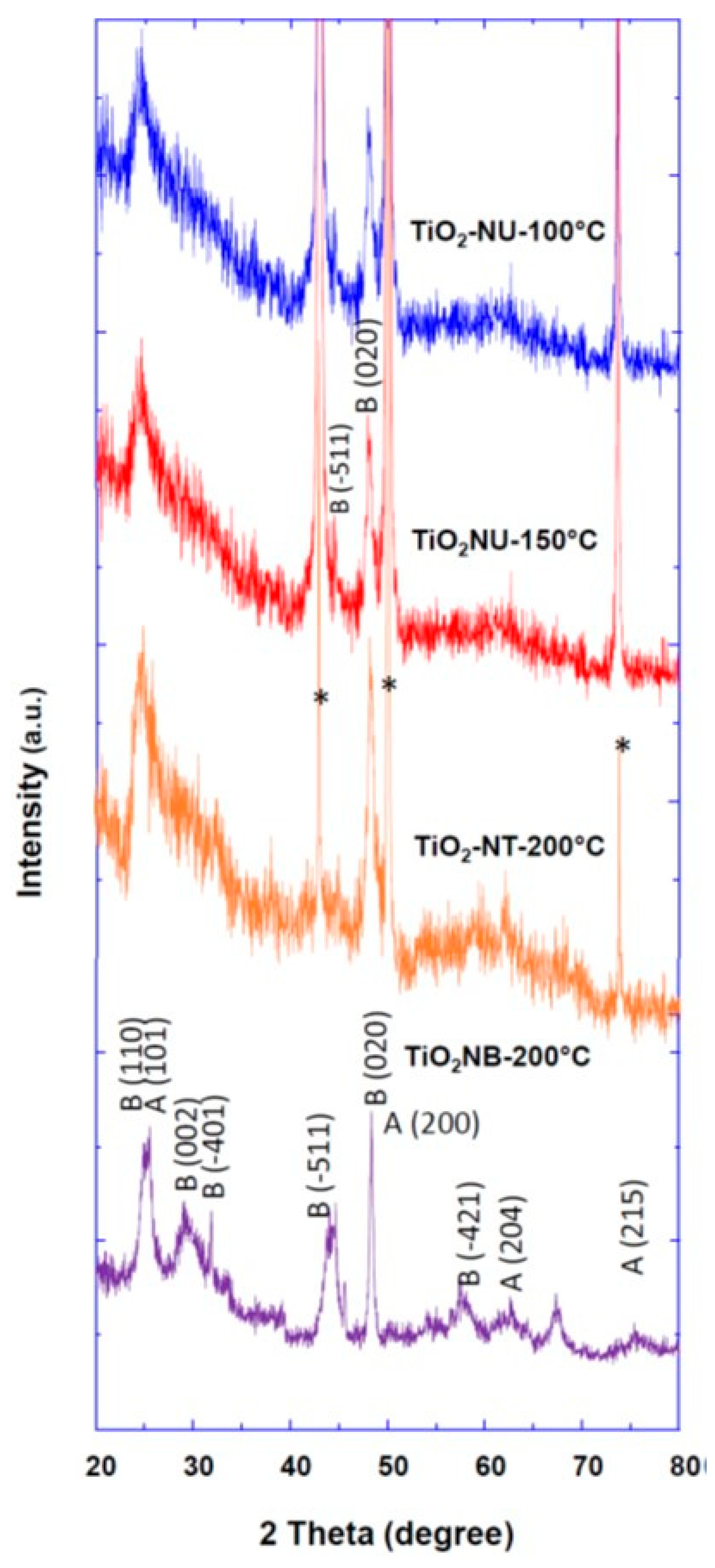
The crystalline structure and phase of prepared TiO
2 powder with different morphologies were investigated by the XRD method, and the obtained patterns are depicted in
Figure 3. In the case of TiO
2 nanourchin and nanotube morphologies (TiO
2-NT-200°C, TiO
2-NU-150°C and TiO
2-NU-100°C), well-pronounced peaks were observed and were assigned to (-511) and (020) crystallographic planes of pure TiO
2 (B) phase (JCPDS No. 35-008) (
Figure 3). In the case of TiO
2 nanobelt morphology (TiO
2-NB-200°C), the well-resolved XRD peaks were attributed to a mixture of anatase (JCPDS 83-2243) and brookite (JCPDS 29-1360) phases (
Figure 3).
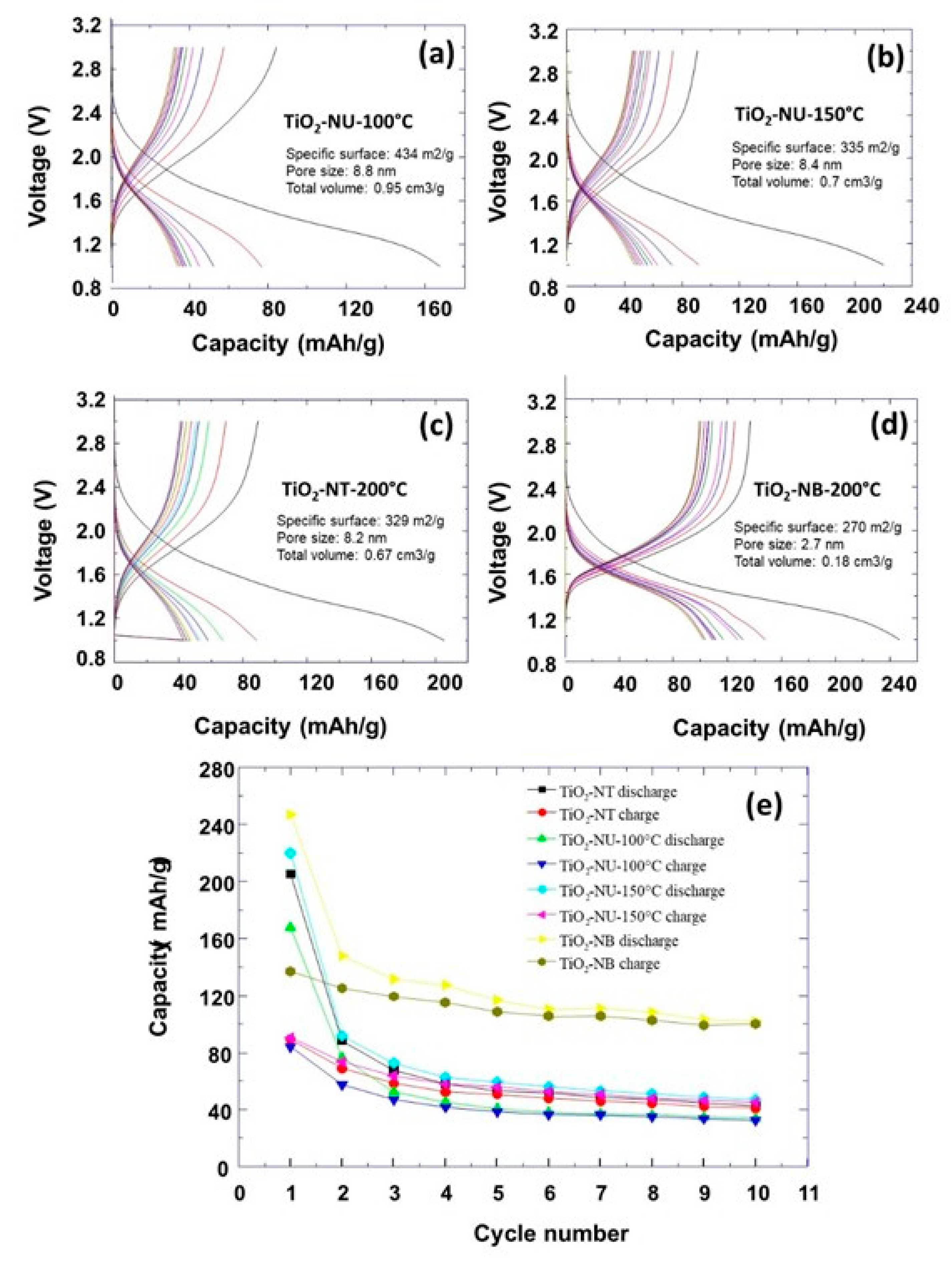
The properties of prepared TiO
2 powders in terms of specific surface area, and the average pore size, were evaluated by analyzing the nitrogen adsorption-desorption isotherms. From the isotherm curves (
Figure 4), the specific surface areas (BET model) were calculated to be 270 m
2g
−1, 329 m
2g
−1, 434 m
2g
−1 and 335 m
2g
−1, for respectively the TiO
2 powder morphology of nanobelt (TiO
2-NB-200°C), nanotube (TiO
2-NT-200°C), nano-urchin((TiO
2-NU-100°C), and (TiO
2-NU-150°C). For most of the samples a multiscale porosity is observed: the smaller pores (2.5-3 nm) are likely due to the intrinsic porosity of the particles (porosity of the nanotube, nanobelt, or nanosheet), while the porosity leading to the second maximum in the pore size distribution (about 5 nm) is probably related to pores resulting from the aggregation of the primary particles. Lastly, the larger pores (between 10 and 20 nm) could result from the flexible porosity formed between particles that are not chemically linked. It is worth noting that the porosity of TiO
2-NU-150°C is like that of TiO
2-NT-200°C, which is consistent with the fact that the nanotube particles are obtained by nanosheets enrolling as reported previously [
19]. It is commonly accepted that the large surface area enhances the material contact surface with the electrolyte, and the large pore size favors fast diffusion and transfer toward the material surface [
5,
16,
18]. These characteristics favor the improvement of LIB rate capability.
The electrochemical characterizations of prepared TiO
2 powders with different morphologies were performed. The obtained discharging/charging curves at a current rate of C/10 are shown in
Figure 5.
It can be observed that the specific capacity decreases as a function of the number of discharging/charging cycles (
Figure 5e). At the first initial discharging process, the highest capacity of about 250 mAh/g was observed for the nanobelt morphology. For the other morphologies, this initial capacity was about 210 mAh/g, 170 mAh/g and 220 mAh/g for respectively the TiO
2 powder morphologies of nanotube (TiO
2-NT-200°C), and nanourchin (TiO
2-NU-100°C and TiO
2-NB-150°C). It is important to note that the specific capacities corresponding to the different morphologies are lower than the theoretical capacity of TiO
2, which is approximately 336 mA h g
-1. One could expect that higher capacities are associated to materials with a higher specific surface, which provide a higher contact surface area with the electrolyte. For example, it can be observed that TiO
2 powders of nanotube and nanourchin-150 morphologies show similar specific capacity, which could be explained by their similar specific surface area. Moreover, TiO
2 powder with nanourchin morphology shows more enrolled nanosheets (TiO
2-NU-150°C) which resembles that of the nanotube morphology. However, the TiO
2-NU-100°C powder has a higher specific surface than nanourchin-150 (434 vs 335 m
2/g), and should, normally, exhibit a higher capacity. The electrochemical measurements show a lower capacity, which is very surprising, if only the specific surface parameter is considered. After the first discharging step, the specific capacity decreases very fast for all prepared TiO
2 powder morphologies, and it reaches a plateau after a few numbers of discharging/charging cycles. To understand this behavior, another phenomenon of individual nanoparticles should be discussed to explain the observed variation of the specific capacity versus the number of discharging/charging cycles. It was reported that the anode material expansion and shrinkage during the lithiation/dilithiation induces the formation of cracks and the initial TiO
2 particles disintegration into small nanoparticles. This in turn provokes the electric disconnection between the current collector and the anode materials. This lowers the LIB cycling stability and specific capacity [
2]. Furthermore, the observed irreversible capacity during the first cycle could be explained mainly by the formation of the passivating layer named solid electrolyte interphase layer (SEI) on the electrode surface because of the electrolyte reduction [
20,
21,
22], and the trapping of the inserted lithium in the crystal lattice defects or on the electrode surface sites [
23]. This explains the low capacity observed than the theoretical calculated capacity which is equal to 335 mA h g
-1 [
12,
24,
25].
Hereafter, we will discuss how the control over the TiO
2 powder morphology may be used to improve the Li-ion batteries' performance. It is well accepted that the pore properties (size and connectivity) and the specific surface depend on the prepared TiO
2 powder morphology, and it plays a crucial role in the optimization of the Li-ion batteries' specific capacity [
26]. To understand how these parameters are behind the observed decrease of the specific capacity, versus the discharging/charging cycles, we must investigate the evolution of the TiO
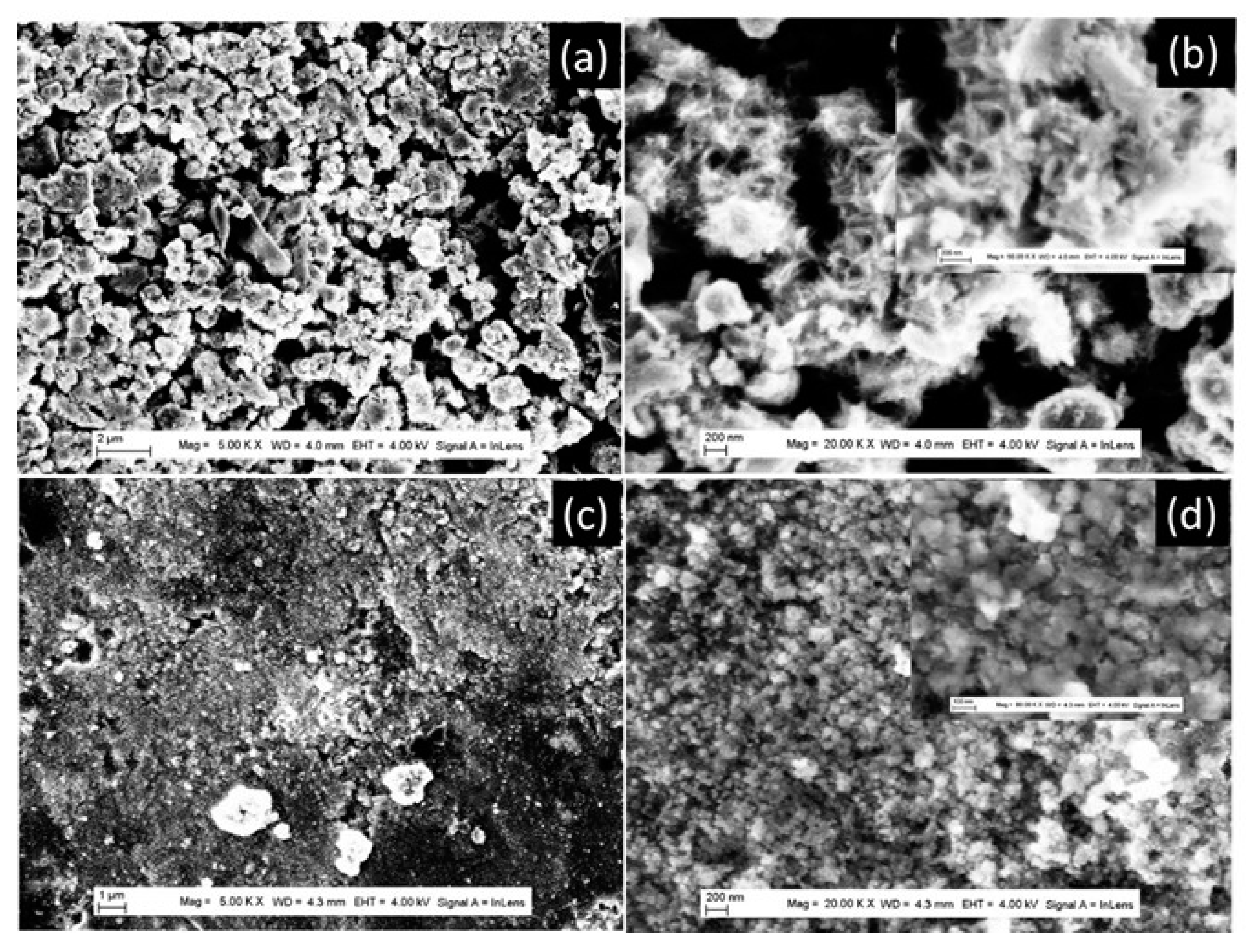
2 powder morphology during the cycling process. To check this point, the FEGSEM characterization was performed just after the preparation of the anode for battery testing and after 10 discharging/charging cycles. The results are presented in
Figure 6,
Figure 7 and
Figure 8, for prepared TiO
2 powders with different morphologies. The TiO
2-NU-100°C powder, with stretched nanosheets, starts to collapse during the preparation of the anode electrode (
Figure 6a,b), inducing the decrease of the anode-specific surface. After the 10th discharging cycle, it can be observed that the nanosheets of nano-urchin morphology did mostly collapse, to form aggregates of around 100 nm diameter (
Figure 6c,d). The observed peculiarity with the TiO
2-NU-100°C powder, in terms of low specific capacity (
Figure 5), despite that it is characterized by the highest specific surface just after synthesis, could be explained by the fact that the stretched sheet forming nano-urchin morphology is easy to collapse, during the battery’s fabrication process. After their preparation, the nanourchin morphology evolves to a denser structure with a lower specific surface than that of nanobelt morphology. Similar nanosheets collapse behavior was previously observed with TiO
2 powders of nano-urchin morphology by Tian-Hui et al [
27].
Similar behavior was observed with TiO
2-NB-200°C powder, in terms of a strong decrease of the LIB-specific capacity between the first and the 10th discharging cycle (
Figure 5). To understand this behavior in the case of nanobelt morphology, we analyzed closely the FEGSEM characterization before batteries testing, and after the 10th discharging cycle (
Figure 8). Just after the preparation of the anode, the TiO
2 powder keeps its nanobelt morphology as it can be identified in the FEGSEM pattern of
Figure 7a,b. After the 10th cycle, only the aggregates with diameters ranging from 50 nm to 200 nm could be observed, in addition to a few nanobelts (
Figure 7c–h). Further analysis of the FEGSEM images (
Figure 7e–h) shows a belt-shaped nanoparticle that is in the process of disintegrating with a coexistence of a part of the particle that is transformed into particle aggregates and another that is not yet. This clearly shows that the aggregates observed are the result of the disintegration of the belt-shaped nanoparticles under the effect of the stress generated by the lithium-ion insertion/extraction process during the charge and discharge cycles. (
Figure 7e–h). It is well known that lithium storage capacity in the anode material induces its expansion during the lithium insertion, which can provoke mechanical fracture in individual nanobelts, and its disintegration into aggregates.
With TiO
2-NT-200°C powder, aggregates are formed during the battery’s fabrication process (
Figure 8a,b). At high magnification in
Figure 8b and the insert, TiO
2 nanotubes could be observed. After the 10th cycle, cracks are formed (
Figure 8c,d), and aggregates of nanotubes could be observed with diameter ranging from 20nm to 50nm (
Figure 8e,f). Regarding, TiO
2-NU-150°C powder, it collapses during the battery’s fabrication process but with less intensity than in the case of TiO
2-NU-100°C. This is due to its nanosheet enrolled structure, which provides more resistance to the change of the powder’s morphology during the fabrication process. TiO
2 nanotubes and their aggregation led to a reduction in surface area, which explains its lower capacity compared to that of nanobelt powder.
It is well known that nanoparticle aggregates are usually a porous materials characterized by pore size, size distribution, connectivity, and specific surface. Furthermore, it is well accepted that these parameters affect the Li-ion diffusion within the LIB electrode and are also behind the volume accommodation during the insertion/extraction cycle of Li-ion [
2,
16,
18]
. The obtained results are very surprising if we consider only the geometrical model in which the reduction of the pore size induces the enhancement of the specific surface, and in turn the specific capacity as previously observed by Lin et al [
14].
In addition, the pore's connectivity should play an important role in the optimization of energy storage of porous electrodes [
28]. However, during the first discharging cycle, the TiO
2 powders are keeping different morphologies and probably different connectivity, which could also explain the observed difference in specific capacities for all the TiO
2 powders, in addition to their specific surface. The small variation in capacity in the form of plateaus observed in
Figure 5e can be explained by the fact that, for all the samples except the nanobelt nanoparticles, the shapes, sizes, and connectivity of the pores are the same for the disintegrated nanoparticles after 10 charge/discharge cycles. In addition, this very small variation in the specific capacity is the signature of a reversible charge/discharge process. This could be explained by the architecture of the anode materials in terms of the pore properties (size, shape, and connectivity), which successfully mitigate the impact of the variation in anode volume on capacity during charge/discharge cycles.
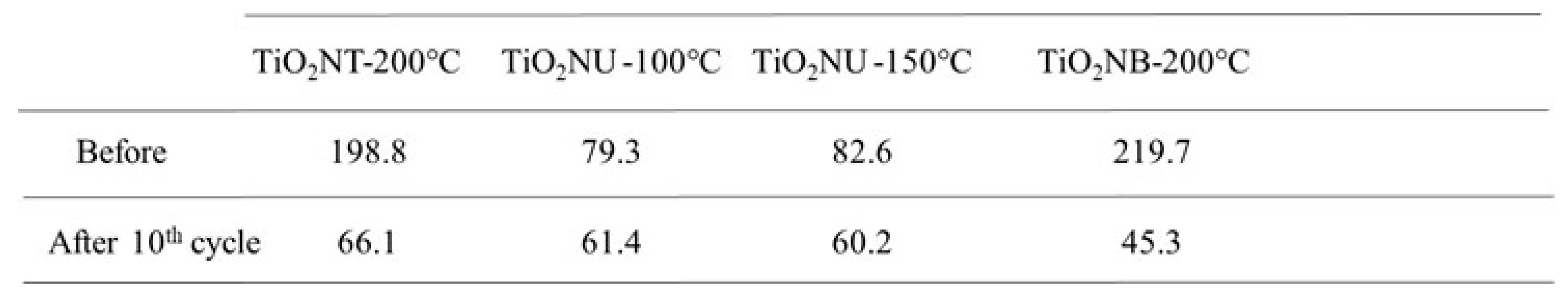
These results were confirmed by XRD experiments (
Table 1), which show that the crystallite size decreases between the 1st and the 10th cycle. This confirms that during the discharging/charging cycles, large particles were disintegrated into small ones. Furthermore, by comparing the crystallite size after the 10th cycle, we note that the nanobelt morphology shows small crystallite, which should have a high specific surface, explaining the corresponding high specific capacity. In addition, the other morphologies show more or less the same crystallite size, which explains their closer specific capacity. These results also confirm that the size of nanoparticles after the same number of LIB charge-discharge cycles depends on the properties of the powders used as active electrode materials, such as morphology, size, crystallinity, and the nature of the phases.